The Prevalence of Previous Coronavirus Disease-19 in Patients with Pulmonary Thromboembolism and Its Effect on Embolism Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
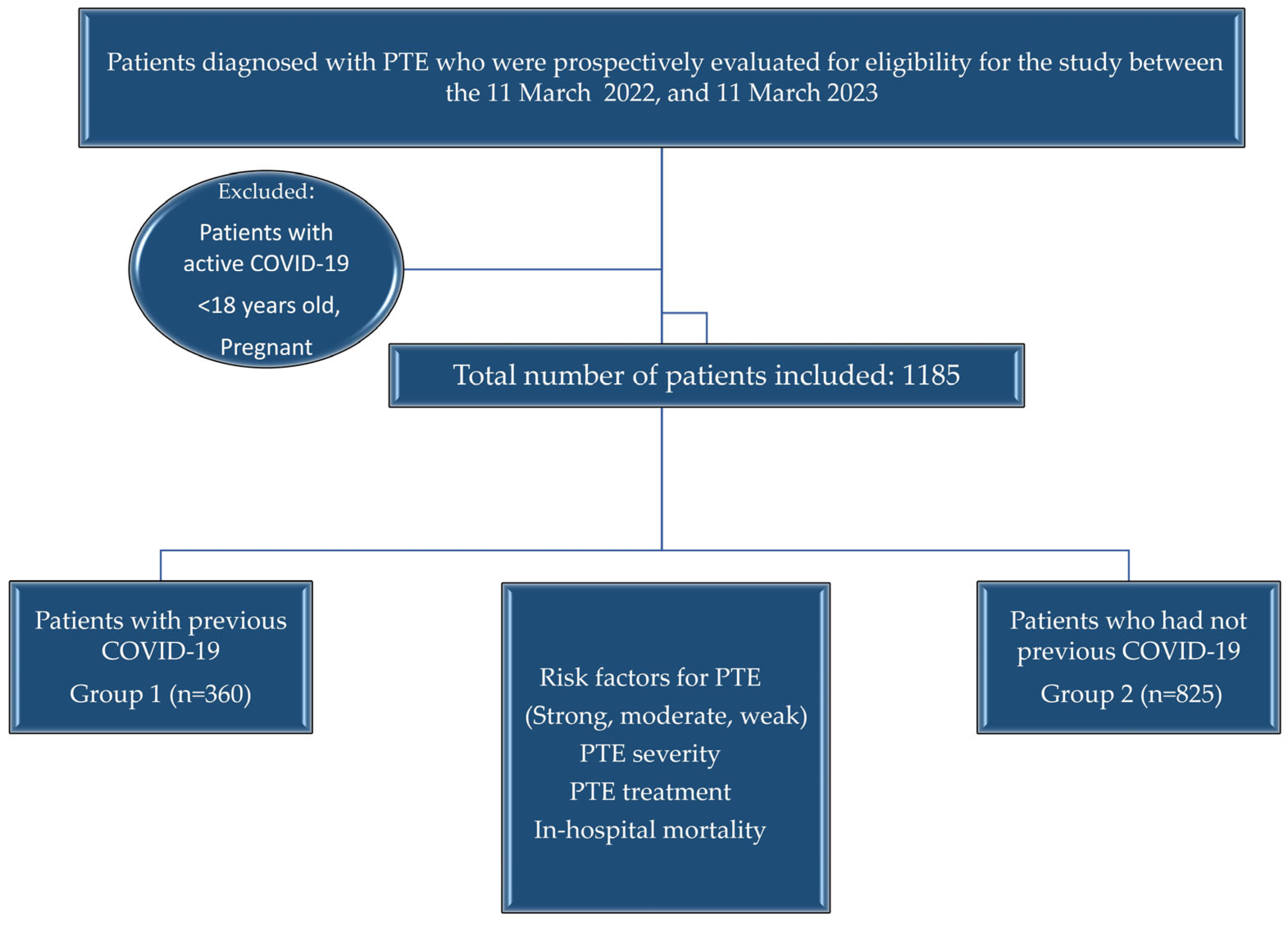
2.2. Patient Selection
2.3. Independent Variables
2.4. Endpoints
2.5. Statistics
2.6. Ethics
3. Results
3.1. Patient Characteristics and the Prevalence of Previous Coronavirus Disease-19
3.2. Risk Factors for Pulmonary Thromboembolism
3.3. The Severity of Pulmonary Thromboembolism in Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| CI | Confidence interval |
| COVID-19 | Coronavirus disease-19 |
| CTPA | Computed tomography pulmonary angiography |
| CUS | Compression venous ultrasonography |
| DVT | Deep vein thrombosis |
| ERS | European Respiratory Society |
| ESC | European Society of Cardiology |
| HF | Heart failure |
| LMWH | Low-molecular weight heparin |
| NOACs | Non-vitamin K antagonist oral anticoagulants |
| OCS | Oral contraceptive |
| ORs | Odds ratios |
| ProBNP | proB-type natriuretic peptide |
| PTE | Pulmonary thromboembolism |
| RV/LV | Right ventricle/left ventricle |
| SARS-CoV-2 | Severe acute respiratory syndrome-Coronavirus-2 |
| sPESI | Simplified pulmonary embolism severity index |
| TTE | Transthoracic echocardiography |
| UFH | Unfractioned heparin |
| VKAs | Vitamin K antagonists |
References
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Díez, J.; Jiménez-García, R.; Jiménez, D.; Monreal, M.; Guijarro, R.; Otero, R.; Hernández-Barrera, V.; Trujillo-Santos, J.; de Andrés, A.L.; Carrasco-Garrido, P. Trends in hospital admissions for pulmonary embolism in Spain from 2002 to 2011. Eur. Respir. J. 2014, 44, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Ageno, W.; Pomero, F.; Fenoglio, L.; Squizzato, A.; Bonzini, M. Time trends and case fatality rate of in-hospital treated pulmonary embolism during 11 years of observation in Northwestern Italy. Thromb. Haemost. 2016, 115, 399–405. [Google Scholar] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart. J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report-22. 11 February 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2 (accessed on 2 February 2022).
- Chi, G.; Lee, J.J.; Jamil, A.; Gunnam, V.; Najafi, H.; Montazerin, S.M.; Shojaei, F.; Marszalek, J. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 2489. [Google Scholar] [CrossRef]
- Henrina, J.; Putra, I.C.S.; Cahyadi, I.; Lawrensia, S.; Gunawan, H.F.H.; Cahyadi, A.; Franke, J.; Suciadi, L.P. Clinical characteristics and outcomes of venous thromboembolism in patients hospitalized for COVID-19: Systematic review and meta-analysis. Thromb. Update 2021, 2, 100037. [Google Scholar] [CrossRef]
- Loo, J.; Spittle, D.A.; Newnham, M. COVID-19, immunothrombosis and venous thromboembolism: Biological mechanisms. Thorax 2021, 76, 412–420. [Google Scholar] [CrossRef]
