Does Oxygen Work? Evidence for Oxygenation During Kidney Graft Preservation: A Review
Abstract
1. Introduction
2. Ischemia-Reperfusion Injury
- (a)
- Decreased ATP synthesis;
- (b)
- Increased lactic acid production (resulting in intracellular acidosis);
- (c)
- Decreased Na+/K+ ATPase, Na+/H+ ATPase, and Ca2+ ATPase pump activity, causing Na+/K+ and Na+/H+ imbalance (resulting in intracellular edema and acidosis) and an increased intracellular Ca2+ concentration;
- (d)
- Cell cycle dysregulation;
- (e)
- Cytoskeletal protein disruption.
3. Methods of Oxygenation
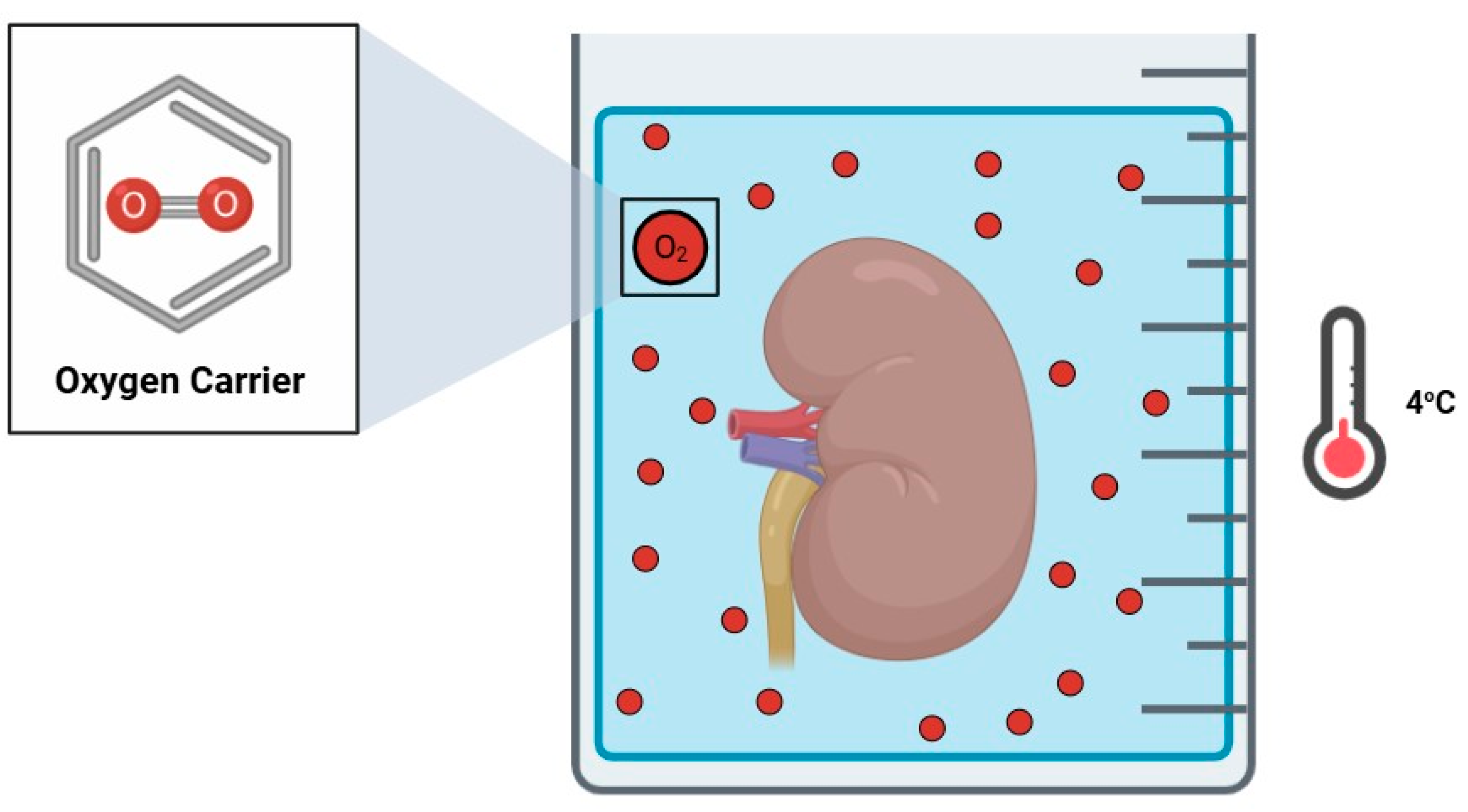
3.1. Oxygen Carriers
- (a)
- A high oxygen affinity and capacity (up to 156 O2 molecules);
- (b)
- Gradient-based oxygen release (a stable oxygen-rich environment);
- (c)
- A wide temperature range (4 °C to 37 °C);
- (d)
- A lack of immunogenicity.
