Real World Sex Differences in Patients Undergoing Ascending Aortic Aneurysm Surgery—A Systematic Review and Meta-Analysis of Reconstructed Time-to-Event Data
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
Included Studies
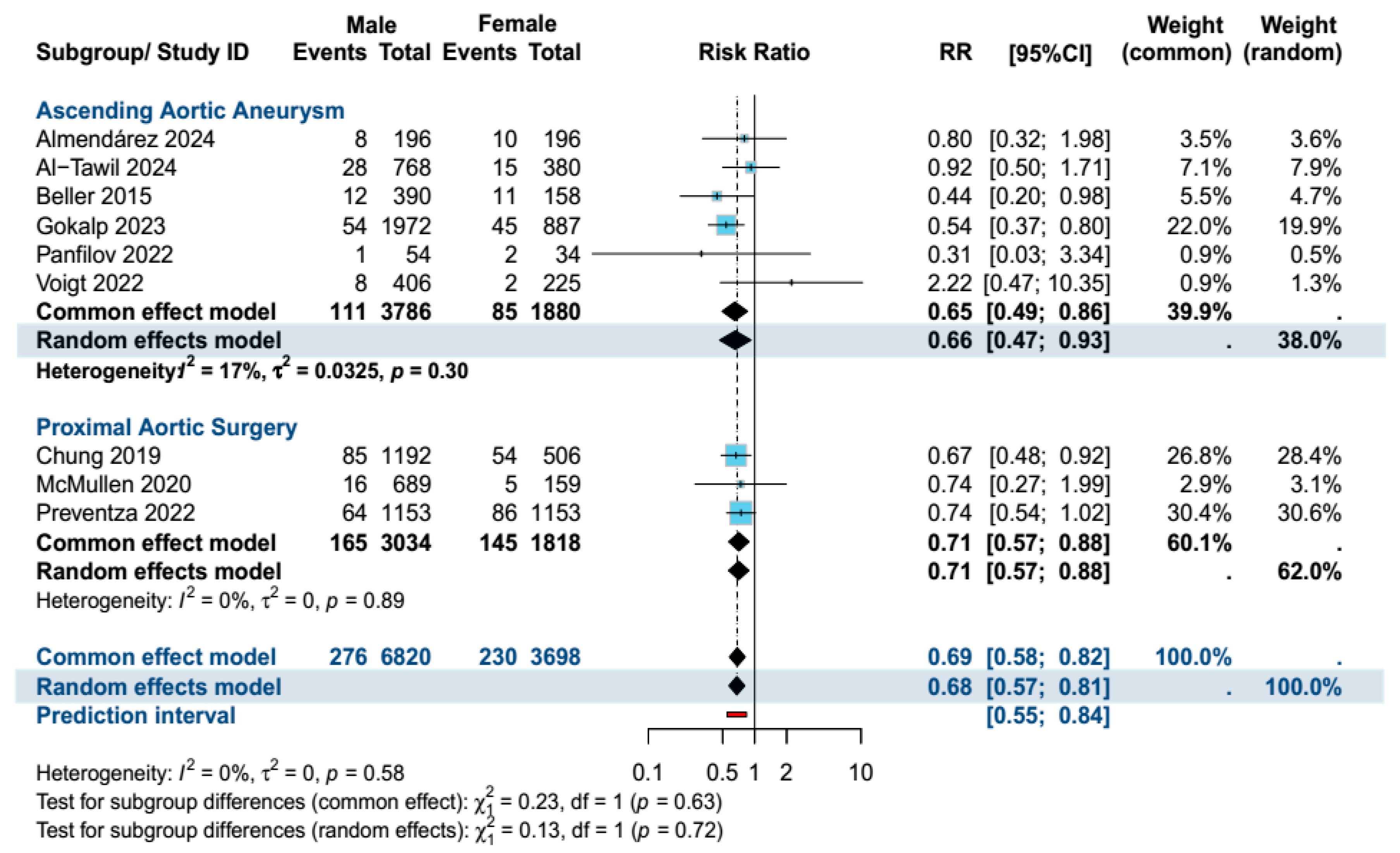
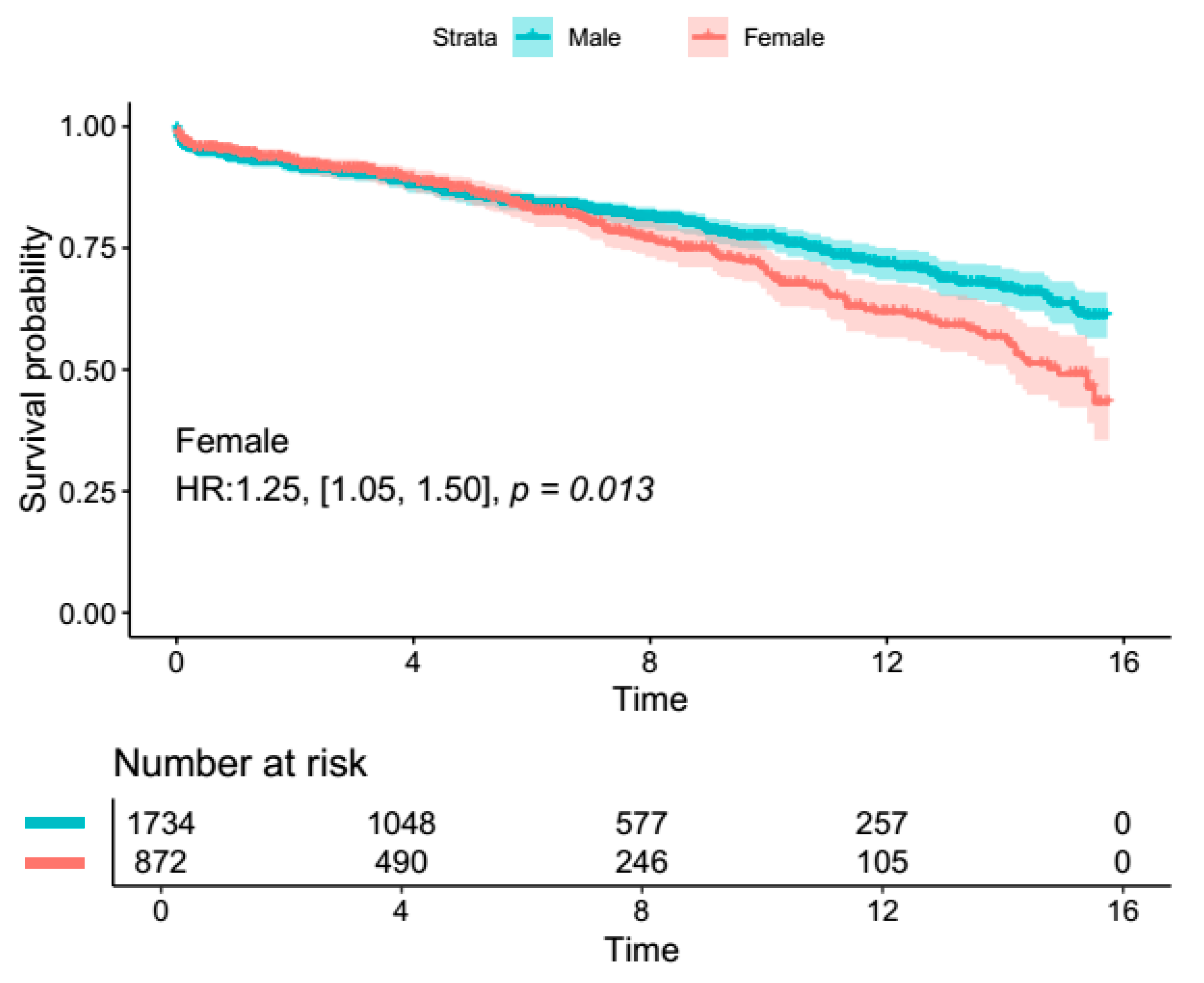
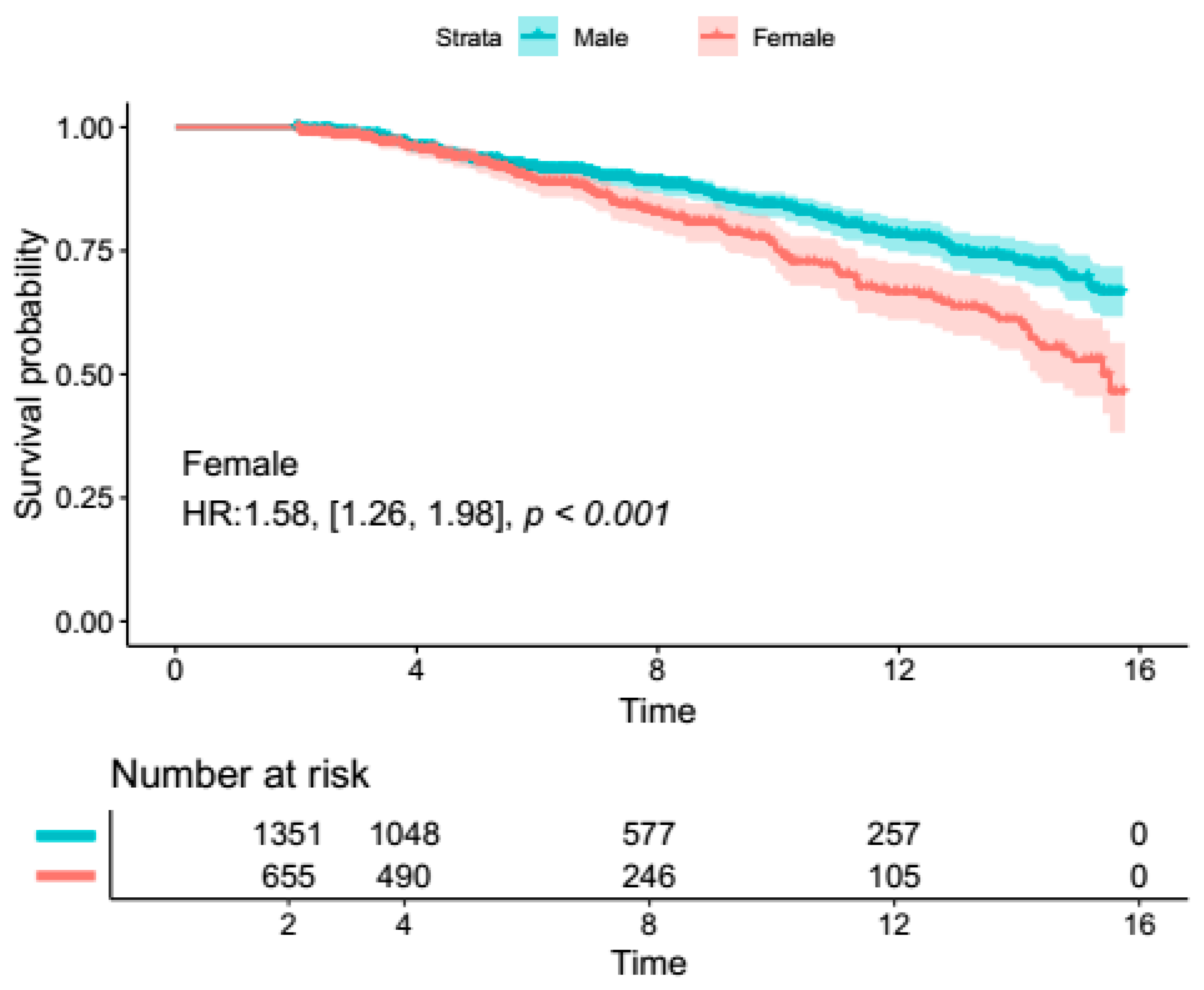
4. Primary Outcome Analysis
4.1. Secondary Outcomes (Overall Proximal Aortic Surgery)
4.2. Secondary Outcomes (Ascending Aortic Aneurysm Surgery Subgroup)
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saravanabavanandan, R.; Jaimalani, A.; Khan, M.A.N.; Riaz, S.; de Moraes Mangas, G.; Ahsan, S.M.; Posani, S.; Patel, T.; Fawad, M.; Al-Tawil, M. Gender-Based Outcome Discrepancies in Patients Who Underwent Alcohol Septal Ablation or Septal Myectomy for Hypertrophic Obstructive Cardiomyopathy: A Systematic Review and Meta-Analysis. Am. J. Cardiol. 2023, 208, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Muppuri, M.C.; Gopinath, L.; Tariq, Z.; Shah, S.; Javier, R.C.; Mahmood, F.; Modi, D.; Joseph, M.; Gopavaram, R.R.; Sharma, S.; et al. The Influence of Biological Sex on Presentation and Outcomes of Acute Myocarditis: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e57325. [Google Scholar]
- DesJardin, J.T.; Chikwe, J.; Hahn, R.T.; Hung, J.W.; Delling, F.N. Sex Differences and Similarities in Valvular Heart Disease. Circ. Res. 2022, 130, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.L.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 2—Isolated valve surgery. Ann. Thorac. Surg. 2009, 88 (Suppl. S1), S23–S42. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.W.; Yin, K.; Connelly, H.L.; Datar, Y.; Brydges, H.; Balasubramaniyan, R.; Karlson, K.J.; Edwards, N.M.; Dobrilovic, N. Sex-based outcomes in surgical repair of acute type A aortic dissection: A meta-analysis and meta-regression. J. Thorac. Cardiovasc. Surg. 2024, 167, 76–85.e13. [Google Scholar] [CrossRef]