- Stelzer, M.; Henes, J.; Saur, S. The role of antiphospholipid antibodies in COVID-19. Curr. Rheumatol. Rep. 2021, 23, 72. [Google Scholar] [CrossRef]
- Vincent, J.L.; Levi, M.; Hunt, B.J. Prevention and management of thrombosis in hospitalised patients with COVID-19 pneumonia. Lancet Respir. Med. 2022, 10, 214–220. [Google Scholar] [CrossRef]
- Knight, R.; Walker, V.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Denholm, R.; Akbavi, A.; Abbasizanjani, H.; Torabi, F.; et al. Association of COVID-19 with major arterial and venous thrombotic diseases: A population-wide cohort study of 48 million adults in England and Wales. Circulation 2022, 146, 892–906. [Google Scholar] [CrossRef]
- Kole, C.; Stefanou, Ε.; Karvelas, N.; Schizas, D.; Toutouzas, K.P. Acute and post-acute COVID-19 cardiovascular complications: A comprehensive review. Cardiovasc. Drugs. Ther. 2024, 38, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Soudet, S.; Basille, D.; Carette, H.; Mercier, M.; Andrejak, C.; Sevestre, M.A. Cardiovascular and venous thromboembolic events after hospital discharge for COVID-19: A prospective single center study. Angiology 2024, 75, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Patell, R.; Bogue, T.; Koshy, A.; Bindal, P.; Merrill, M.; Aird, W.C.; Bauer, K.A.; Zwicker, J.I. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood 2020, 136, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Gandet, F.F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Miró, Ò.; Jiménez, S.; Mebazaa, A.; Freund, Y.; Burillo-Putze, G.; Martín, A.; Martín-Sánchez, F.J.; García-Lamberechts, E.J.; Alquézar-Arbé, A.; Jacob, J.; et al. Pulmonary embolism in patients with COVID-19: Incidence, risk factors, clinical characteristics, and outcome. Eur. Heart J. 2021, 42, 3127–3142. [Google Scholar] [CrossRef]
- Chamorro, E.M.; Ostolaza, T.Y.R.; Núñez, M.P.; Nacenta, S.B.; Rodríguez-Guerra, C.C.C.; Sanz, L.I. Pulmonary embolisms in patients with COVID-19: A prevalence study in a tertiary hospital. Radiologia 2021, 63, 13–21. [Google Scholar]
- Sutanta, H.; Soegiarto, G. Risk of Thrombosis during and after a SARS-CoV-2 infection: Pathogenesis, diagnostic approach, and management. Hematol. Rep. 2023, 15, 225–243. [Google Scholar] [CrossRef]
- Mouzarou, A.; Ioannou, M.; Leonidou, E.; Chaziri, I. Pulmonary Embolism in Post-COVID-19 Patients, a Literature Review: Red Flag for Increased Awareness? SN Compr. Clin. Med. 2022, 4, 190. [Google Scholar] [CrossRef]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Connolly, A.M.F. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: Nationwide self-controlled cases series and matched cohort study. BMJ 2022, 377, e069590. [Google Scholar] [CrossRef]