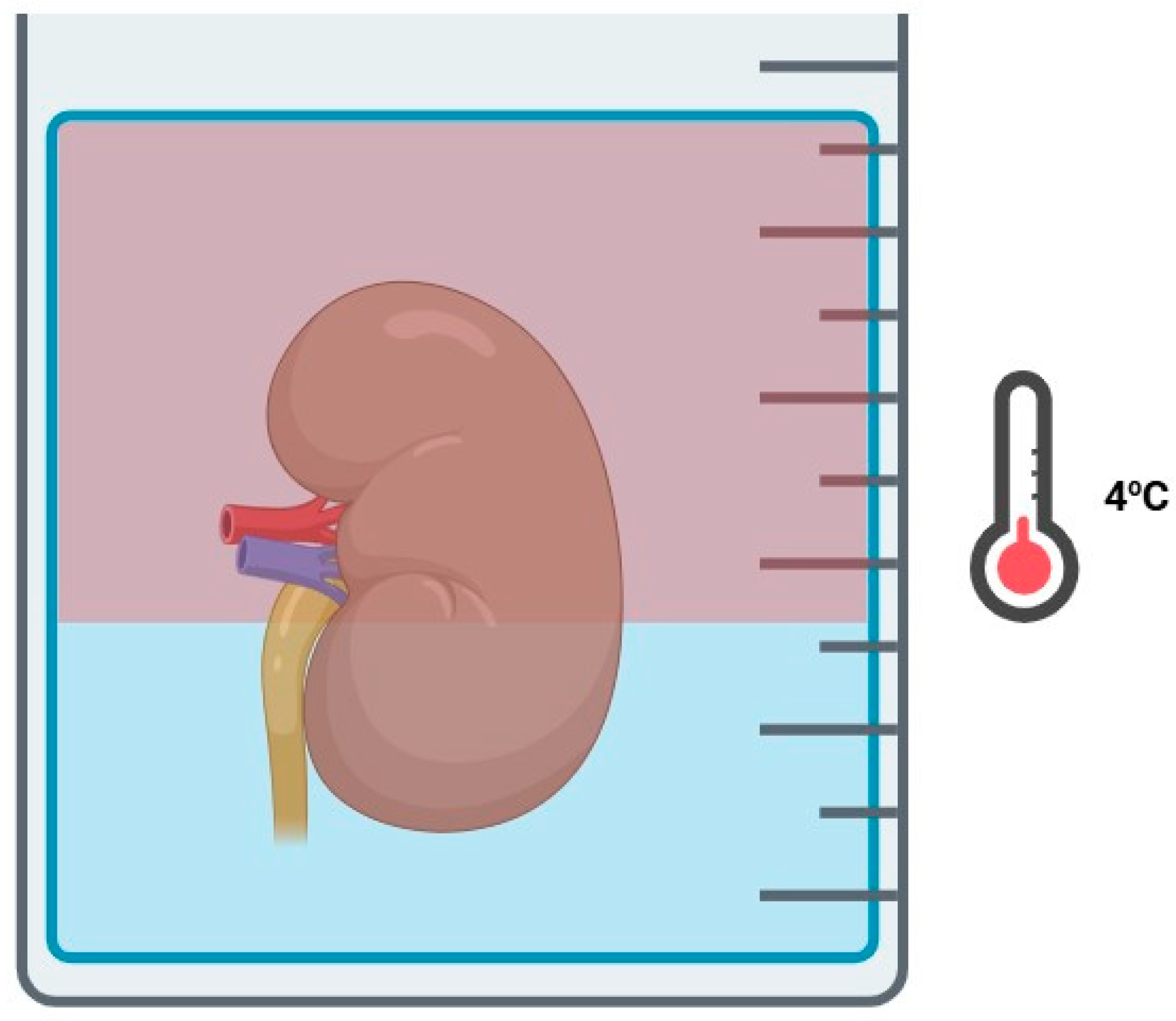
3.2. Hyperbaric Oxygenation
3.3. Two-Layer Method
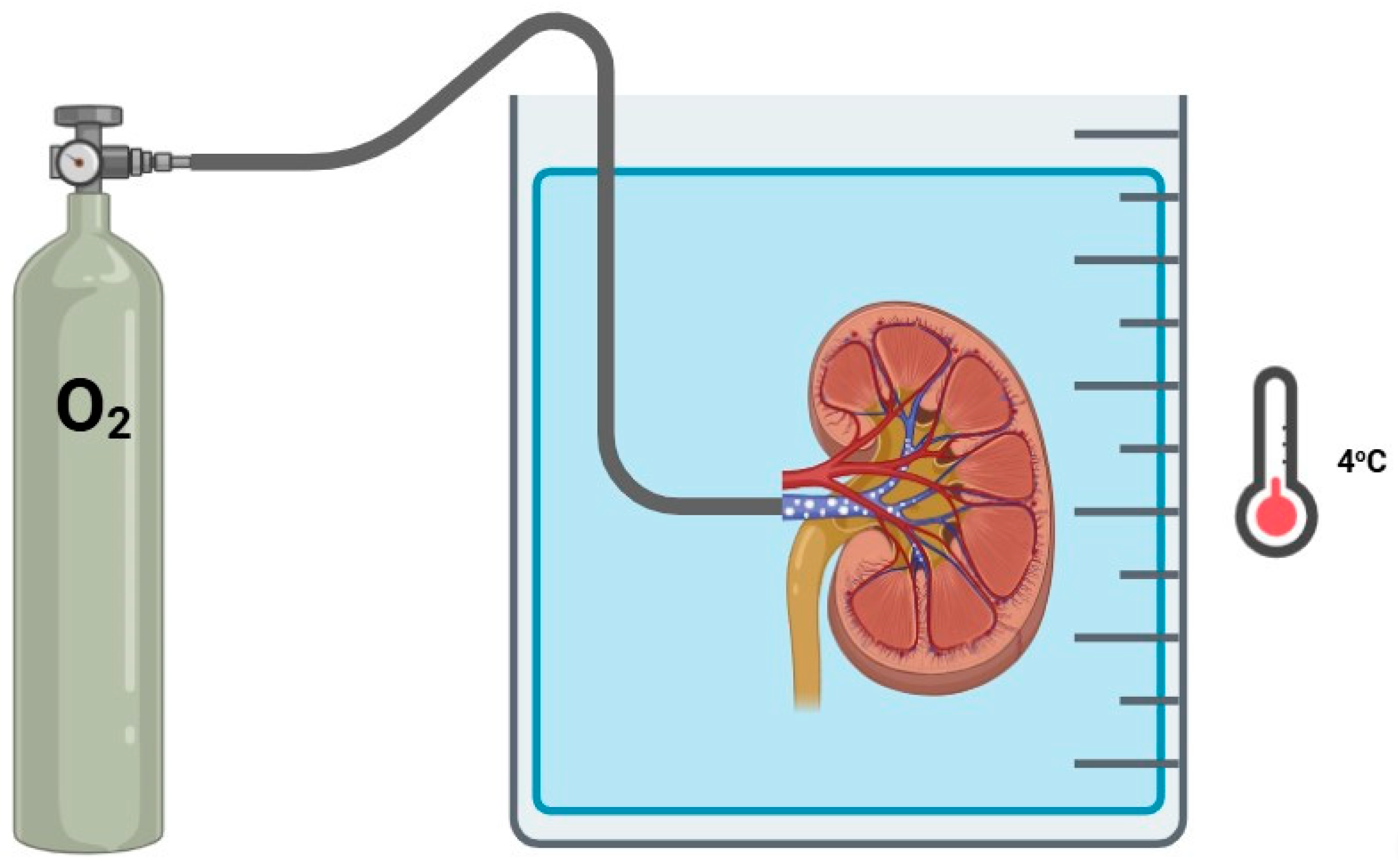
3.4. Retrograde Oxygen Persufflation
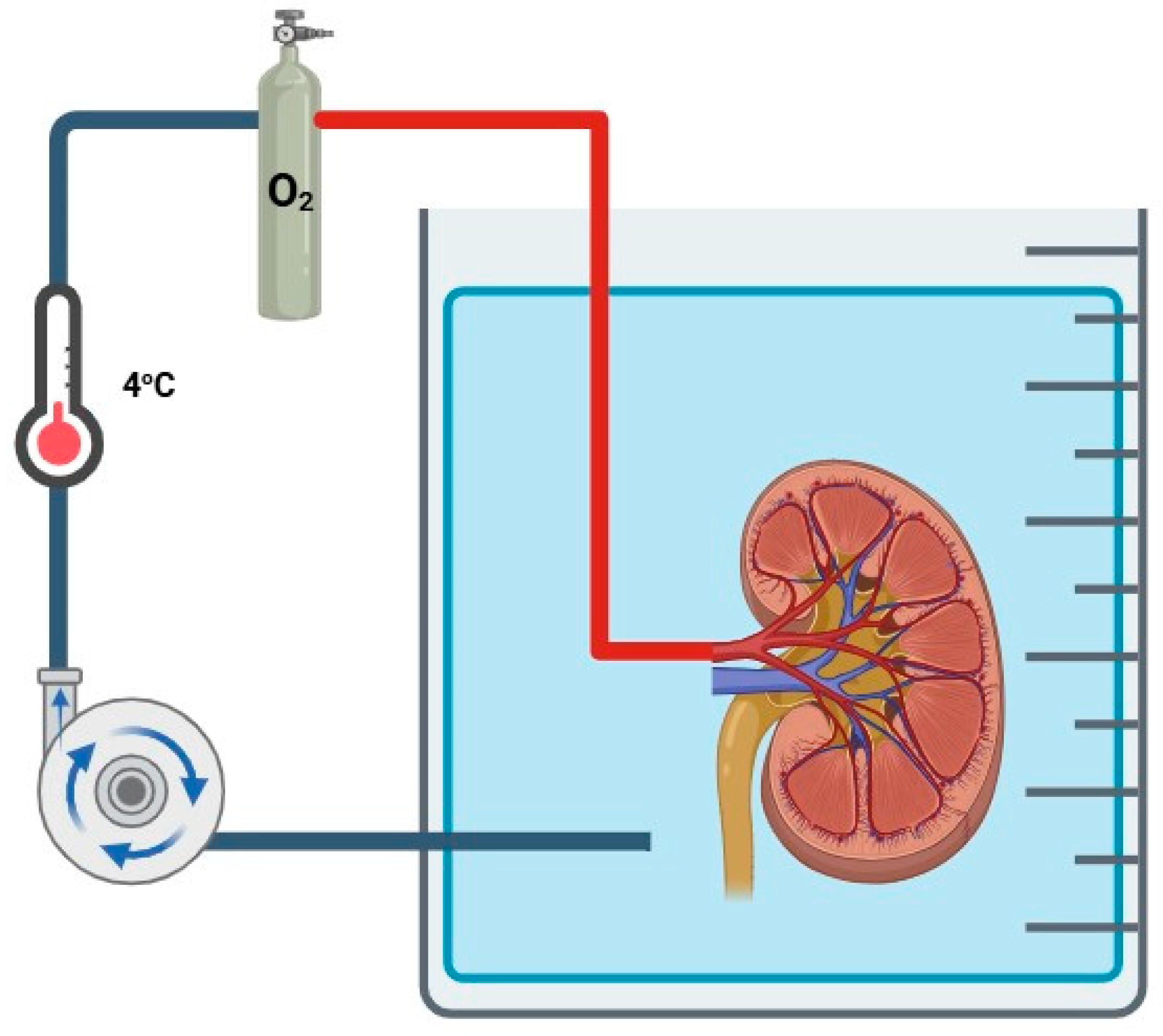
3.5. Hypothermic Oxygenated Machine Perfusion
3.5.1. Animal Studies
3.5.2. Human Clinical Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD Mortality Causes of Death Collaborators. Global, Regional, and National Age—Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Nicholson, H.F.L.; Nicholson, M.L. Oxygenated Kidney Preservation Techniques. Transplantation 2012, 93, 455–459. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2017, 7, 113–117. [Google Scholar] [CrossRef]
- Ponticelli, C.E. The impact of cold ischemia time on renal transplant outcome. Kidney Int. 2015, 87, 272–275. [Google Scholar] [CrossRef]
- Lasorsa, F.; Rutigliano, M.; Milella, M.; D’Amati, A.; Crocetto, F.; Pandolfo, S.D.; Barone, B.; Ferro, M.; Spilotros, M.; Battaglia, M.; et al. Ischemia–Reperfusion Injury in Kidney Transplantation: Mechanisms and Potential Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 4332. [Google Scholar] [CrossRef] [PubMed]
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated Necrosis in Kidney Ischemia-Reperfusion Injury. Kidney Int. 2019, 96, 291–301. [Google Scholar] [CrossRef]
- Rousselot, M.; Delpy, E.; La Rochelle, C.D.; Lagente, V.; Pirow, R.; Rees, J.F.; Hagege, A.; Le Guen, D.; Hourdez, S.; Zal, F. Arenicola Marina Extracellular Hemoglobin: A New Promising Blood Substitute. Biotechnol. J. 2006, 1, 333–345. [Google Scholar] [CrossRef]
- Toulmond, A. Blood Oxygen Transport and Metabolism of the Confined Lugworm Arenicola marina (L.). J. Exp. Biol. 1975, 63, 647–660. [Google Scholar] [CrossRef]
- Thuillier, R.; Dutheil, D.; Trieu, M.T.N.; Mallet, V.; Allain, G.; Rousselot, M.; Denizot, M.; Goujon, J.M.; Zal, F.; Hauet, T. Supplementation with a New Therapeutic Oxygen Carrier Reduces Chronic Fibrosis and Organ Dysfunction in Kidney Static Preservation. Am. J. Transplant. 2011, 11, 1845–1860. [Google Scholar] [CrossRef]
- Mallet, V.; Dutheil, D.; Polard, V.; Rousselot, M.; Leize, E.; Hauet, T.; Goujon, J.M.; Zal, F. Dose-Ranging Study of the Performance of the Natural Oxygen Transporter HEMO2Life in Organ Preservation. Artif. Organs 2014, 38, 691–701. [Google Scholar] [CrossRef]