- Sá, M.P.; Tasoudis, P.; Jacquemyn, X.; Ahmad, D.; Diaz-Castrillón, C.E.; Brown, J.A.; Yousef, S.; Zhang, D.; Dufendach, K.; Serna-Gallegos, D.; et al. Long-term sex-based outcomes after surgery for acute type A aortic dissection: Meta-analysis of reconstructed time-to-event data. Am. J. Surg. 2024, 228, 159–164. [Google Scholar] [CrossRef]
- Cheung, K.; Boodhwani, M.; Chan, K.L.; Beauchesne, L.; Dick, A.; Coutinho, T. Thoracic Aortic Aneurysm Growth: Role of Sex and Aneurysm Etiology. J. Am. Heart Assoc. 2017, 6, e003792. [Google Scholar] [CrossRef]
- Zafar, M.A.; Li, Y.; Rizzo, J.A.; Charilaou, P.; Saeyeldin, A.; Velasquez, C.A.; Mansour, A.M.; Bin Mahmood, S.U.; Ma, W.G.; Brownstein, A.J.; et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J. Thorac. Cardiovasc. Surg. 2018, 155, 1938–1950. [Google Scholar] [CrossRef]
- Tedjawirja, V.N.; Nieuwdorp, M.; Yeung, K.K.; Balm, R.; de Waard, V. A Novel Hypothesis: A Role for Follicle Stimulating Hormone in Abdominal Aortic Aneurysm Development in Postmenopausal Women. Front. Endocrinol. 2021, 12, 726107. [Google Scholar] [CrossRef]
- Beller, C.J.; Farag, M.; Wannaku, S.; Seppelt, P.; Arif, R.; Ruhparwar, A.; Karck, M.; Weymann, A.; Kallenbach, K. Gender-specific differences in outcome of ascending aortic aneurysm surgery. PLoS ONE 2015, 10, e0124461. [Google Scholar] [CrossRef]
- Chung, J.; Stevens, L.M.; Ouzounian, M.; El-Hamamsy, I.; Bouhout, I.; Dagenais, F.; Cartier, A.; Peterson, M.D.; Boodhwani, M.; Guo, M.; et al. Sex-Related Differences in Patients Undergoing Thoracic Aortic Surgery. Circulation 2019, 139, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Voigt, K.R.; Gökalp, A.L.; Papageorgiou, G.; Bogers, A.; Takkenberg, J.J.M.; Mokhles, M.M.; Bekkers, J.A. Male-Female Differences in Ascending Aortic Aneurysm Surgery: 25-Year Single Center Results. Semin. Thorac. Cardiovasc. Surg. 2023, 35, 300–308. [Google Scholar] [CrossRef]