- Eswaran, H.; Jarmul, J.A.; Shaheen, A.W.; Meaux, D.; Long, T.; Saccoccio, D.; Moll, S. Vascular thromboembolic events following COVID-19 hospital discharge: Incidence and risk factors. Res. Pract. Thromb. Haemost. 2021, 5, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Aktaa, S.; Wu, J.; Nadarajah, R.; Rashid, M.; de Belder, M.; Deanfield, J.; Mamas, M.A.; Gale, C.P. Incidence and mortality due to thromboembolic events during the COVID-19 pandemic: Multi-sourced population-based health records cohort study. Thromb. Res. 2021, 202, 17–23. [Google Scholar] [CrossRef]
- Ho, F.K.; Man, K.K.C.; Toshner, M.; Church, C.; Celis-Morales, C.; Wong, I.C.K.; Berry, C.; Sattar, N.; Pell, J.P. Thromboembolic risk in hospitalized and nonhospitalized COVID-19 patients: A self-controlled case series analysis of a nationwide cohort. Mayo Clin. Proc. 2021, 96, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.N.; Whyte, M.B.; Georgiou, L.; Giron, G.; Czuprynska, J.; Rea, C.; Vadher, B.; Patel, R.K.; Gee, E.; Arya, R. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood 2020, 136, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, W.; Kaatz, S.; Latack, K.; Schultz, L.; Poisson, L. Factors associated with risk of postdischarge thrombosis in patients with COVID-19. JAMA Netw. Open 2021, 4, e2135397. [Google Scholar] [CrossRef]
- Giannis, D.; Allen, S.L.; Tsang, J.; Flint, S.; Pinhasov, T.; Williams, S.; Tan, G.; Thakur, R.; Leung, C.; Snyder, M. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: The CORE-19 registry. Blood 2021, 137, 2838–2847. [Google Scholar] [CrossRef]
- Salisbury, R.; Iotchkova, V.; Jaafar, S.; Morton, J.; Sangha, G.; Shah, A.; Untiveros, P.; Curry, N.; Shapiro, S. Incidence of symptomatic, image-confirmed venous thromboembolism following hospitalization for COVID-19 with 90-day follow-up. Blood Adv. 2020, 24, 6230–6239. [Google Scholar] [CrossRef]
- Gómez, C.A.; Sun, C.K.; Tsai, I.T.; Chang, Y.P.; Lin, M.C.; Hung, I.Y.; Chang, Y.J.; Wang, L.K.; Lin, Y.T.; Hung, K.C. Mortality and risk factors associated with pulmonary embolism in coronavirus disease 2019 patients: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16025. [Google Scholar] [CrossRef]
- Thachil, J.; Srivastava, A. SARS-2 Coronavirus–associated hemostatic lung abnormality in COVID-19: Is it pulmonary thrombosis or pulmonary embolism? Semin. Thromb. Hemost. 2020, 46, 777–780. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J. D-dimer is associated with severity of coronavirus disease 2019: A pooled analysis. Thromb. Haemost. 2020, 120, 876–878. [Google Scholar] [CrossRef]
- Vechi, H.T.; Maia, L.R.; do Monte Alves, M. Late acute pulmonary embolism after mild Coronavirus Disease 2019 (COVID-19): A case series. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e63. [Google Scholar] [CrossRef]
