Oral Tongue Reconstruction with a Bozola Flap According to the Ansarin Glossectomies Classification
Abstract
1. Introduction
2. Materials and Methods
2.1. The Ansarin Classification of Glossectomies
2.2. The Anatomy of the Cheek
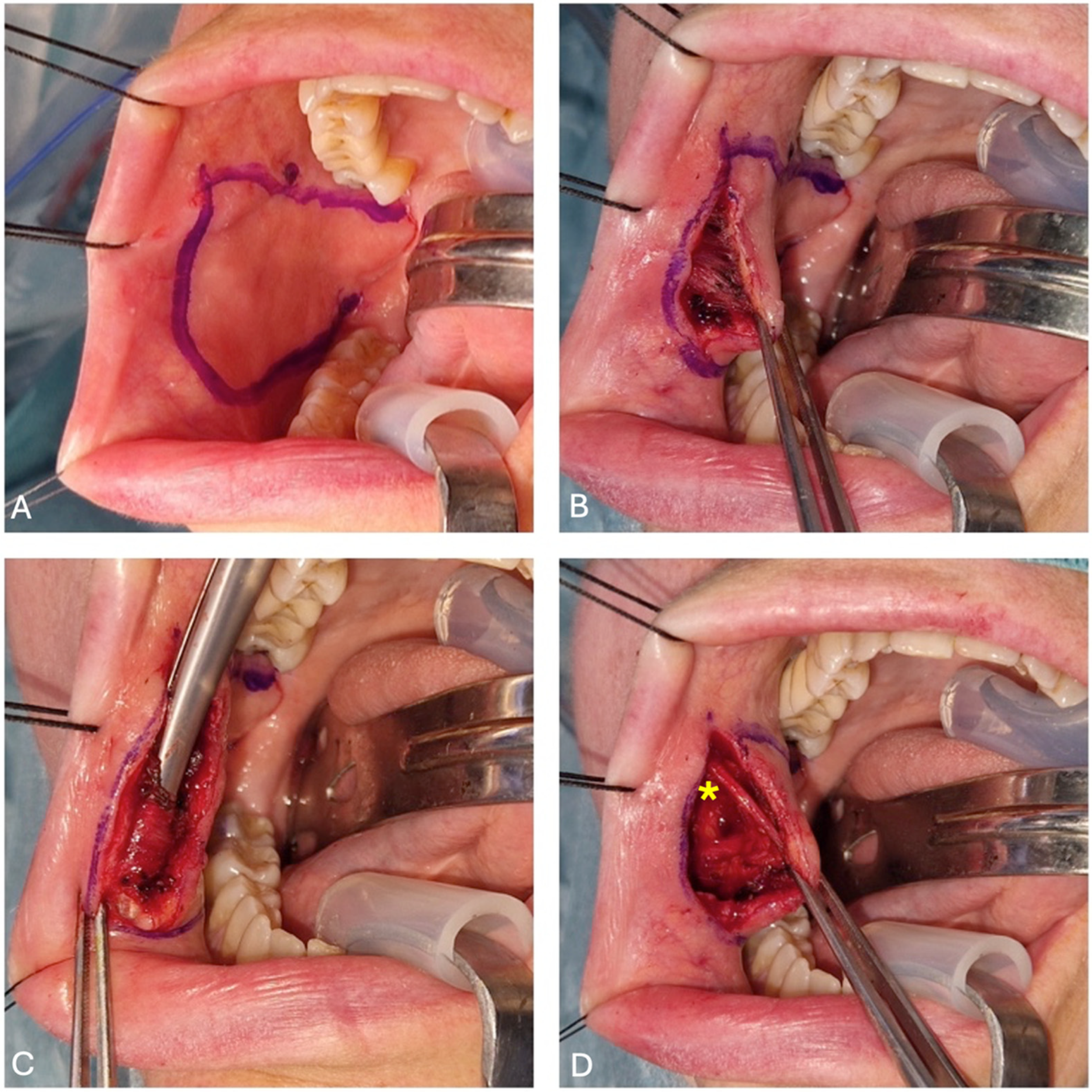
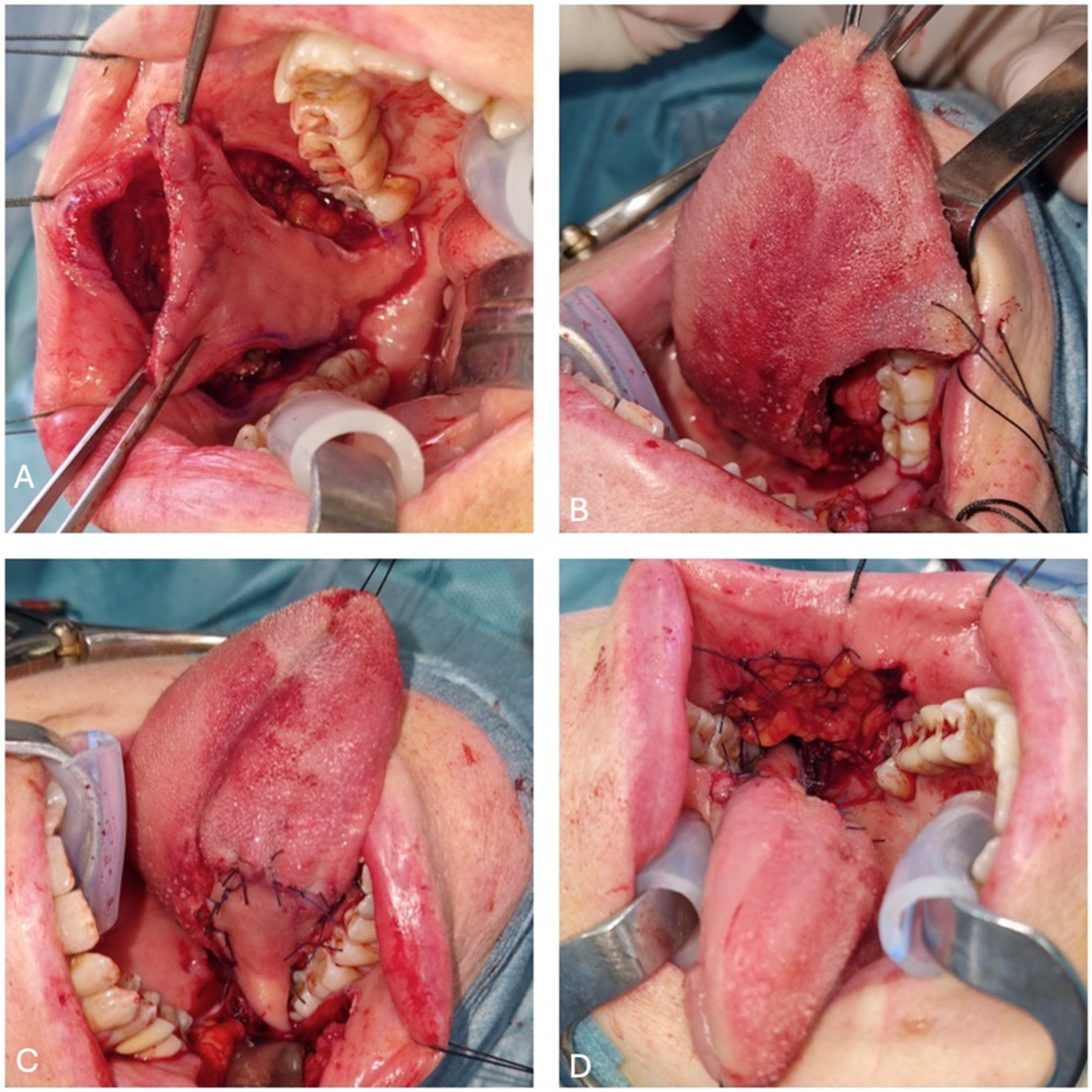
2.3. Flap Harvesting
2.4. Post-Operative Care
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molteni, G.; Ghirelli, M.; Molinari, G.; Presutti, L. Microvascular reconstruction two years after subtotal glossectomy: Is it worth it? J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, K.; Shao, Z.; Shang, Z.J. Individual design of the anterolateral thigh flap for functional reconstruction after hemiglossectomy: Experience with 238 patients. Int. J. Oral Maxillofac. Surg. 2016, 45, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Canis, M.; Weiss, B.G.; Ihler, F.; Hummers-Pradier, E.; Matthias, C.; Wolff, H.A. Quality of life in patients after resection of pT3 lateral tongue carcinoma: Microvascular reconstruction versus primary closure. Head Neck 2016, 38, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Cortina, L.E.; Moverman, D.J.; Zhao, Y.; Goss, D.; Zenga, J.; Puram, S.V.; Varvares, M.A. Functional considerations between flap and non-flap reconstruction in oral tongue cancer: A systematic review. Oral Oncol. 2023, 147, 106596. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, O.; Baj, A.; Gobbi, R.; Soma, D.; Marelli, S.; De Riu, G.; Tullio, A.; Giannì, A.B. Cheek mucosa: A versatile donor site of myomucosal flaps. Technical and functional considerations. Head Neck 2013, 35, 109–117. [Google Scholar] [CrossRef]