- Panfilov, D.; Saushkin, V.; Sazonova, S.; Kozlov, B. Ascending Aortic Surgery for Small Aneurysms in Men and Women. Braz. J. Cardiovasc. Surg. 2023, 39, e20220179. [Google Scholar] [CrossRef]
- Al-Tawil, M.; Friedrich, C.; Broll, A.; Salem, M.; Schoettler, J.; de Silva, N.; Kolat, P.; Schoeneich, F.; Haneya, A. Sex-based disparities in ascending aortic aneurysm surgery outcomes: A comprehensive analysis of 1148 consecutive patients with propensity-score matching. J. Cardiothorac. Surg. 2024, 19, 331. [Google Scholar] [CrossRef]
- Gökalp, A.L.; Thijssen, C.G.E.; Bekkers, J.A.; Roos-Hesselink, J.W.; Bogers, A.; Geuzebroek, G.S.C.; Houterman, S.; Takkenberg, J.J.M.; Mokhles, M.M. Male-female differences in contemporary elective ascending aortic surgery: Insights from the Netherlands Heart Registration. Ann. Cardiothorac. Surg. 2023, 12, 577–587. [Google Scholar] [CrossRef]
- Almendárez, M.; Formica, F.; Gutierrez Sáenz de Santamaría, J.; Avanzas, P.; Escalera, A.; Alvarez-Velasco, R.; Pascual, I.; Silva, J.; Díaz, R.; Alperi, A.; et al. Sex-Related Differences in Life Expectancy Compared to General Population after Surgery for Ascending Aortic Aneurysm. J. Clin. Med. 2024, 13, 4554. [Google Scholar] [CrossRef]
- McMullen, H.; Yamabe, T.; Zhao, Y.; Kurlansky, P.; Sanchez, J.; Kelebeyev, S.; Bethancourt, C.R.; George, I.; Smith, C.R.; Takayama, H. Sex-related difference in outcomes after aortic root replacement. J. Card. Surg. 2020, 35, 1010–1020. [Google Scholar] [CrossRef]
- van Kampen, A.; Haunschild, J.; von Aspern, K.; Dietze, Z.; Misfeld, M.; Saeed, D.; Borger, M.A.; Etz, C.D. Sex-Related Differences After Proximal Aortic Surgery: Outcome Analysis of 1773 Consecutive Patients. Ann. Thorac. Surg. 2023, 116, 1186–1193. [Google Scholar] [CrossRef]