| Variables | Group 1 (n = 360) | Group 2 (n = 825) | p-Value |
|---|---|---|---|
| Age, mean (SD) | 64.1 (16.5) | 64.1 (15.7) | 0.99 * |
| Sex, n (%) | |||
| Female | 190 (52.8) | 417 (50.5) | 0.48 |
| Male | 170 (47.2) | 408 (49.5) | |
| Strong risk factors (n = 341), n (%) | |||
| Fracture of lower limb | 11 (3.1) | 36 (4.4) | 0.29 |
| Hospitalization for HF or AF/flatter (within previous 3 months) | 19 (5.3) | 23 (2.8) | 0.033 |
| Hip or knee replacement | 14 (3.9) | ||
| Major trauma | 7 (1.9) | 41 (5.0) | 0.42 |
| MI (within previous 3 months) | 5 (1.4) | 26 (3.2) | 0.25 |
| Previous VTE | 32 (8.9) | 9 (1.1) | 0.66 |
| Spinal cord injury | 4 (1.1) | 68 (8.2) | 0.71 |
| Moderate risk factors (n = 372), n (%) | |||
| Arthroscopic knee surgery | 7 (1.9) | 8 (1.0) | 0.17 |
| Autoimmune diseases | 10 (2.8) | 20 (2.4) | 0.72 |
| Blood transfusion | 14 (3.9) | 16 (1.9) | 0.049 |
| Central venous lines | 7 (1.9) | 8 (1.0) | 0.17 |
| Intravenous catheters and leads | 20 (5.6) | 31 (3.8) | 0.16 |
| Chemotherapy | 22 (6.1) | 67 (8.1) | 0.23 |
| Congestive HF or respiratory failure | 79 (21.9) | 141 (17.1) | 0.048 |
| Erythropoiesis-stimulating agents | 2 (0.6) | 2 (0.2) | 0.39 |
| Hormone replacement therapy | 2 (0.6) | 5 (0.6) | 0.91 |
| In vitro fertilization | 0 (0.0) | 1 (0.1) | 0.51 |
| Oral contraceptive therapy | 18 (5.0) | 18 (2.2) | 0.009 |
| Post-partum period | 3 (0.8) | 8 (1.0) | 0.82 |
| Infection | 76 (21.1) | 115 (14.0) | 0.002 |
| Inflammatory bowel disease | 1 (0.3) | 6 (0.7) | 0.35 |
| Cancer | 43 (11.9) | 161 (19.5) | 0.001 |
| Paralytic stroke | 14 (3.9) | 28 (3.4) | 0.67 |
| Superficial vein thrombosis | 4 (1.1) | 10 (1.2) | 0.88 |
| Thrombophilia | 7 (1.9) | 12 (1.5) | 0.54 |
| Weak risk factors (n = 293), n (%) | |||
| Bed rest >3 days | 140 (39.0) | 294 (35.6) | 0.27 |
| Diabetes mellitus | 80 (22.2) | 148 (17.9) | 0.09 |
| Arterial hypertension | 152 (42.2) | 334 (40.5) | 0.59 |
| Immobility due to sitting | 89 (24.7) | 214 (25.9) | 0.66 |
| Increasing age | 119 (33.1) | 289 (35.0) | 0.51 |
| Laparoscopic surgery | 9 (2.5) | 29 (3.5) | 0.36 |
| Obesity | 48 (13.3) | 93 (11.3) | 0.31 |
| Varicose veins | 26 (7.2) | 40 (4.8) | 0.10 |
| No identifiable risk factor (n = 47), n (%) | 47 (13.1) | 132 (16.0) | 0.19 |
| Variables | Group 1 (n = 360) | Group 2 (n = 825) | p-Value |
|---|---|---|---|
| Symptoms, n (%) | |||
| Hemoptysis | 30 (8.3) | 73 (8.8) | 0.76 |
| Dyspnea | 314 (87.2) | 683 (82.8) | 0.07 |
| Chest pain | 202 (56.1) | 438 (53.1) | 0.38 |
| Syncope | 23 (6.4) | 86 (10.4) | 0.06 |
| Cough | 106 (29.4) | 186 (22.5) | 0.012 |
| DVT symptoms | 55 (15.3) | 153 (18.5) | 0.16 |
| Laboratory, n (%) | |||
| Age-adjusted D-Dimer (high) | 289 (89.5) | 648 (94.5) | 0.004 |
| Troponin (high) | 123 (40.7) | 301 (43.4) | 0.44 |