- Kaminski, J.; Hannaert, P.; Kasil, A.; Thuillier, R.; Leize, E.; Delpy, E.; Steichen, C.; Goujon, J.M.; Zal, F.; Hauet, T. Efficacy of the Natural Oxygen Transporter HEMO2life® in Cold Preservation in a Preclinical Porcine Model of Donation after Cardiac Death. Transpl. Int. 2019, 32, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Kasil, A.; Giraud, S.; Couturier, P.; Amiri, A.; Danion, J.; Donatini, G.; Matillon, X.; Hauet, T.; Badet, L. Individual and Combined Impact of Cxygen and Cxygen Transporter Supplementation during Kidney Machine Preservation in a Porcine Preclinical Kidney Transplantation Model. Int. J. Mol. Sci. 2019, 20, 1992. [Google Scholar] [CrossRef]
- Le Meur, Y.; Badet, L.; Essig, M.; Thierry, A.; Büchler, M.; Drouin, S.; Deruelle, C.; Morelon, E.; Pesteil, F.; Delpech, P.O.; et al. First-in-Human Use of a Marine Oxygen Carrier (M101) for Organ Preservation: A Safety and Proof-of-Principle Study. Am. J. Transplant. 2020, 20, 1729–1738. [Google Scholar] [CrossRef]
- Le Meur, Y.; Delpy, E.; Renard, F.; Hauet, T.; Badet, L.; Rerolle, J.P.; Thierry, A.; Büchler, M.; Zal, F.; Barrou, B. HEMO2life® Improves Renal Function Independent of Cold Ischemia Time in Kidney Recipients: A Comparison with a Large Multicenter Prospective Cohort Study. Artif. Organs 2022, 46, 597–605. [Google Scholar] [CrossRef]
- Le Meur, Y.; Nowak, E.; Barrou, B.; Thierry, A.; Badet, L.; Buchler, M.; Rerolle, J.P.; Golbin, L.; Duveau, A.; Dantal, J.; et al. Evaluation of the Efficacy of HEMO2life®, a Marine OXYgen Carrier for Organ Preservation (OxyOp2) in Renal Transplantation: Study Protocol for a Multicenter Randomized Trial. Trials 2023, 24, 1–13. [Google Scholar] [CrossRef]
- Manax, W.G.; Bloch, J.H.; Longerbeam, J.K.; Lillehei, R.C. Successful 24 hour in vitro preservation of canine kidneys by the combined use of hyperbaric oxygenation and hypothermia. Surgery 1964, 56, 275–282. [Google Scholar] [PubMed]
- Manax, W.G.; Block, J.H.; Eyal, Z. Hypothermia and Hyperbaria: Simple Method for Whole Organ Preservation. JAMA 1965, 192, 755–759. [Google Scholar] [CrossRef]
- Ladaga, L.G.; Nabseth, D.C.; Besznyak, I.; Hendry, W.F.; McLeod, G.; Deterling, R.A. Preservation of Canine Kidneys by Hypothermia and Hyperbaric Oxygen: Long-Term Survival of Autografts Following 24-Hour Storage. Ann. Surg. 1966, 163, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Calne, R.Y.; Hopkinson, W.I. Renal Preservation with Hyperbaric Oxygenation and Hypothermia. Br. J. Urol. 1967, 39, 276–288. [Google Scholar] [CrossRef]
- Rudolf, L.E.; Mandel, S. Supercooling, Intermitent Perfusion and High Pressure Oxygen in Whole Organ Preservation. Transplantation 1967, 5, 1159–1166. [Google Scholar] [CrossRef]
