How to Limit Interdialytic Weight Gain in Patients on Maintenance Hemodialysis: State of the Art and Perspectives
Abstract
1. Introduction
2. To Limit IDWG: State of the Art
- 1.
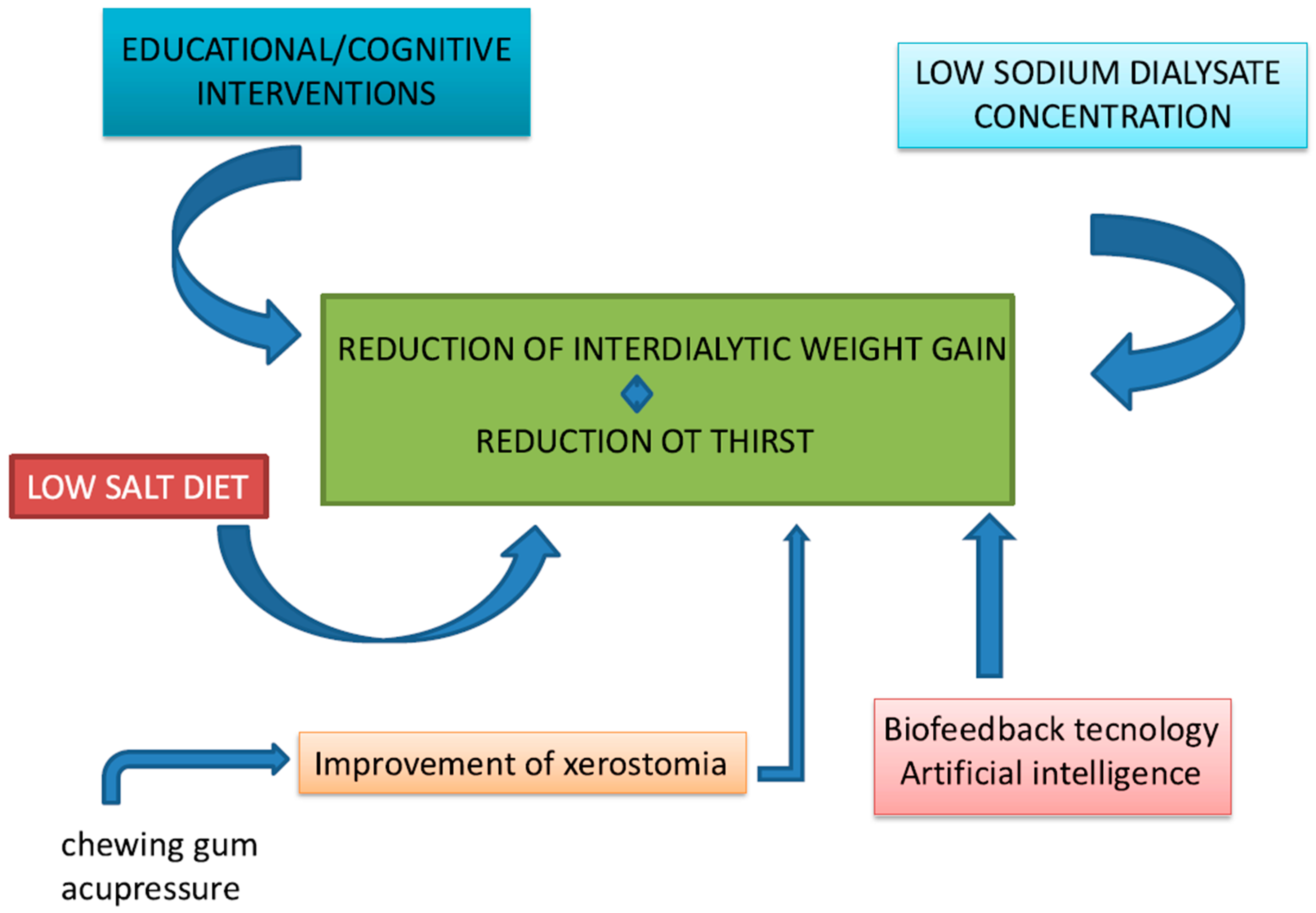
- Educational/cognitive interventions, as well as counseling/behavioral and psychological/affective, have been employed to inform patients about their condition, encourage active participation in their care, and promote an appropriate lifestyle. These interventions aim to improve emotional and social aspects, fostering motivation. Studies have demonstrated that these approaches yield significant benefits in reducing IDWG in hemodialysis patients. The patient’s compliance is still the most important, as is the renal kidney function, on which the patient’s survival depends. However, these strategies also face challenges, including time constraints, high costs, and the need for patient compliance, as they require multiple training sessions, regular feedback, and homework. Even when good adherence is achieved, there remains a risk of relapsing [10,11,12,13]. Additionally, psychological interventions are limited by biases, such as the availability of qualified psychologists who may not be present in all dialysis centers. Moreover, the optimal duration of these interventions to achieve clinically meaningful effects remains unclear [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27].
- 2.
- Another strategy to reduce IDWG involves lowering the sodium concentration in dialysis fluid. By reducing the sodium load in the dialysate, better sodium removal is achieved, resulting in lower overall sodium levels in circulation. This alleviates thirst, leading to reduced fluid intake between dialysis sessions. Moreover, sodium in the dialysis fluid is a source of sodium entry that can be adjusted independently of biosensors in monitors, mainly in HDF online, with a high infusion rate. Although the benefits of this approach have been well-documented in numerous studies, especially in Europe and the United States, it has also sparked considerable debate. Three recent systematic reviews reported conflicting results regarding the effects of dialysate sodium concentrations on IDWG [28,29]. Basile et al. found that most studies included in their analysis reported significantly higher IDWG in patients treated with high-sodium dialysate. However, their review of three interventional studies revealed no substantial differences in IDWG between treatment groups. Additionally, another study documented a reduction in total body weight in patients undergoing dialysis with both low (135 mmol/L) and high (140 mmol/L) sodium dialysate, although the difference between groups was not statistically significant [28]. In contrast, Dunlop et al. reported that low-sodium dialysate was associated with a significant reduction in IDWG compared to neutral or high sodium concentrations. However, the authors noted that the magnitude of this reduction was modest from a clinical perspective [29].
- 3.