- Preventza, O.; Cekmecelioglu, D.; Chatterjee, S.; Green, S.Y.; Amarasekara, H.; Zhang, Q.; LeMaire, S.A.; Coselli, J.S. Sex Differences in Ascending Aortic and Arch Surgery: A Propensity-Matched Comparison of 1153 Pairs. Ann. Thorac. Surg. 2022, 113, 1153–1158. [Google Scholar] [CrossRef]
- Vignac, M.; Björck, H.M.; Olsson, C.; Eriksson, M.J.; Jouven, X.; Michos, E.D.; Franco-Cereceda, A.; Eriksson, P.; Gaye, B. Sex Differences in Aortopathy and Valve Diseases Among Patients Undergoing Cardiac Surgical Procedure. Ann. Thorac. Surg. 2022, 114, 1665–1670. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Paparella, D.; Ivanov, J.; Buchanan, M.R.; Brister, S.J. Gender-related differences in morbidity and mortality during combined valve and coronary surgery. J. Thorac. Cardiovasc. Surg. 2003, 126, 959–964. [Google Scholar] [CrossRef]
- Lo, R.C.; Bensley, R.P.; Hamdan, A.D.; Wyers, M.; Adams, J.E.; Schermerhorn, M.L. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J. Vasc. Surg. 2013, 57, 1261–1268.e5. [Google Scholar] [CrossRef]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010, 121, e266–e369. [Google Scholar] [CrossRef] [PubMed]
- Elefteriades, J.A. Natural history of thoracic aortic aneurysms: Indications for surgery, and surgical versus nonsurgical risks. Ann. Thorac. Surg. 2002, 74, S1877–S1880, discussion S1878–S1892. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.; Freundt, M.; Salem, M.A.; Panholzer, B.; Huenges, K.; Puehler, T.; Cremer, J.; Haneya, A. Sex-Specific Outcome after Ascending Aortic Surgery in Moderate Hypothermic Circulatory Arrest. Thorac. Cardiovasc. Surg. 2021, 69, 314–321. [Google Scholar] [CrossRef]
- Kim, J.B.; Spotnitz, M.; Lindsay, M.E.; MacGillivray, T.E.; Isselbacher, E.M.; Sundt, T.M., 3rd. Risk of Aortic Dissection in the Moderately Dilated Ascending Aorta. J. Am. Coll. Cardiol. 2016, 68, 1209–1219. [Google Scholar] [CrossRef]
- Sokolis, D.P.; Iliopoulos, D.C. Impaired mechanics and matrix metalloproteinases/inhibitors expression in female ascending thoracic aortic aneurysms. J. Mech. Behav. Biomed. Mater. 2014, 34, 154–164. [Google Scholar] [CrossRef]