- Ferrari, S.; Balestreri, A.; Bianchi, B.; Multinu, A.; Ferri, A.; Sesenna, E. Buccinator myomucosal island flap for reconstruction of the floor of the mouth. J. Oral Maxillofac. Surg. 2008, 66, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Bozola, A.R.; Gasques, J.A.; Carriquiry, C.E.; Cardoso de Oliveira, M. The buccinator musculomucosal flap: Anatomic study and clinical application. Plast. Reconstr. Surg. 1989, 84, 250–257. [Google Scholar] [CrossRef]
- Ferrari, S.; Copelli, C.; Bianchi, B.; Ferri, A.; Sesenna, E. The Bozola flap in oral cavity reconstruction. Oral Oncol. 2012, 48, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Ansarin, M.; Bruschini, R.; Navach, V.; Giugliano, G.; Calabrese, L.; Chiesa, F.; Medina, J.E.; Kowalski, L.P.; Shah, J.P. Classification of GLOSSECTOMIES: Proposal for tongue cancer resections. Head Neck 2019, 41, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Iocca, O.; Copelli, C.; Ramieri, G.; Zocchi, J.; Savo, M.; Di Maio, P. Antibiotic prophylaxis in head and neck cancer surgery: Systematic review and Bayesian network meta-analysis. Head Neck 2022, 44, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Sittitrai, P.; Ruenmarkkaew, D.; Klibngern, H. Pedicled Flaps versus Free Flaps for Oral Cavity Cancer Reconstruction: A Comparison of Complications, Hospital Costs, and Functional Outcomes. Int. Arch. Otorhinolaryngol. 2022, 27, e32–e42. [Google Scholar] [CrossRef] [PubMed]