- An additional factor contributing to IDWG is excessive dietary salt intake, which increases thirst and subsequently leads to higher IDWG. According to international guidelines [40], the recommended daily sodium chloride intake for dialysis patients should not exceed 5 g (equivalent to 2.0 g [85 mmol] of sodium). For hypertensive dialysis patients, a stricter limitation of 2.5–3.8 g (1–1.5 g [43–65 mmol] of sodium) per day is advised. However, the average daily salt intake among dialysis patients remains significantly higher, ranging from 7.9 to 14.1 g/day [41,42,43,44,45,46,47,48]. Adherence to a low-sodium diet is often hindered by cultural, social, and economic factors. Moreover, the need to limit foods rich in phosphorus and potassium, which are often less palatable, may discourage adherence to a low-sodium diet [49]. Our systematic review and meta-analysis compared the effects of low-sodium diets versus normal or high-sodium diets, as well as the impact of nutritional counseling versus no counseling on IDWG in chronic hemodialysis patients [50]. The results revealed a significantly lower risk of experiencing IDWG > 2.5 kg among patients adhering to a low-sodium diet or receiving nutritional counseling compared to controls [51,52]. However, there are concerns about potential adverse effects associated with low-sodium diets, including pre-dialysis hyponatremia, malnutrition, and an increased risk of all-cause and cardiovascular mortality, that in the group of HD patients it is up to 33x the frequency [53,54,55,56]. Recent studies have also highlighted a correlation between low daily sodium intake and insufficient consumption of calories, proteins, minerals, trace elements, and vitamin B1 [53]. Hyponatremia can adversely affect the brain, heart, bones, musculoskeletal system, and immune system [57]. Overall, it seems that low-sodium diets or nutritional counseling for sodium reduction are effective strategies for achieving significant reductions in IDWG among chronic hemodialysis patients. Nevertheless, the limited availability of studies and the potential risks associated with these interventions underscore the need for further research. Prospective randomized trials with long-term follow-up are essential to clarify the effects of low-sodium diets, with particular attention to monitoring the development of hyponatremia, malnutrition, and other related complications.
3. Thirst Is the Problem
Focus on Dialysis Patients
4. To Limit the IDWG: Perspectives
- ○
- The presence of a psychologist in a multidisciplinary team comprising professionals such as nurses, dieticians, pharmacists, or physicians to develop patients’ skills and promote better adherence;
- ○
- Integration of collective and individual practices, achieving more beneficial effects compared to a single approach;
- ○
- Combination of biological/clinical outcomes (objective measures) and psychosocial criteria (subjective measures) to evaluate interventions targeting improvements in therapeutic adherence and lifestyle recommendations;
- ○
- Continuous follow-up, accounting for short-, medium-, and long-term effects of interventions, as some results may decline over time [78].
- 1.
- Regarding chewing gum consumption, the potential mechanisms by which chewing gum may reduce thirst are
- -
- Salivation stimulation: Chewing stimulates saliva production, which may help alleviate the sensation of dry mouth and thirst. Indeed, an increase in saliva could reduce the perception of thirst, which is a common issue in hemodialysis patients due to fluid restrictions between dialysis sessions [86];
- -
- Psychological distraction: Chewing gum may serve as a psychological distraction, reducing awareness of thirst, which is often acute in dialysis patients due to the need to limit fluid intake between dialysis sessions [87];Unlike previously mentioned studies, Chen et al. found that the sensation of thirst was alleviated in the treated patient group, although no effect was observed on saliva production or IDWG in this population [85]. Similarly, Allida et al. demonstrated its short- and long-term effectiveness [88]. Dehghanmehr et al. found a significant difference between thirst and dry mouth and chewing sugar-free gum before and after the intervention [89]. Similarly, a significant reduction in thirst was observed by Bots et al. [90], Fan et al. [91], and Duruk and Eser [92]. Nonetheless, it is important to note that while chewing gum may provide temporary relief from thirst, it does not address the underlying issues related to fluid balance in dialysis patients.
- 2.
- Acupressure: Keskin et al. reported increased salivation, a reduction in the severity of thirst on the visual analogue scale (VAS), and an enhanced quality of life for hemodialysis patients when applied at the CV-23 (Ren-23, Lianquan - "Corner Spring"), SJ-17 (SJ-17 (San Jiao-17, Yifeng - "Wind Screen"), and Kid-1 (Kidney-1, Yongquan - "Gushing Spring") points. In particular, they examined the effect of 15 min of acupressure three times a week for six weeks, considering an intervention group and a control group, finding significant differences in the mental component sub-dimension of the “Quality of Life” scale at both the first and sixth weeks [93]. These findings are supported by the study of Yang et al. [94], which demonstrated that acupressure improved the salivary flow rate and the mean thirst intensity, although no statistically significant difference was observed in pre- and post-program salivary flow rate.Acupuncture could improve thirst through several mechanisms:
- Regulation of body fluids: Acupuncture stimulates specific points in the body that may influence fluid balance. It could help improve the equilibrium of body fluids, thereby reducing the sensation of thirst, which is often problematic for dialysis patients [95];
- Effects on the nervous system: Acupuncture may have a positive impact on the autonomic nervous system, which is responsible for involuntary body functions such as thirst regulation. By stimulating certain points, acupuncture might reduce excessive thirst and improve the quality of life for dialysis patients [95];
- Improvement of blood flow and fluid retention: Acupuncture could enhance blood circulation, reduce fluid retention, and improve kidney function. These effects might contribute to better fluid balance, thereby reducing the sensation of thirst [96].
- Reduction in stress and pain: Acupuncture is also known for its effects in reducing stress and pain, and since dialysis patients may experience discomfort and frustration related to their condition, reducing stress could help minimize the need to drink excessively as an emotional response [97].
- 3.
- Frequency and duration of HD: increasing the frequency of dialysis sessions and extending session duration are two promising strategies for optimizing fluid management and improving patient outcomes. Evidence highlights that more frequent dialysis regimens—such as daily or nocturnal sessions—consistently reduce interdialytic weight gain (IDWG) compared to the conventional thrice-weekly schedule [98]. Notably, findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS) underscore the superior efficacy of frequent dialysis in controlling fluid overload, an improvement intricately tied to better cardiovascular health and reduced mortality [98]. The compelling results of the Frequent Hemodialysis Network (FHN) trial further emphasize the clinical advantages of increased frequency. Researchers reported that patients undergoing in-center hemodialysis six times per week experienced significant reductions in IDWG, improved blood pressure control, and better overall fluid balance compared to those receiving standard thrice-weekly treatments [99]. This is particularly critical given the robust association between elevated IDWG and adverse cardiovascular consequences, including left ventricular hypertrophy and heightened hospitalization rates [100,101]. Equally important is the role of session duration in fluid management. Prolonging dialysis sessions allows for a more gradual ultrafiltration process, effectively reducing the risk of intradialytic hypotension and minimizing complications associated with rapid fluid shifts [102,103]. Longer session lengths have been strongly associated with better blood pressure control, enhanced cardiovascular outcomes, and lower IDWG [98,104]. These outcomes highlight the potential of extended session duration to complement increased frequency in achieving optimal fluid management. Rather than viewing increased frequency or extended duration as isolated strategies, clinicians should consider an individualized approach that integrates both, tailored to the patient’s specific needs. For patients with high IDWG, frequent, shorter sessions might be more practical, whereas those prone to hemodynamic instability may benefit more from longer, less frequent sessions. By balancing these factors, it is possible to achieve a more patient-centered dialysis regimen that improves both immediate and long-term outcomes.