| ProBNP (high) | 111 (64.9) | 208 (61.9) | 0.51 |
| Hemoglobin (gr/dL), median (min-max) | 12.8 (7.4–17.5) | 12.6 (4.8–19.4) | 0.36 * |
| Hematocrit (%), median (min-max) | 39.0 (24.0–53.6) | 39.0 (17.0–59.0) | 0.39 * |
| WBC (count/µL), median (min-max) | 8810(1040–91800) | 9420(2000–75,300) | 0.043 * |
| PLT (count/µLx103), median (min-max) | 250 (22–819) | 244 (198–751) | 0.64 * |
| Urea (mg/dL), median (min-max) | 37.0 (8.0–149.0) | 38.0 (10.0–215.0) | 0.19 * |
| Creatinine (mg/dL), median (min-max) | 0.86 (0.38–5.50) | 0.88 (0.07–8.14) | 0.32 * |
| Diagnosis, n (%) | |||
| CTPA (n = 1097) | 335 (93.1) | 762 (92.4) | |
| Lung scintigraphy (n = 76) | 22 (6.9) | 54 (7.6) | 0.77 |
| Bilateral thrombus (CTPA), n (%) | 202 (59.2) | 455 (57.9) | 0.67 |
| CTPA thrombus localization, n (%) | |||
| Main pulmonary artery | 82 (23.8) | 226 (28.9) | |
| Saddle embolism | 20 (5.8) | 35 (4.5) | |
| Lobar | 89 (25.8) | 214 (27.3) | 0.13 |
| Segmental | 105 (30.4) | 229 (29.2) | |
| Subsegmental | 49 (14.2) | 79 (10.1) | |
| Main pulmonary artery/aorta diameter > 1 | 67 (19.3) | 192 (24.5) | 0.06 |
| RV/LV ratio > 1 (CTPA), n (%) | 68 (19.7) | 219 (27.9) | 0.003 |
| TTE parameters (n = 1069), n (%) | |||
| RV/LV ratio > 1 | 54 (16.2) | 144 (19.6) | 0.19 |
| D shape | 20 (6.0) | 70 (9.5) | 0.06 |
| Flattened intraventricular septum | 12 (3.6) | 48 (6.5) | 0.06 |
| Tricuspid insufficiency | 158 (47.4) | 353 (48.0) | 0.88 |
| Mobile right heart thrombus | 7 (2.1) | 8 (1.1) | 0.19 |
| Systolic PAP (mmHg), median (min-max) | 35.0 (20.0–83.0) | 37.5 (15.0–100.0) | 0.79 * |
| TAPSE (cm), median (min-max) | 1.9 (0.0–2.7) | 2.2 (0.0–2.8) | 0.12 * |
| LVEF (%), median (min-max) | 60.0 (30.0–70.0) | 60.0 (30.0–66.0) | 0.59 * |
| Lower limb CUS (n = 873), n (%) | |||
| No DVT | 208 (73.0) | 386 (65.6) | |
| Unilateral thrombus | 65 (22.6) | 166 (28.2) | |
| Bilateral thrombus | 12 (4.2) | 36 (6.1) | 0.004 |
| Variables | Group 1 (n = 360) | Group 2 (n = 825) | p-Value |
|---|---|---|---|
| sPESI score, median (min-max) | 1.0 (0.0–4.0) | 1.0 (0.0–5.0) | 0.025 * |
| PTE risk stratification, n (%) | |||
| High | 24 (6.7) | 65 (7.9) | |
| Intermediate-high | 81 (22.5) | 207 (25.1) | 0.16 |
| Intermediate-low | 106 (29.4) | 268 (32.5) | |
| Low | 149 (41.4) | 285 (34.5) | |
| Treatment, n (%) | |||
| LMWH | 316 (87.8) | 740 (89.7) | 0.33 |
| UFH | 11 (3.1) | 39 (4.7) | 0.19 |
| VKAs | 119 (33.1) | 277 (33.6) | 0.86 |
| NOACs | 116 (32.2) | 200 (24.2) | 0.004 |
| Thrombolytic treatment | 27 (7.5) | 93 (11.3) | 0.048 |
| Initial treatment place, n (%) | |||
| Intensive care unit | 53 (14.7) | 193 (23.4) | |
| Treatment at hospital | 256 (71.1) | 554 (67.2) | 0.001 |
| Treatment at home | 51 (14.2) | 78 (9.5) | |
| In-hospital mortality (n = 1056), n (%) | 10 (3.2) | 31 (4.1) | 0.45 |