- Schuster, V.; Eggersmann, T.K.; Eifert, S.; Ueberfuhr, P.; Zugenmaier, B.; Kolben, T.M.; Thaler, C.J.; Kublickiene, K.; Rieger, A.; Reichart, B.; et al. Ascending Aortic Disease is Associated with Earlier Menopause and Shorter Reproductive Life Span. J. Womens Health 2016, 25, 912–919. [Google Scholar] [CrossRef]
- Hannawa, K.K.; Eliason, J.L.; Upchurch, G.R. Gender Differences in Abdominal Aortic Aneurysms. Vascular 2009, 17 (Suppl. S1), 30–39. [Google Scholar] [CrossRef]
| Study ID | Location | Study Duration | Population | Patients | FU (y) | Long Term Survival | |||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | p Value | |||||
| Beller et al., 2015 [11] | Germany | 1994–2011 | Ascending aneurysm | 390 | 158 | _ | _ | _ | _ |
| Voigt et al., 2022 [18] | Netherlands | 1991–2016 | Ascending aneurysm | 406 | 224 | 15-year | 72.9% | 48.1% | <0.001 * |
| Panfilov et al., 2022 [19] | Russia | 2013–2021 | Ascending aneurysm | 54 | 34 | 3-year | 83.5 | 94.3% | 0.43 |
| Al-Tawil et al., 2024 [20] | Germany | 2002–2021 | Ascending aneurysm | 768 | 380 | 15-year | 59% | 51% | 0.15 |
| Gokalp et al., 2023 [21] | Netherlands | 2013–2017 | Ascending aneurysm | 1972 | 887 | _ | _ | _ | _ |
| Almendárez et al., 2024 [22] | Spain | 2000–2019 | Ascending aneurysm | 506 | 232 | 8-year | 76.1% | 75.8% | 0.23 |
| Chung et al., 2019 [12] | Canada | 2002–2017 | Proximal aortic surgery | 1155 | 498 | _ | _ | _ | _ |
| McMullen et al., 2020 [23] | USA | 2005–2018 | Proximal aortic surgery | 689 | 159 | 5-year | 93.6% | 91.3% | 0.43 |
| Kampen et al., 2022 [24] | Germany | 2000–2018 | Proximal aortic surgery | 1331 | 442 | 5-year | 91.7% | 87.6% | 0.02 * |
| Preventza et al., 2022 [25] | USA | 1990–2020 | Proximal aortic surgery | 1153 | 1153 | 5-year | 67.1% | 66.3% | 0.40 |