- 4.
- Biofeedback technology: the utilization of biofeedback systems in hemodialysis has emerged as a promising approach to assess and manage volume overload and interdialytic weight gain (IDWG) in patients undergoing this treatment. Biofeedback systems facilitate real-time monitoring of blood volume and other physiological parameters, allowing for tailored interventions that can mitigate these risks. One of the primary advantages of biofeedback systems is their ability to guide ultrafiltration (UF) rates based on continuous blood volume monitoring. This real-time feedback can help prevent excessive fluid removal, which is a common cause of intradialytic hypotension (IDH) [105,106]. Studies have shown that patients using biofeedback-guided UF experience fewer episodes of IDH compared to those receiving conventional hemodialysis [107,108]. A randomized controlled trial demonstrated that blood volume monitoring significantly reduced the frequency of symptomatic IDH, thereby improving overall patient stability during dialysis sessions [106]. Moreover, biofeedback systems can play a crucial role in managing IDWG, which is often a reflection of fluid retention between dialysis sessions. By providing real-time data on fluid status, biofeedback systems enable clinicians to make informed decisions regarding fluid removal and dietary recommendations, potentially reducing IDWG [109]. The study of Mohamed et al. found that patients who received biofeedback on their fluid status were better able to adhere to fluid restrictions, resulting in lower IDWG [110].
- 5.
- Wearable Devices and Artificial Intelligence Integration: the impact of wearable devices on clinical outcomes in hemodialysis patients is a rapidly evolving area of research with significant implications for patient management and health outcomes. Wearable technologies have the potential to enhance patient engagement, facilitate self-management, and provide real-time data that can inform clinical decision-making. One of the primary benefits of wearable devices is their ability to monitor physiological parameters continuously, which is crucial for managing conditions such as fluid overload in hemodialysis patients. For instance, wearable technologies can track vital signs and fluid status, enabling patients to receive immediate feedback on their health status. This real-time monitoring can help patients adhere to fluid restrictions and dietary guidelines, which are essential for minimizing IDWG and preventing complications associated with fluid overload [112]. Studies have indicated that the use of wearable devices can lead to improved adherence to treatment protocols, resulting in better clinical outcomes, including reduced hospitalization rates and improved quality of life [113]. These devices utilize advanced sensors to assess various physiological parameters, including bioimpedance and thoracic fluid levels, which correlate strongly with fluid changes in the body [112,114]. For instance, a study demonstrated that a wearable bioimpedance device could effectively monitor fluid overload and provide feedback to patients, allowing for timely interventions [114]. This capability is particularly crucial as traditional methods often rely on periodic assessments in clinical settings, which may not capture fluctuations in fluid status between dialysis sessions [112]. Research has shown that wearable bioimpedance devices can effectively track changes in fluid volume, thereby facilitating better fluid management strategies [115,116]. Integration of mobile applications that track dietary intake and fluid consumption can further enhance patient engagement and adherence to prescribed regimens [115,117]. Bioimpedance measures the resistance of body tissues to electrical currents, providing insights into hydration levels and fluid overload [118]. Recent advancements in textile electrodes have improved the comfort and usability of these devices, enabling long-term monitoring without compromising patient mobility [118]. The ability to track fluid status continuously can help patients adhere to fluid restrictions and prevent complications associated with fluid overload, such as cardiovascular events. Moreover, the integration of artificial intelligence and digital health technologies into these wearable devices can enhance their functionality. AI algorithms can analyze data collected from patients to provide personalized recommendations for fluid intake and dietary adjustments, thereby promoting adherence to treatment protocols [112]. This is particularly relevant given that non-adherence to fluid restrictions is a common issue among hemodialysis patients, often exacerbated by factors such as thirst and psychological stress [49]. Wearable technologies can also incorporate features that support psychological well-being, such as reminders and motivational prompts, which can enhance adherence to fluid management strategies [117].
Author Contributions
Funding
Conflicts of Interest
References
- Wijayanti, L.; Winoto, P.M.P.; Nursalam, N. How to control interdialytic weight gain (Idwg) among hemodialysis patients. Nurse Health J. Keperawatan 2021, 10, 214–221. [Google Scholar] [CrossRef]
- Wong, M.M.; McCullough, K.P.; Bieber, B.A.; Bommer, J.; Hecking, M.; Levin, N.W.; McClellan, W.M.; Pisoni, R.L.; Saran, R.; Tentori, F.; et al. Interdialytic weight gain: Trends, predictors, and associated outcomes in the international dialysis outcomes and practice patterns study (DOPPS). Am. J. Kidney Dis. 2017, 69, 367–379. [Google Scholar] [CrossRef]