- Remangeon, F.; Hivelin, M.; Maurice, D.; Lantieri, L.; Laccourreye, O. The posterior-based buccinator myomucosal flap (Bozola’s flap). Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 59–62. [Google Scholar] [CrossRef] [PubMed]
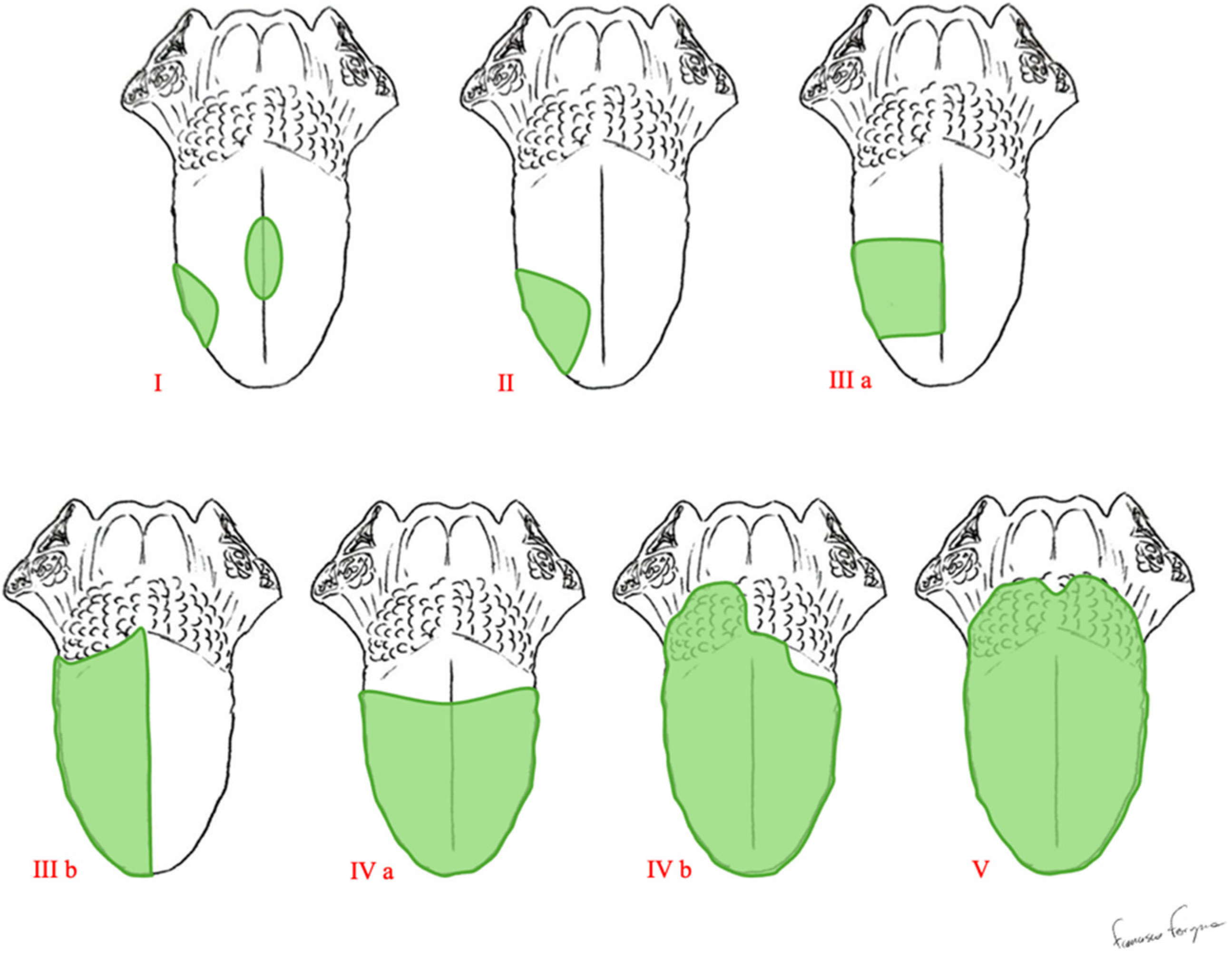

| Type of Glossectomy | Anatomical Structures Included in Surgery | |
|---|---|---|
| Type I | Mucosectomy | Mucosa and submucosa, including a thin layer of the intrinsic muscles. |
| Type II | Partial Glossectomy | Mucosa, submucosa, and intrinsic muscles up to the surface of the extrinsic muscles. |
| Type IIIa | Hemi-glossectomy | Surgery extends to all of the ipsilateral extrinsic muscles, including the lingual artery and the lingual and hypoglossal nerves. |
| Type IIIb | Compartmental Hemi-glossectomy | Surgery extends to all of the ipsilateral extrinsic muscles, including the genioglossus muscle, hyoglossus muscle, styloglossus muscle, and the lower portion of the palatoglossus muscle. The lingual nerve is resected as much as possible cranially, the hypoglossal nerve is removed after the ansa, and the lingual artery and vein are ligated in proximity to the hyoid bone. |
| Type IVa | Subtotal Glossectomy | This is an anterior-subtotal glossectomy with the preservation of both sides of the tongue base. |