- Bao, D.S.; Wu, Y.K.; Fu, S.J.; Wang, G.Y.; Yang, S.J.; Liang, G.B.; Xie, Z.H.; Rong, S. Hyperbaric Oxygenation Protects Against Ischemia-Reperfusion Injury in Transplanted Rat Kidneys by Triggering Autophagy and Inhibiting Inflammatory Response. Ann. Transplant. 2017, 22, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Bayrakci, B. Preservation of organs from brain dead donors with hyperbaric oxygen. Pediatr. Transplant. 2008, 12, 506–509. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. The Role of Perfluorocarbon in Organ Preservation. Transplantation 2010, 89, 1169–1175. [Google Scholar] [CrossRef]
- Maluf, D.G.; Mas, V.R.; Yanek, K.; Stone, J.J.; Weis, R.; Massey, D.; Spiess, B.; Posner, M.P.; Fisher, R.A. Molecular Markers in Stored Kidneys Using Perfluorocarbon-based Preservation Solution: Preliminary Results. Transplant. Proc. 2006, 38, 1243–1246. [Google Scholar] [CrossRef]
- Marada, T.; Zacharovova, K.; Saudek, F. Perfluorocarbon Improves Post-Transplant Survival and Early Kidney Function following Prolonged Cold Ischemia. Eur. Surg. Res. 2010, 44, 170–178. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Mohamed, I.H.; Nicholson, M.L. The two layer method does not improve the preservation of porcine kidneys. Med. Sci. Monit. 2011, 17, 27–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flatmark, A.; Slaattelid, O.; Woxholt, G. Gaseous persufflation during machine perfusion of human kidneys before transplantation. Eur. Surg. Res. 1975, 7, 83–90. [Google Scholar] [CrossRef]
- Rolles, K.; Foreman, J.; Pegg, D.E. Preservation of ischemically injured canine kidneys by retrograde oxygen persufflation. Transplantation 1984, 38, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Pegg, D.E.; Foreman, J.; Hunt, C.J.; Diaper, M.P. The mechanism of action of retrograde oxygen persufflation in renal preservation. Transplantation 1989, 48, 210–217. [Google Scholar] [CrossRef]
- Treckmann, J.W.; Paul, A.; Saad, S.; Hoffmann, J.; Waldmann, K.H.; Broelsch, C.E.; Nagelschmidt, M. Primary organ function of warm ischaemically damaged porcine kidneys after retrograde oxygen persufflation. Nephrol. Dial. Transplant. 2006, 21, 1803–1808. [Google Scholar] [CrossRef]
- Treckmann, J.W.; Nagelschmidt, M.; Minor, T.; Saner, F.; Saad, S.; Paul, A. Function and quality of kidneys after cold storage, machine perfusion, or retrograde oxygen persufflation: Results from a porcine autotransplantation model. Cryobiology 2009, 59, 19–23. [Google Scholar] [CrossRef]
- Minor, T.; Efferz, P.; Lüer, B. Hypothermic reconditioning by gaseous oxygen persufflation after cold storage of porcine kidneys. Cryobiology 2012, 65, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Moláček, J.; Opatrný, V.; Matějka, R.; Baxa, J.; Třeška, V. Retrograde Oxygen Persufflation of Kidney—Experiment on an Animal. In Vivo 2016, 30, 801–805. [Google Scholar] [CrossRef]