- Vr, V.; Kaur Kang, H. The Worldwide Prevalence of Nonadherence to Diet and Fluid Restrictions Among Hemodialysis Patients: A Systematic Review and Meta-analysis. J. Ren. Nutr. 2022, 32, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Kurita, N.; Hayashino, Y.; Yamazaki, S.; Akizawa, T.; Akiba, T.; Saito, A.; Fukuhara, S. Revisiting Interdialytic Weight Gain and Mortality Association With Serum Albumin Interactions: The Japanese Dialysis Outcomes and Practice Pattern Study. J. Ren. Nutr. 2017, 27, 421–429. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Regidor, D.L.; Kovesdy, C.P.; Van Wyck, D.; Bunnapradist, S.; Horwich, T.B.; Fonarow, G.C. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 2009, 119, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, P.L.; Varela, M.P.; Peterson, R.A.; Weihs, K.L.; Simmens, S.J.; Alleyne, S.; Amarashinge, A.; Mishkin, G.J.; Cruz, I.; Veis, J.H. Interdialytic weight gain and survival in hemodialysis patients: Effects of duration of ESRD and diabetes mellitus. Kidney Int. 2000, 57, 1141–1151. [Google Scholar] [CrossRef]
- Cabrera, C.; Brunelli, S.M.; Rosenbaum, D.; Anum, E.; Ramakrishnan, K.; Jensen, D.E.; Stålhammar, N.-O. A retrospective, longitudinal study estimating the association between interdialytic weight gain and cardiovascular events and death in hemodialysis patients. BMC Nephrol. 2015, 16, 113. [Google Scholar] [CrossRef]
- Lee, M.J.; Doh, F.M.; Kim, C.H.; Koo, H.M.; Oh, H.J.; Park, J.T.; Han, S.H.; Yoo, T.-H.; Kim, Y.-L.; Kim, Y.S.; et al. Interdialytic weight gain and cardiovascular outcome in incident hemodialysis patients. Am. J. Nephrol. 2014, 39, 427–435. [Google Scholar] [CrossRef]
- Saran, R.; Bragg-Gresham, J.L.; Rayner, H.C.; Goodkin, D.A.; Keen, M.L.; Van Dijk, P.C.; Kurokawa, K.; Piera, L.; Saito, A.; Fukuhara, S.; et al. Nonadherence in hemodialysis: Associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003, 64, 254–262. [Google Scholar] [CrossRef]
- Murali, K.M.; Lonergan, M. Breaking the adherence barriers: Strategies to improve treatment adherence in dialysis patients. Semin. Dial. 2020, 33, 475–485. [Google Scholar] [CrossRef]
- De Bleser, L.; Matteson, M.; Dobbels, F.; Russell, C.; De Geest, S. Interventions to improve medication-adherence after transplantation: A systematic review. Transpl. Int. 2009, 22, 780–797. [Google Scholar] [CrossRef] [PubMed]
- Cummings, K.M.; Becker, M.H.; Kirscht, J.P.; Levin, N.W. Intervention strategies to improve compliance with medical regimens by ambulatory hemodialysis patients. J. Behav. Med. 1981, 4, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.L.; Craig, C.B.; Bartolucci, A.A.; Allon, M.; Fox, L.M.; Geiger, B.F.; Wilson, N.P. The effect of a self-monitoring tool on self-efficacy, health beliefs, and adherence in patients receiving hemodialysis. J. Ren. Nutr. 1998, 8, 203–211. [Google Scholar] [CrossRef]
- Christensen, A.J.; Moran, P.J.; Wiebe, J.S.; Ehlers, S.L.; Lawton, W.J. Effect of a behavioral self-regulation intervention on patient adherence in hemodialysis. Health Psychol. 2002, 21, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Molaison, E.F.; Yadrick, M.K. Stages of change and fluid intake in dialysis patients. Patient Educ. Couns. 2003, 49, 5–12. [Google Scholar] [CrossRef]
- Tsay, S.L. Self-efficacy training for patients with end-stage renal disease. J. Adv. Nurs. 2003, 43, 370–375. [Google Scholar] [CrossRef]
- Sharp, J.; Wild, M.R.; Gumley, A.I.; Deighan, C.J. A cognitive behavioral group approach to enhance adherence to hemodialysis fluid restrictions: A randomized controlled trial. Am. J. Kidney Dis. 2005, 45, 1046–1057. [Google Scholar] [CrossRef]
- Kauric-Klein, Z. Improving blood pressure control in end stage renal disease through a supportive educative nursing intervention. Nephrol. Nurs. J. 2012, 39, 217–228. [Google Scholar]
- Cho, M.K. Effect of health contract intervention on renal dialysis patients in Korea. Nurs. Health Sci. 2013, 15, 86–93. [Google Scholar] [CrossRef]
- Welch, J.L.; Astroth, K.S.; Perkins, S.M.; Johnson, C.S.; Connelly, K.; Siek, K.A.; Jones, J.; Scott, L.L. Using a mobile application to self-monitor diet and fluid intake among adults receiving hemodialysis. Res. Nurs. Health 2013, 36, 284–298. [Google Scholar] [CrossRef]
- Cukor, D.; Ver Halen, N.; Asher, D.R.; Coplan, J.D.; Weedon, J.; Wyka, K.E.; Saggi, S.J.; Kimmel, P.L. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J. Am. Soc. Nephrol. 2014, 25, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Griva, K.; Nandakumar, M.; Ng, J.H.; Lam, K.F.Y.; McBain, H.; Newman, S.P. Hemodialysis Self-management Intervention Randomized Trial (HED-SMART): A. Practical Low-Intensity Intervention to Improve Adherence and Clinical Markers in Patients Receiving Hemodialysis. Am. J. Kidney Dis. 2018, 71, 371–381. [Google Scholar] [CrossRef]
- Başer, E.; Mollaoğlu, M. The effect of a hemodialysis patient education program on fluid control and dietary compliance. Hemodial Int. 2019, 23, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.M.; Hu, P.C.; Liang, Y.P.; Mo, Z.Y. Effects of rational-emotive therapy on adherence to fluid restrictions of patients maintained on hemodialysis prior to and after kidney transplantation. J. Clin. Rehab. Tissue Eng. Res. 2020, 31, 5869–5872. [Google Scholar]
- Pasyar, N.; Rambod, M.; Sharif, F.; Rafii, F.; Pourali-Mohammadi, N. Improving adherence and biomedical markers in hemodialysis patients: The effects of relaxation therapy. Complement. Ther. Med. 2015, 23, 38–45. [Google Scholar] [CrossRef]
- Bellomo, G.; Coccetta, P.; Pasticci, F.; Rossi, D.; Selvi, A. The effect of psychological intervention on thirst and interdialytic weight gain in patients on chronic hemodialysis: A randomized controlled trial. J. Ren. Nutr. 2015, 25, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Kellerman, Q.D.; Hillis, S.L.; Cvengros, J.; Lawton, W.; Christensen, A.J. Effect of a Behavioral Self-Regulation Intervention on Patient Adherence to Fluid-Intake Restrictions in Hemodialysis: A Randomized Controlled Trial. Ann. Behav. Med. 2016, 50, 167–176. [Google Scholar] [CrossRef]