| Vignac et al., 2022 [26] | Sweden | 2007–2017 | Proximal aortic surgery | 700 | 345 | _ | _ | _ | _ |
| Overall Proximal Aortic Surgery | Ascending Aortic Aneurysm Subgroup | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients Analyzed | Effect Estimate | M% vs. F% | p Value | Patients Analyzed | Effect Estimate | M% vs. F% | p Value | |
| Age (mean ± SD) # | 13,637 | −3.94 [−5.58, −2.31] | / | p < 0.001 | 6012 | −4.25 [−6.94, −1.55] | / | p = 0.002 |
| Absolute AscA diameter (mean ± SD) (mm) # | 7624 | −0.88 [−2.46, 0.69] | / | p = 0.27 | 3153 | −0.74 [−1.73, 0.24] | / | p = 0.14 |
| Indexed/normalized ascending aortic diameter (mean ± SD) (mm/m2) # | 7624 | −3.19 [−4.06, −2.31] | / | p < 0.001 | 3153 | −3.21 [−4.58, −1.84] | / | p < 0.001 |
| Bicuspid aortic valve | 9014 | 1.51 [1.09, 2.08] | 36% vs. 22% | p = 0.01 | 434 | 1.36 [1.00, 1.85] | 23% vs. 17% | p = 0.05 |
| Aortic stenosis | 9097 | 1.16 [0.86, 1.56] | 34% vs. 28% | p = 0.32 | 2,517 | 1.13 [0.84, 1.52] | 26% vs. 23% | p = 0.41 |
| Aortic regurgitation | 9097 | 1.25 [0.95, 1.64] | 43% vs. 30% | p = 0.10 | 2517 | 0.93 [0.76, 1.15] | 43% vs. 48% | p = 0.51 |
| Hypertension | 12,489 | 0.94 [0.88, 1.00] | 54% vs. 59% | p = 0.05 | 4864 | 0.90 [0.78, 1.04] | 29% vs. 32% | p = 0.17 |
| Diabetes | 13,637 | 1.01 [0.88, 1.16] | 9% vs. 9% | p = 0.85 | 6012 | 1.05 [0.87, 1.26] | 8% vs. 8% | p = 0.63 |
| Operative details | ||||||||
| Elective | 7336 | 1.00 [0.99, 1.01] | 90% vs. 91% | p = 0.92 | 4638 | 1.00 [1.00, 1.00] | 98% vs. 98% | p = 0.99 |
| Valve-sparing root replacement | 9993 | 1.16 [0.90, 1.49] | 11% vs. 10% | p = 0.26 | 5186 | 1.04 [0.65, 1.67] | 8% vs. 8% | p = 0.87 |
| Isolated supra-coronary aortic replacement | 5924 | 0.67 [0.51, 0.88] | 22% vs. 36% | p = 0.004 | 5924 | 0.67 [0.51, 0.88] | 22% vs. 36% | p = 0.004 |
| Concomitant CABG | 10,081 | 1.46 [1.21, 1.77] | 21% vs. 14.5% | p < 0.001 | 5274 | 1.64 [1.19, 2.26] | 22% vs. 13% | p = 0.003 |