- Kalenski, J.; Mancina, E.; Paschenda, P.; Beckers, C.; Bleilevens, C.; Tóthová, L.; Boor, P.; Gross, D.; Tolba, R.H.; Doorschodt, B.M. Comparison of Aerobic Preservation by Venous Systemic Oxygen Persufflation or Oxygenated Machine Perfusion of Warm-Ischemia-Damaged Porcine Kidneys. Eur. Surg. Res. 2016, 57, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Rolles, K.; Foreman, J.; Pegg, D.E. A pilot clinical study of retrograde oxygen persufflation in renal preservation. Transplantation 1989, 48, 339–342. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.-H.J.; Treckmann, J.; Van Gelder, F.; Napieralski, B.P.; Van Kasterop-Kutz, M.; Homan Van Der Heide, J.J.; Squifflet, J.-P.; Van Heurn, E.; et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Pirenne, J.; Paul, A.; Ploeg, R.J. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2012, 366, 770–771. [Google Scholar] [CrossRef]
- Treckmann, J.; Moers, C.; Smits, J.M.; Gallinat, A.; Maathuis, M.H.J.; Van Kasterop-Kutz, M.; Jochmans, I.; Homan Van Der Heide, J.J.; Squifflet, J.P.; Van Heurn, E.; et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl. Int. 2011, 24, 548–554. [Google Scholar] [CrossRef]
- Gill, J.; Dong, J.; Eng, M.; Landsberg, D.; Gill, J.S. Pulsatile perfusion reduces the risk of delayed graft function in deceased donor kidney transplants, irrespective of donor type and cold ischemic time. Transplantation 2014, 97, 668–674. [Google Scholar] [CrossRef]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.G.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2019, 3, CD011671. [Google Scholar] [CrossRef]
- Peng, P.; Ding, Z.; He, Y.; Zhang, J.; Wang, X.; Yang, Z. Hypothermic Machine Perfusion Versus Static Cold Storage in Deceased Donor Kidney Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Artif. Organs 2019, 43, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Tingle, S.J.; Thompson, E.R.; Figueiredo, R.S.; Moir, J.A.G.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Normothermic and hypothermic machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2024, CD011671. [Google Scholar] [CrossRef]
- Minor, T.; Sitzia, M.; Dombrowski, F. Kidney transplantation from non-heart-beating donors after oxygenated low-flow machine perfusion preservation with histidine-tryptophan-ketoglutarate solution. Transpl. Int. 2004, 17, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Paul, A.; Efferz, P.; Wohlschlaeger, J.; Rauen, U.; Gallinat, A. Kidney transplantation after oxygenated machine perfusion preservation with Custodiol-N solution. Transpl. Int. 2015, 28, 1102–1108. [Google Scholar] [CrossRef]