- Basile, C.; Pisano, A.; Lisi, P.; Rossi, L.; Lomonte, C.; Bolignano, D. High versus low dialysate sodium concentration in chronic haemodialysis patients: A systematic review of 23 studies. Nephrol. Dial Transpl. 2016, 31, 548–563. [Google Scholar] [CrossRef]
- Dunlop, J.L.; Vandal, A.C.; Marshall, M.R. Low dialysate sodium levels for chronic haemodialysis. Cochrane Database Syst. Rev. 2019, 1, CD011204. [Google Scholar] [CrossRef]
- Marshall, M.R.; Vandal, A.C.; de Zoysa, J.R.; Gabriel, R.S.; Haloob, I.A.; Hood, C.J.; Irvine, J.H.; Matheson, P.J.; McGregor, D.O.R.; Rabindranath, K.S.; et al. Effect of Low-Sodium versus Conventional Sodium Dialysate on Left Ventricular Mass in Home and Self-Care Satellite Facility Hemodialysis Patients: A Randomized Clinical Trial. J. Am. Soc. Nephrol. 2020, 31, 1078–1091. [Google Scholar] [CrossRef]
- Bossola, M.; Mariani, I.; Sacco, M.; Antocicco, M.; Pepe, G.; Di Stasio, E. Interdialytic weight gain and low dialysate sodium concentration in patients on chronic hemodialysis: A systematic review and meta-analysis. Int. Urol. Nephrol. 2024, 56, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Henrich, W.L.; Woodard, T.D.; McPhaul, J.J., Jr. The chronic efficacy and safety of high sodium dialysate: Double-blind, crossover study. Am. J. Kidney Dis. 1982, 2, 349–353. [Google Scholar] [CrossRef]
- Farmer, C.; Donohoe, P.; Dallyn, P. Low-sodium hemodialysis without fluid removal improves blood pressure control in chronic hemodialysis patients. Nephrology 2000, 5, 237–241. [Google Scholar] [CrossRef]
- de Paula, F.M.; Peixoto, A.J.; Pinto, L.V.; Dorigo, D.; Patricio, P.J.; Santos, S.F. Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int. 2004, 66, 1232–1238. [Google Scholar] [CrossRef]
- Munoz Mendoza, J.; Bayes, L.Y.; Sun, S.; Doss, S.; Schiller, B. Effect of lowering dialysate sodium concentration on interdialytic weight gain and blood pressure in patients undergoing thrice-weekly in-center nocturnal hemodialysis: A quality improvement study. Am. J. Kidney Dis. 2011, 58, 956–963. [Google Scholar] [CrossRef]
- Eftimovska-Otovic, N.; Stojceva-Taneva, O.; Grozdanovski, R.; Stojcev, S. Clinical Effects of Standard and Individualized Dialysate Sodium in Patients on Maintenance Hemodialysis. Open Access Maced. J. Med. Sci. 2016, 4, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Manji, S.; Shah, J.; Twahir, A.; Sokwala, A. Association between dialysate sodium concentration and interdialytic weight gain in patients undergoing twice weekly haemodialysis. BMC Nephrol. 2021, 22, 225. [Google Scholar] [CrossRef] [PubMed]
- Inrig, J.K.; Molina, C.; D’Silva, K.; Kim, C.; Van Buren, P.; Allen, J.D.; Toto, R. Effect of low versus high dialysate sodium concentration on blood pressure and endothelial-derived vasoregulators during hemodialysis: A randomized crossover study. Am. J. Kidney Dis. 2015, 65, 464–473. [Google Scholar] [CrossRef]
- Beduschi, G.C.; Telini, L.S.; Caramori, J.C.; Martin, L.C.; Barretti, P. Effect of dialysate sodium reduction on body water volume, blood pressure, and inflammatory markers in hemodialysis patients—A prospective randomized controlled study. Ren. Fail. 2013, 35, 742–747. [Google Scholar] [CrossRef]
- Nkf Kdoqi Guidelines. Clinical Practice Guidelines for Hemodialysis Adequacy, Update 2006. Am. J. Kidney Dis. 2006, 48, S2–S90. [Google Scholar] [CrossRef]
- Walsh, E.; Lehane, E. An exploration of the relationship between adherence with dietary sodium restrictions and health beliefs regarding these restrictions in Irish patients receiving haemodialysis for end-stage renal disease. J. Clin. Nurs. 2011, 20, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Nerbass, F.B.; Morais, J.G.; dos Santos, R.G.; Kruger, T.S.; Sczip, A.C.; da Luz Filho, H.A. Factors associated to salt intake in chronic hemodialysis patients. J. Bras. Nefrol. 2013, 35, 87–92. [Google Scholar] [CrossRef]
- Kimura, G.; Kojima, S.; Saito, F.; Kawano, Y.; Imanishi, M.; Kuramochi, M.; Omae, T. Quantitative estimation of dietary intake in patients on hemodialysis. Int. Artif. Organs. 1988, 11, 161–168. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Maduell, F.; Navarro, V. Assessment of salt intake in hemodialysis. Nefrologia 2001, 21, 71–77. [Google Scholar] [PubMed]
- Wyskida, K.; Wajda, J.; Klein, D.; Witkowicz, J.; Ficek, R.; Rotkegel, S.; Spiechowicz-Zatoń, U.; Kocemba-Dyczek, J.; Ciepał, J.; Olszanecka-Glinianowicz, M.; et al. Nutrient intake assessed with Diet History Questionnaire II, in relation to long-term calcium-phosphate control in hemodialysis patients with end-stage renal failure. Adv. Clin. Exp. Med. 2018, 27, 217–224. [Google Scholar] [CrossRef]
- Gkza, A.; Davenport, A. Estimated dietary sodium intake in haemodialysis patients using food frequency questionnaires. Clin. Kidney J. 2017, 10, 715–720. [Google Scholar] [CrossRef]
- Xie, Z.; McLean, R.; Marshall, M. Dietary sodium and other nutrient intakes among patients undergoing hemodialysis in New Zealand. Nutrients 2018, 10, 502. [Google Scholar] [CrossRef]
- Bossola, M.; Pepe, G.; Vulpio, C. The frustrating attempt to limit the interdialytic weight gain in patients on chronic hemodialysis: New insights into an old problem. J. Ren. Nutr. 2018, 28, 293–301. [Google Scholar] [CrossRef]
- Bossola, M.; Mariani, I.; Antocicco, M.; Pepe, G.; Spoliti, C.; Di Stasio, E. Interdialytic weight gain and low-salt diet in patients on chronic hemodialysis: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2024, 63, 105–112. [Google Scholar] [CrossRef]