| Concomitant AVR | 7960 | 1.04 [0.85, 1.26] | 28% vs. 24% | p = 0.72 | 3153 | 1.01 [0.84, 1.23] | 38% vs. 35% | p = 0.88 |
| Total arch replacement | 6494 | 0.67 [0.50, 0.91] | 3% vs. 7% | p = 0.009 | 2535 | 0.49 [0.25, 0.98] | 11% vs. 16% | p = 0.04 |
| Operation time (mean ± SD) # | 3557 | 16.60 [1.32, 31.87] | / | p = 0.03 | 1784 | 18.01 [−6.54, 42.56] | / | p = 0.15 |
| Circulatory arrest time (mean ± SD) # | 7959 | −0.73 [−2.74, 1.29] | / | p = 0.48 | 3152 | −0.82 [−4.27, 2.62] | / | p = 0.64 |
| CPB bypass time (mean ± SD) # | 9733 | 10.61 [4.55, 16.68] | / | p < 0.001 | 3153 | 9.47 [−1.18, 20.12] | / | p = 0.08 |
| Aortic cross-clamp time, (mean ± SD) # | 7339 | 10.36 [4.50, 16.21] | / | p < 0.001 | 3065 | 9.21 [0.03, 18.39] | / | p = 0.05 |
| Postoperative Outcomes | ||||||||
| 30-day mortality | 10,518 | 0.68 [0.57, 0.81] | 4% vs. 6% | p < 0.001 | 5666 | 0.66 [0.47, 0.93] | 3% vs. 5% | p = 0.02 |
| Neurological complications (stroke/TIA) | 11,899 | 0.81 [0.61, 1.07] | 5% vs. 4% | p = 0.13 | 5274 | 0.74 [0.38, 1.43] | 4% vs. 6% | p = 0.38 |
| Re-thoracotomy | 9278 | 1.11 [1.00, 1.23] | 13% vs. 14% | p = 0.05 | 5274 | 1.03 [0.83, 1.28] | 8% vs. 8% | p = 0.78 |
| Ascending aortic reoperation | 2544 | 0.99 [0.60, 1.64] | 4% vs. 3% | p = 0.97 | 1686 | 0.76 [0.10, 5.98] | 0.5% vs. 0.6% | p = 0.79 |
| New AKI, or new dialysis | 11,899 | 0.87 [0.66, 1.15] | 5% vs. 6% | p = 0.34 | 5274 | 0.95 [0.57, 1.58] | 5% vs. 5% | p = 0.84 |
| New myocardial infarction | 8730 | 1.10 [0.75, 1.60] | 1.6% vs. 1.3% | p = 0.63 | 4726 | 1.34 [0.85, 2.10] | 2% vs. 1.6% | p = 0.21 |
| New pacemaker | 5787 | 0.92 [0.65, 1.30] | 20% vs. 22% | p = 0.63 | 4939 | 1.04 [0.78, 1.40] | 23% vs. 23% | p = 0.78 |
| Chest tube bleeding, mL # | 1784 | 0.09 [−0.01, 0.19] | / | p = 0.07 | 1784 | 0.09 [−0.01, 0.19] | / | p = 0.07 |
| Invasive ventilation time, h # | 3557 | −0.11 [−0.28, 0.06] | / | p = 0.22 | 1784 | −0.04 [−0.14, 0.06] | / | p = 0.39 |