| Type IVb | Near-Total Glossectomy | This is an anterior-subtotal glossectomy, including the ipsilateral base of the tongue; it preserves only the contralateral tongue base. |
| Type V | Total Glossectomy | It includes the mobile tongue, both sides of the tongue base, intrinsic and extrinsic muscles, both lingual arteries, hypoglossal, lingual nerves, and the floor of the mouth. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzano, G.; Ferragina, F.; Cocis, S.; Maglitto, F.; Manfuso, A.; Copelli, C. Oral Tongue Reconstruction with a Bozola Flap According to the Ansarin Glossectomies Classification. J. Clin. Med. 2025, 14, 1965. https://doi.org/10.3390/jcm14061965
Salzano G, Ferragina F, Cocis S, Maglitto F, Manfuso A, Copelli C. Oral Tongue Reconstruction with a Bozola Flap According to the Ansarin Glossectomies Classification. Journal of Clinical Medicine. 2025; 14(6):1965. https://doi.org/10.3390/jcm14061965
Chicago/Turabian StyleSalzano, Giovanni, Francesco Ferragina, Stefan Cocis, Fabio Maglitto, Alfonso Manfuso, and Chiara Copelli. 2025. "Oral Tongue Reconstruction with a Bozola Flap According to the Ansarin Glossectomies Classification" Journal of Clinical Medicine 14, no. 6: 1965. https://doi.org/10.3390/jcm14061965
APA StyleSalzano, G., Ferragina, F., Cocis, S., Maglitto, F., Manfuso, A., & Copelli, C. (2025). Oral Tongue Reconstruction with a Bozola Flap According to the Ansarin Glossectomies Classification. Journal of Clinical Medicine, 14(6), 1965. https://doi.org/10.3390/jcm14061965