- Kayikcioglu, M.; Tumuklu, M.; Ozkahya, M.; Ozdogan, O.; Asci, G.; Duman, S.; Toz, H.; Can, L.H.; Basci, A.; Ok, E. The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol. Dial. Transpl. 2009, 24, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.M.; Fang, H.Y.; Ashrafi, S.A.; Burrows, B.T.; King, A.C.; Larsen, R.J.; Sutton, B.P.; Wilund, K.R. Pilot study to reduce interdialytic weight gain by provision of low-sodium, home-delivered meals in hemodialysis patients. Hemodial. Int. 2021, 25, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Di Stasio, E.; Viola, A.; Cenerelli, S.; Leo, A.; Santarelli, S.; Monteburini, T. Dietary Daily Sodium Intake Lower than 1500 mg Is Associated with Inadequately Low Intake of Calorie, Protein, Iron, Zinc and Vitamin B1 in Patients on Chronic Hemodialysis. Nutrients 2020, 12, 260. [Google Scholar] [CrossRef]
- Li, J.; Song, P.; Yang, D.; Liu, Y. A Systematic Review and Meta-Analysis: Hyponatremia Predicted All-Cause and Cardiovascular Mortality in Dialysis Population. Blood Purif. 2022, 51, 345–354. [Google Scholar]
- Ikenoue, T.; Koike, K.; Fukuma, S.; Ogata, S.; Iseki, K.; Fukuhara, S. Salt Intake and All-Cause Mortality in Hemodialysis Patients. Am. J. Nephrol. 2018, 48, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hitomi, Y.; Takata, H.; Ushiya, S.; Yamada, M.; Sakai, Y.; Konishi, T.; Takeda, Y.; Sumino, Y.; Mizo, M.; et al. Association between salt intake and long-term mortality in hemodialysis patients: A retrospective cohort study. PLoS ONE 2021, 16, e0260671. [Google Scholar] [CrossRef]
- Rhee, C.M.; Ravel, V.A.; Ayus, J.C.; Sim, J.J.; Streja, E.; Mehrotra, R.; Amin, A.N.; Nguyen, D.V.; Brunelli, S.M.; Kovesdy, C.P.; et al. Pre-dialysis serum sodium and mortality in a national incident hemodialysis cohort. Nephrol. Dial. Transpl. 2016, 31, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Todini, L.; Fantuz, F. Thirst: Neuroendocrine regulation in mammals. Vet. Res. Commun. 2023, 47, 1085–1101. [Google Scholar] [CrossRef]
- Leib, E.D.; Zimmerman, A.C.; Knight, A.Z. Thirst. Curr Biol. 2016, 26, R1260–R1265. [Google Scholar] [CrossRef]
- Mount, D.B.; Sayegh, M.H.; Singh, A.K. Core Concepts in the Disorders of Fluid, Electrolytes and Acid-Base Balance; Chapter: The Physiology of Water Homeostasis; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Gizowski, C.; Bourque, W.C. The neural basis of homeostatic and anticipatory thirst. Nat. Rev. Nephrol. 2018, 14, 11–25. [Google Scholar] [CrossRef]
- Zimmerman, A.C.; Leib, E.D.; Knight, A.Z. Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 2017, 18, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.; Mythen, M.; Montgomery, H. The sensitivity of the human thirst response to changes in plasma osmolality: A systematic review. Perioper. Med. 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Calvani, R.; Marzetti, E.; Picca, A.; Antocicco, E. Thirst in patients on chronic hemodialysis: What do we know so far? Int. Urol. Nephrol. 2020, 52, 697–711. [Google Scholar] [CrossRef]
- Bossola, M.; Tazza, L. Xerostomia in patients on chronic hemodialysis. Nat. Rev. Nephrol. 2012, 8, 176–182. [Google Scholar] [CrossRef]
- Bruzda-Zwiech, A.; Szczepańska, J.; Zwiech, R. Sodium gradient, xerostomia, thirst and inter-dialytic excessive weight gain: A possible relationship with hyposalivation in patients on maintenance hemodialysis. Int. Urol. Nephrol. 2014, 46, 1411–1417. [Google Scholar] [CrossRef][Green Version]
- Kao, C.H.; Hsieh, J.F.; Tsai, S.C.; Ho, Y.J.; Chang, H.R. Decreased salivary function in patients with end-stage renal disease requiring hemodialysis. Am. J. Kidney Dis. 2000, 36, 1110–1114. [Google Scholar] [CrossRef]
- Kaya, M.; Fikret Çermik, T.; Üstün, F.; Şen, S.; Berkarda, S. Salivary function in patients with chronic renal failure undergoing hemodialysis. Ann. Nucl. Med. 2002, 16, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Postorino, M.; Catalano, C.; Martorano, C.; Cutrupi, S.; Marino, C.; Cozzupoli, P.; Scudo, P.; Zoccali, C. Salivary and lacrimal secretion is reduced in patients with ESRD. Am. J. Kidney Dis. 2003, 42, 722–728. [Google Scholar] [CrossRef]
- Bergdahl, M.; Bergdahl, J. Low unstimulated salivary flow and subjective oral dryness: Association with medication, anxiety, depression, and stress. J. Dent. Res. 2000, 79, 1652–1658. [Google Scholar] [CrossRef]
- Anttila, S.S.; Knuuttila, M.L.; Sakki, T.K. Depressive symptoms as an underlying factor of the sensation of dry mouth. Psychosom. Med. 1998, 60, 215–218. [Google Scholar] [CrossRef]
- Warren, R.E.; Deary, I.J.; Frier, B.M. The symptoms of hyperglycaemia in people with insulin-treated diabetes: Classification using principal components analysis. Diabetes Metab. Res. Rev. 2003, 19, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Allida, S.M.; Hayward, C.S.; Newton, P.J. Thirst in heart failure: What do we know so far. Curr. Opin. Support. Palliat. Care 2018, 12, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Liu, Y.Q.; Qian, S.L.; Dou, J.; Zheng, X.; Xu, Q.-S.; Li, M. Enhancing Oral Mucosal Barrier, Mitigating Microinflammation, and Addressing Malnutrition in Dialysis Patients: The Impact of Tangerine Peel Lemon Glycerin. Altern. Ther. Health Med. 2024, 30, 168–173. [Google Scholar]
- Welch, J.L. Development of the thirst distress scale. Nephrol. Nurs. J. 2022, 29, 337–341. [Google Scholar]
- Bots, C.P.; Brand, H.S.; Veerman, E.C.I.; Valentijn-Benz, M.; Van Amerongen, B.M.; Valentijn, R.M.; Vos, P.F.; Bijlsma, J.A.; Bezemer, P.D.; Wee, P.M.T.; et al. Interdialytic weight gain in patients on hemodialysis is associated with dry mouth and thirst. Kidney Int. 2004, 66, 1662–1668. [Google Scholar] [CrossRef]
- Zhianfar, L.; Nadrian, H.; Shaghaghi, A. Enhancement of Adherence to Therapeutic and Lifestyle Recommendations Among Hemodialysis Patients: An Umbrella Review of Interventional Strategies. Ther. Clin. Risk Manag. 2020, 16, 233–243. [Google Scholar] [CrossRef]
- World |Health Organization. Therapeutic Patient Education: Continuing Education Programmes for Health Care Providers in the Field of Prevention of Chronic Diseases: Report of a WHO Working Group; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 1998. [Google Scholar]