| Length of hospital stay (mean no. of days) # | 11,811 | −0.58 [−1.03, −0.12] | / | p = 0.01 | 5186 | −0.33 [−1.03, 0.38] | / | p = 0.36 |
| Length of ICU stay (mean no. of days) # | 9040 | −0.48 [−0.84, −0.13] | / | p = 0.008 | 2415 | −0.06 [−0.29, 0.18] | / | p = 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Tawil, M.; Geragotellis, A.; Alroobi, A.; Aboabdo, M.; Alaila, D.; Sulaiman, W.A.; Ghaben, N.; Salim, H.T.; Friedrich, C.; Rusch, R.; et al. Real World Sex Differences in Patients Undergoing Ascending Aortic Aneurysm Surgery—A Systematic Review and Meta-Analysis of Reconstructed Time-to-Event Data. J. Clin. Med. 2025, 14, 1908. https://doi.org/10.3390/jcm14061908
Al-Tawil M, Geragotellis A, Alroobi A, Aboabdo M, Alaila D, Sulaiman WA, Ghaben N, Salim HT, Friedrich C, Rusch R, et al. Real World Sex Differences in Patients Undergoing Ascending Aortic Aneurysm Surgery—A Systematic Review and Meta-Analysis of Reconstructed Time-to-Event Data. Journal of Clinical Medicine. 2025; 14(6):1908. https://doi.org/10.3390/jcm14061908
Chicago/Turabian StyleAl-Tawil, Mohammed, Alexander Geragotellis, Ahmad Alroobi, Mohammad Aboabdo, Doa’a Alaila, Wafaa A. Sulaiman, Nour Ghaben, Heba T. Salim, Christine Friedrich, René Rusch, and et al. 2025. "Real World Sex Differences in Patients Undergoing Ascending Aortic Aneurysm Surgery—A Systematic Review and Meta-Analysis of Reconstructed Time-to-Event Data" Journal of Clinical Medicine 14, no. 6: 1908. https://doi.org/10.3390/jcm14061908
APA StyleAl-Tawil, M., Geragotellis, A., Alroobi, A., Aboabdo, M., Alaila, D., Sulaiman, W. A., Ghaben, N., Salim, H. T., Friedrich, C., Rusch, R., & Haneya, A. (2025). Real World Sex Differences in Patients Undergoing Ascending Aortic Aneurysm Surgery—A Systematic Review and Meta-Analysis of Reconstructed Time-to-Event Data. Journal of Clinical Medicine, 14(6), 1908. https://doi.org/10.3390/jcm14061908