| Variables | Wald | df | p-Value | ORs | 95% CI for ORs |
|---|---|---|---|---|---|
| Age | 0.011 | 1 | 0.92 | 1.001 | 0.989–1.012 |
| Sex | 0.062 | 1 | 0.80 | 1.044 | 0.746–1.460 |
| Cough | 1.970 | 1 | 0.16 | 1.298 | 0.902–1.867 |
| No identifiable risk factor (idiopathic) | 9.070 | 1 | 0.003 | 0.456 | 0.275–0.760 |
| Age-adjusted D-Dimer (high) | 2.343 | 1 | 0.13 | 0.620 | 0.337–1.143 |
| RV/LV ratio > 1 (CTPA) | 3.874 | 1 | 0.049 | 0.603 | 0.365–0.998 |
| Main Pulmonary artery/Aorta ratio > 1 (CTPA) | 1.317 | 1 | 0.25 | 1.340 | 0.813–2.210 |
| D-shape (TTE) | 0.026 | 1 | 0.87 | 0.943 | 0.463–1.920 |
| Lower limb CUS | 4.104 | 2 | 0.13 | ||
| Lower limb CUS (1) | 1.549 | 1 | 0.21 | 0.776 | 0.520–1.157 |
| Lower limb CUS (2) | 3.140 | 1 | 0.08 | 0.491 | 0.223–1.078 |
| sPESI score | 1.747 | 1 | 0.19 | 0.887 | 0.743–1.060 |
| NOACs | 0.635 | 1 | 0.42 | 1.161 | 0.804–1.676 |
| Systemic thrombolytic treatment | 0.487 | 1 | 0.49 | 1.314 | 0.610–2.829 |
| Initial treatment place | 2.025 | 2 | 0.36 | ||
| Initial treatment place (1) | 0.079 | 1 | 0.78 | 0.919 | 0.509–1.659 |
| Initial treatment place (2) | 1.391 | 1 | 0.24 | 0.621 | 0.282–1.370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durmuş Koçak, N.; Tutar, N.; Çil, G.; Afşin, E.; Şentürk, A.; Aydın, D.; Mermit, B.; Torun Parmaksız, E.; Çolak, M.; Yıldırım, E.; et al. The Prevalence of Previous Coronavirus Disease-19 in Patients with Pulmonary Thromboembolism and Its Effect on Embolism Severity. J. Clin. Med. 2025, 14, 1909. https://doi.org/10.3390/jcm14061909
Durmuş Koçak N, Tutar N, Çil G, Afşin E, Şentürk A, Aydın D, Mermit B, Torun Parmaksız E, Çolak M, Yıldırım E, et al. The Prevalence of Previous Coronavirus Disease-19 in Patients with Pulmonary Thromboembolism and Its Effect on Embolism Severity. Journal of Clinical Medicine. 2025; 14(6):1909. https://doi.org/10.3390/jcm14061909
Chicago/Turabian StyleDurmuş Koçak, Nagihan, Nuri Tutar, Gizem Çil, Emine Afşin, Ayşegül Şentürk, Derya Aydın, Buket Mermit, Elif Torun Parmaksız, Mustafa Çolak, Elif Yıldırım, and et al. 2025. "The Prevalence of Previous Coronavirus Disease-19 in Patients with Pulmonary Thromboembolism and Its Effect on Embolism Severity" Journal of Clinical Medicine 14, no. 6: 1909. https://doi.org/10.3390/jcm14061909
APA StyleDurmuş Koçak, N., Tutar, N., Çil, G., Afşin, E., Şentürk, A., Aydın, D., Mermit, B., Torun Parmaksız, E., Çolak, M., Yıldırım, E., Özyurt, S., Polat, G., Tanrıverdi, E., Kaya, İ., Yetkin, N. A., Yılmazel Uçar, E., Doğru, S., Kilic, T., Uçar, H. A., ... Pala, A. (2025). The Prevalence of Previous Coronavirus Disease-19 in Patients with Pulmonary Thromboembolism and Its Effect on Embolism Severity. Journal of Clinical Medicine, 14(6), 1909. https://doi.org/10.3390/jcm14061909