- Doorschodt, B.M.; Schreinemachers, M.-C.J.M.; Florquin, S.; Lai, W.; Sitzia, M.; Zernecke, A.; Tolba, R.H. Evaluation of a novel system for hypothermic oxygenated pulsatile perfusion preservation. Int. J. Artificlal Organs 2009, 32, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Gallinat, A.; Fox, M.; Lüer, B.; Efferz, P.; Paul, A.; Minor, T. Role of Pulsatility in Hypothermic Reconditioning of Porcine Kidney Grafts by Machine Perfusion After Cold Storage. Transplantation 2013, 96, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Buchs, J.B.; Lazeyras, F.; Ruttimann, R.; Nastasi, A.; Morel, P. Oxygenated hypothermic pulsatile perfusion versus cold static storage for kidneys from non heart-beating donors tested by in-line ATP resynthesis to establish a strategy of preservation. Perfusion 2011, 26, 159–165. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; De Rougemont, O.; Oberkofler, C.E.; Clavien, P.A.; Dutkowski, P. Short, cool, and well oxygenated—HOPE for kidney transplantation in a rodent model. Ann. Surg. 2016, 264, 815–822. [Google Scholar] [CrossRef]
- Darius, T.; Gianello, P.; Vergauwen, M.; Mourad, N.; Buemi, A.; De Meyer, M.; Mourad, M. The effect on early renal function of various dynamic preservation strategies in a preclinical pig ischemia—Reperfusion autotransplant model. Am. J. Transplant. 2019, 19, 752–762. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.B.; Patel, K.; Craps, J.; Joris, V.; Aydin, S.; Ury, B.; Buemi, A.; De Meyer, M.; et al. Influence of Different Partial Pressures of Oxygen During Continuous Hypothermic Machine Perfusion in a Pig Kidney Ischemia-reperfusion Autotransplant Model. Transplantation 2020, 104, 731–743. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Mueller, M.; Aydin, S.; Dutkowski, P.; Gianello, P.; Mourad, M. Brief Bubble and Intermittent Surface Oxygenation Is a Simple and Effective Alternative for Membrane Oxygenation During Hypothermic Machine Perfusion in Kidneys. Transplant. Direct 2020, 6, e571. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.; Frotscher, C.; Minor, T. Hypothermic reconditioning after cold storage improves postischemic graft function in isolated porcine kidneys. Transpl. Int. 2010, 23, 538–542. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; Muller, X.; Gaspert, A.; Clavien, P.A.; Dutkowski, P. Hypothermic Oxygenated Perfusion: A Simple and Effective Method to Modulate the Immune Response in Kidney Transplantation. Transplantation 2019, 103, e128–e136. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Sijbrand Hofker, H.; van de Leemkolk, F.E.; Leuvenink, H.G.; Knight, S.R.; Pirenne, J.; Ploeg, R.J. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef]