- Fava, G.A.; Cosci, F.; Sonino, N. Current psychosomatic practice. Psychother. Psychosom. 2017, 86, 13–30. [Google Scholar] [CrossRef]
- Pu, J.; Jiang, Z.H.; Wu, W.; Zhang, L.; Li, Y.; Liu, Q.; Ou, S. Efficacy and safety of intradialytic exercise in haemodialysis patients: A systematic review and meta-analysis. BMJ Open 2019, 9, e020633. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Song, J.K.; Hong, S.C.; Choi, J.W.; Jeon, H.J.; Shin, D.H.; Ji, E.H.; Choi, E.-H.; Lee, J.; Kim, A.; et al. Intradialytic exercise improves physical function and reduces intradialytic hypotension and depression in hemodialysis patients. Korean J. Intern. Med. 2019, 34, 588–598. [Google Scholar] [CrossRef]
- Barcellos, F.C.; Santos, I.S.; Umpierre, D.; Bohlke, M.; Hallal, P.C. Effects of exercise in the whole spectrum of chronic kidney disease: A systematic review. Clin. Kidney J. 2015, 8, 753–765. [Google Scholar] [CrossRef]
- Gomes Neto, M.; de Lacerda, F.F.R.; Lopes, A.A.; Martinez, B.P.; Saquetto, M.B. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: Systematic review and meta-analysis. Clin. Rehabil. 2018, 32, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Nissenson, A.R. Improving outcomes for ESRD patients: Shifting the quality paradigm. Clin. J. Am. Soc. Nephrol. 2014, 9, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Wefer, F.; Krüger, L.; Waldréus, N.; Köpke, S. Non-pharmacological interventions to reduce thirst in patients with heart failure or hemodialysis: A systematic review and meta-analysis. Heart Lung 2024, 67, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Bots, C.P.; Brand, H.S.; Veerman, E.C.I.; van Amerongen, B.M.; Nieuw Amerongen, A.V. Preferences and saliva stimulation of eight different chewing gums. Int. Dent. J. 2004, 54, 143–148. [Google Scholar] [CrossRef]
- Scholey, A.; Haskell, C.; Robertson, B.; Kennedy, D.; Milne, A.; Wetherell, M. Chewing gum alleviates negative mood and reduces cortisol during acute laboratory psychological stress. Physiol. Behav. 2009, 97, 304–312. [Google Scholar] [CrossRef]
- Allida, S.M.; Shehab, S.; Inglis, S.C.; Davidson, P.M.; Hayward, C.S.; Newton, P.J. A RandomisEd ControLled TrIal of ChEwing Gum to RelieVE Thirst in Chronic Heart Failure (RELIEVE-CHF). Heart Lung Circ. 2021, 30, 516–524. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Wang, C.L.; Chiu, A.H.; Yeh, M.C.; Chiang, T.I. Chewing Gum May Alleviate Degree of Thirst in Patients on Hemodialysis. Medicina 2023, 60, 2. [Google Scholar] [CrossRef]
- Bots, C.P.; Brand, H.S.; Veerman, E.C.; Valentijn-Benz, M.; Van Amerongen, B.M.; Nieuw Amerongen, A.V.; Valentijn, R.M.; Vos, P.F.; Bijlsma, J.A.; Bezemer, P.D.; et al. The management of xerostomia in patients on haemodialysis: Comparison of artificial saliva and chewing gum. Palliat. Med. 2005, 19, 202–207. [Google Scholar] [CrossRef]
- Fan, W.F.; Zhang, Q.; Luo, L.H.; Niu, J.Y.; Gu, Y. Study on the clinical significance and related factors of thirst and xerostomia in maintenance hemodialysis patients. Kidney Blood Press Res. 2013, 37, 464–474. [Google Scholar] [CrossRef]
- Duruk, N.; Eşer, I. The Null Effect of Chewing Gum During Hemodialysis on Dry Mouth. Clin. Nurse Spec. 2016, 30, E12–E23. [Google Scholar] [CrossRef]
- Yıldırım Keskin, A.; Taşci, S. The Effect of Acupressure Applied to Individuals Receiving Hemodialysis Treatment on Severity of Thirst and Quality of Life. Altern. Ther. Health Med. 2021, 27, 20–30. [Google Scholar] [PubMed]
- Yang, L.Y.; Yates, P.; Chin, C.C.; Kao, T.K. Effect of Acupressure on Thirst in Hemodialysis Patients. Kidney Blood Press Res. 2010, 33, 260–265. [Google Scholar] [CrossRef]
- Oh, J.E.; Kim, S.N. Anti-Inflammatory Effects of Acupuncture at ST36 Point: A Literature Review in Animal Studies. Front. Immunol. 2022, 12, 813748. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Hu, J. Acupuncture: An Overview on Its Functions, Meridian Pathways and Molecular Mechanisms. Am. J. Chin. Med. 2024, 52, 1215–1244. [Google Scholar]
- Wang, S.J.; Zhang, J.J.; Yang, H.Y.; Wang, F.; Li, S.T. Acupoint specificity on acupuncture regulation of hypothalamic- pituitary-adrenal cortex axis function. BMC Complement. Altern. Med. 2015, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Tentori, F.; Zhang, J.; Li, Y.; Karaboyas, A.; Kerr, P.; Saran, R.; Bommer, J.; Port, F.; Akiba, T.; Pisoni, R.; et al. Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: Results from the dialysis. outcomes and practice patterns study (DOPPS). Nephrol. Dial. Transpl. 2012, 27, 4180–4188. [Google Scholar] [CrossRef]
- FHN Trial Group; Chertow, G.M.; Levin, N.W.; Beck, G.J.; Depner, T.A.; Eggers, P.W.; Gassman, J.J.; Gorodetskaya, I.; Greene, T.; James, S.; et al. In-center hemodialysis six times per week versus three times per week. N. Engl. J. Med. 2010, 363, 2287–2300. [Google Scholar] [CrossRef]
- Dasgupta, I.; Thomas, G.N.; Clarke, J.; Sitch, A. Associations between Hemodialysis Facility Practices to Manage Fluid Volume and Intradialytic Hypotension and Patient Outcomes. Clin. J. Am. Soc. Nephrol. 2019, 14, CJN.08240718. [Google Scholar] [CrossRef]
- Shawky, S.; Khalifa, M.; Amin, K.; Hassan, M. Correlation between interdialytic weight gain, left ventricular hypertrophy and FGF-23 in prevalent hemodialysis patients. J. Clin. Nephrol. 2020, 4, 036–043. [Google Scholar] [CrossRef]
- Flythe, J.; Kimmel, S.; Brunelli, S. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011, 79, 250–257. [Google Scholar] [CrossRef]
- Flythe, J.; Curhan, G.; Brunelli, S. Shorter length dialysis sessions are associated with increased mortality, independent of body weight. Kidney Int. 2013, 83, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, S.; Chertow, G.; Ankers, E.; Lowrie, E.; Thadhani, R. Shorter dialysis times are associated with higher mortality among incident hemodialysis patients. Kidney Int. 2010, 77, 630–636. [Google Scholar] [CrossRef]