- Houtzager, J.H.E.; Hemelrijk, S.D.; Post, I.C.J.H.; Idu, M.M.; Bemelman, F.J.; Van Gulik, T.M. The Use of the Oxygenated AirdriveTMMachine Perfusion System in Kidney Graft Preservation: A Clinical Pilot Study. Eur. Surg. Res. 2021, 61, 153–162. [Google Scholar] [CrossRef]
- Meister, F.A.; Czigany, Z.; Rietzler, K.; Miller, H.; Reichelt, S.; Liu, W.J.; Boecker, J.; Moeller, M.J.; Tolba, R.H.; Hamesch, K.; et al. Decrease of renal resistance during hypothermic oxygenated machine perfusion is associated with early allograft function in extended criteria donation kidney transplantation. Sci. Rep. 2019, 10, 17726. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; De Pace, V.; Angeletti, A.; Comai, G.; Vasuri, F.; Baldassarre, M.; Maroni, L.; Odaldi, F.; Fallani, G.; Caraceni, P.; et al. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci. Rep. 2020, 10, 6063. [Google Scholar] [CrossRef]
- Husen, P.; Boffa, C.; Jochmans, I.; Krikke, C.; Davies, L.; Mazilescu, L.; Brat, A.; Knight, S.; Wettstein, D.; Cseprekal, O.; et al. Oxygenated End-Hypothermic Machine Perfusion in Expanded Criteria Donor Kidney Transplant: A Randomized Clinical Trial. JAMA Surg. 2021, 156, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Pravisani, R.; Baccarani, U.; Molinari, E.; Cherchi, V.; Bacchetti, S.; Terrosu, G.; Avital, I.; Ekser, B.; Adani, G.L. PO2 21% oxygenated hypothermic machine perfusion in kidney transplantation: Any clinical benefit? Int. J. Artif. Organs 2022, 45, 666–671. [Google Scholar] [CrossRef]
- Darius, T.; Devresse, A.; Buemi, A.; De Meyer, M.; Mourad, M. First clinical kidney transplantation series using brief bubble and direct surface oxygenation as alternative for membrane oxygenation during hypothermic machine perfusion. Artif. Organs 2023, 47, 777–785. [Google Scholar] [CrossRef]
- Robb, E.L.; Hall, A.R.; Prime, T.A.; Eaton, S.; Szibor, M.; Viscomi, C.; James, A.M.; Murphy, M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018, 293, 9869–9879. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O2 uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transplant. 2020, 20, 2030–2043. [Google Scholar] [CrossRef]

| Author (Year) | Country | Study Design | Population | Outcomes |
|---|---|---|---|---|
| Jochmans (2020) [54] | Belgium Netherlands United Kingdom | Randomized controlled phase III study Kidney Assist™ | DCD ≥ 50 years old; HOPE n = 106 HMP n = 106 | Significant differences in eGFR 1 year after transplantation. Less severe complications and kidney graft failure 1 year after transplantation. |
| Houtzager (2021) [55] | Netherlands | Case series Airdive™ | n = 5; DBD n = 4 DCD n = 1 | No differences in adverse events or safety 1 month after transplantation. |