- Leung, K.; Quinn, R.; Ravani, P.; MacRae, J. Ultrafiltration biofeedback guided by blood volume monitoring to reduce intradialytic hypotensive episodes in hemodialysis: Study protocol for a randomized controlled trial. Trials 2014, 15, 483. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Quinn, R.; Ravani, P.; Duff, H.; MacRae, J. Randomized crossover trial of blood volume monitoring–guided ultrafiltration biofeedback to reduce intradialytic hypotensive episodes with hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1831–1840. [Google Scholar] [CrossRef]
- Kondo, K.; Noonan, K.; Freeman, M.; Ayers, C.; Morasco, B.; Kansagara, D. Efficacy of biofeedback for medical conditions: An evidence map. J. Gen. Intern. Med. 2019, 34, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.; Bang, K.; Lee, S.; Han, B.; Kim, J.K.; Kim, Y.O.; Song, H.C.; Kwon, Y.J.; Kim, Y.-S. Efficacy of hemocontrol biofeedback system in intradialytic hypotension-prone hemodialysis patients. J. Korean Med. Sci. 2014, 29, 805. [Google Scholar] [CrossRef] [PubMed]
- Flythe, J.; Assimon, M.; Overman, R. Target weight achievement and ultrafiltration rate thresholds: Potential patient implications. BMC Nephrol. 2017, 18, 185. [Google Scholar] [CrossRef]
- Mohamed, M. Effect of sugar free gum chewing on thirst and interdialytic weight gain among patients undergoing hemodialysis. Menoufia Nurs. J. 2023, 8, 309–326. [Google Scholar] [CrossRef]
- Mambelli, E. Comparison of blood volume biofeedback hemodialysis and conventional hemodialysis on cardiovascular stability and blood pressure control in hemodialysis patients: A systematic review and meta-analysis of randomized controlled trials. J. Nephrol. 2024, 37, 897–909. [Google Scholar] [CrossRef]
- Sandys, V.; Sexton, D.; O’Seaghdha, C. Artificial intelligence and digital health for volume maintenance in hemodialysis patients. Hemodial. Int. 2022, 26, 480–495. [Google Scholar] [CrossRef]
- Chiauzzi, E.; Rodarte, C.; DasMahapatra, P. Patient-centered activity monitoring in the self-management of chronic health conditions. BMC Med. 2015, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Schoutteten, M.; Vranken, J.; Lee, S.; Smeets, C.J.P.; De Cannière, H.; Van Hoof, C.; Peeters, J.; Groenendaal, W.; Vandervoort, P.M. Towards personalized fluid monitoring in haemodialysis patients: Thoracic bioimpedance signal shows strong correlation with fluid changes, a cohort study. BMC Nephrol. 2020, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Kosa, S.; Monize, J.; D’Souza, M.; Joshi, A.; Philip, K.; Reza, S.; Samra, S.; Serrago, B.; Thabane, L.; Gafni, A.; et al. Nutritional mobile applications for ckd patients: Systematic review. Kidney Int. Rep. 2019, 4, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Sulistyaningrum, D.; Septianingtyas, N.; Nurhanifah, T.; Izza, N. The effect of using fluid balance application on interdialytic weight gain in stage v chronic kidney disease patients undergoing hemodialysis. Babali Nurs. Res. 2023, 4, 161–169. [Google Scholar] [CrossRef]
- Park, O.; Kim, S. Integrated self-management program effects on hemodialysis patients: A quasi-experimental study. Jpn. J. Nurs. Sci. 2019, 16, 396–406. [Google Scholar] [CrossRef]
- Delano, M.; Ganapati, V.; Kamal, R.; Le, B.; Le, J.; Mendoza, R. Evaluating research grade bioimpedance hardware using textile electrodes for long-term fluid status monitoring. Front. Electron. 2022, 2, 762442. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, L.; Shen, G. Recent advances in smart wearable sensing systems. Adv. Mater. Technol. 2018, 3, 1800444. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, J.; Wu, W. Scalably nanomanufactured atomically thin materials-based wearable health sensors. Small Struct. 2021, 3, 2100120. [Google Scholar] [CrossRef]
- Toh, S.; González, P.; Fong, K. Usability of a wearable device for home-based upper limb telerehabilitation in persons with stroke: A mixed-methods study. Digit. Health 2023, 9, 20552076231153737. [Google Scholar] [CrossRef]
- Hojs, N.; Fissell, W.; Roy, S. Ambulatory hemodialysis-technology landscape and potential for patient-centered treatment. Clin. J. Am. Soci. Nephrol. 2019, 15, 152–159. [Google Scholar] [CrossRef]
- Lin, Y. Evaluating the effectiveness of a novel personalized health education approach for hemodialysis patients: A four-week study using a widely-used communication app in taiwan. Stud. Health Technol. Inform. 2024, 316, 511–512. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossola, M.; Mariani, I.; Strizzi, C.T.; Piccinni, C.P.; Di Stasio, E. How to Limit Interdialytic Weight Gain in Patients on Maintenance Hemodialysis: State of the Art and Perspectives. J. Clin. Med. 2025, 14, 1846. https://doi.org/10.3390/jcm14061846
Bossola M, Mariani I, Strizzi CT, Piccinni CP, Di Stasio E. How to Limit Interdialytic Weight Gain in Patients on Maintenance Hemodialysis: State of the Art and Perspectives. Journal of Clinical Medicine. 2025; 14(6):1846. https://doi.org/10.3390/jcm14061846
Chicago/Turabian StyleBossola, Maurizio, Ilaria Mariani, Camillo Tancredi Strizzi, Carlo Pasquale Piccinni, and Enrico Di Stasio. 2025. "How to Limit Interdialytic Weight Gain in Patients on Maintenance Hemodialysis: State of the Art and Perspectives" Journal of Clinical Medicine 14, no. 6: 1846. https://doi.org/10.3390/jcm14061846
APA StyleBossola, M., Mariani, I., Strizzi, C. T., Piccinni, C. P., & Di Stasio, E. (2025). How to Limit Interdialytic Weight Gain in Patients on Maintenance Hemodialysis: State of the Art and Perspectives. Journal of Clinical Medicine, 14(6), 1846. https://doi.org/10.3390/jcm14061846