| Meister (2019) [56] | Germany | Retrospective cohort study Kidney Assist™ | ECD; End-HOPE n = 15 SCS n = 30 | No differences in DGF 6 months after transplantation. Inverse association between renal resistance and creatinine levels, especially in grafts with PNF. |
| Ravaioli (2020) [57] | Italy | Non-randomized controlled phase II study | ECD; End-HOPE n = 10 SCS n = 30 | No differences in adverse events or safety, PNF or graft survival 3 months after transplantation. HOPE of more than 2 h prevents DGF. |
| Husen (2021) [58] | Belgium Germany Hungary Netherlands United Kingdom | Randomized controlled phase III study Kidney Assist™ | ECD; End-HOPE n = 127 SCS n = 135 | No differences in 1-year graft survival. Lower incidence of DGF with HOPE. |
| Pravisani (2022) [59] | Italy | Retrospective cohort study LifePort® Kidney Transporter | KT recipients (n = 103) | No differences in incidence of DGF, acute rejection, or creatinine levels 1 year after transplantation. |
| Darius (2023) [60] | Belgium | Case series LifePort® Kidney Transporter | n = 5; Continuous surface HOPE (n = 3) Intermittent surface HOPE (n = 2) | No differences in adverse events or safety. No incidences of DGF or acute rejection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calva Lopez, A.; Robles Garcia, J.E.; Yanez Ruiz, C.A.; Tapia Tapia, M.D.; Talavera Cobo, V.; Muñoz Bastidas, C.A.; Miñana Lopez, B.; Sanchez Zalabardo, D. Does Oxygen Work? Evidence for Oxygenation During Kidney Graft Preservation: A Review. J. Clin. Med. 2025, 14, 1927. https://doi.org/10.3390/jcm14061927
Calva Lopez A, Robles Garcia JE, Yanez Ruiz CA, Tapia Tapia MD, Talavera Cobo V, Muñoz Bastidas CA, Miñana Lopez B, Sanchez Zalabardo D. Does Oxygen Work? Evidence for Oxygenation During Kidney Graft Preservation: A Review. Journal of Clinical Medicine. 2025; 14(6):1927. https://doi.org/10.3390/jcm14061927
Chicago/Turabian StyleCalva Lopez, Andres, Jose Enrique Robles Garcia, Carlos Andres Yanez Ruiz, Mario Daniel Tapia Tapia, Vanessa Talavera Cobo, Carmina Alejandra Muñoz Bastidas, Bernardino Miñana Lopez, and Daniel Sanchez Zalabardo. 2025. "Does Oxygen Work? Evidence for Oxygenation During Kidney Graft Preservation: A Review" Journal of Clinical Medicine 14, no. 6: 1927. https://doi.org/10.3390/jcm14061927
APA StyleCalva Lopez, A., Robles Garcia, J. E., Yanez Ruiz, C. A., Tapia Tapia, M. D., Talavera Cobo, V., Muñoz Bastidas, C. A., Miñana Lopez, B., & Sanchez Zalabardo, D. (2025). Does Oxygen Work? Evidence for Oxygenation During Kidney Graft Preservation: A Review. Journal of Clinical Medicine, 14(6), 1927. https://doi.org/10.3390/jcm14061927






