Clinical Utilization and Performance of Bempedoic Acid in an Italian Real-World Setting: Insight from Campania Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Protocol
2.3. Study Objectives
2.4. Statistical Analysis
3. Results
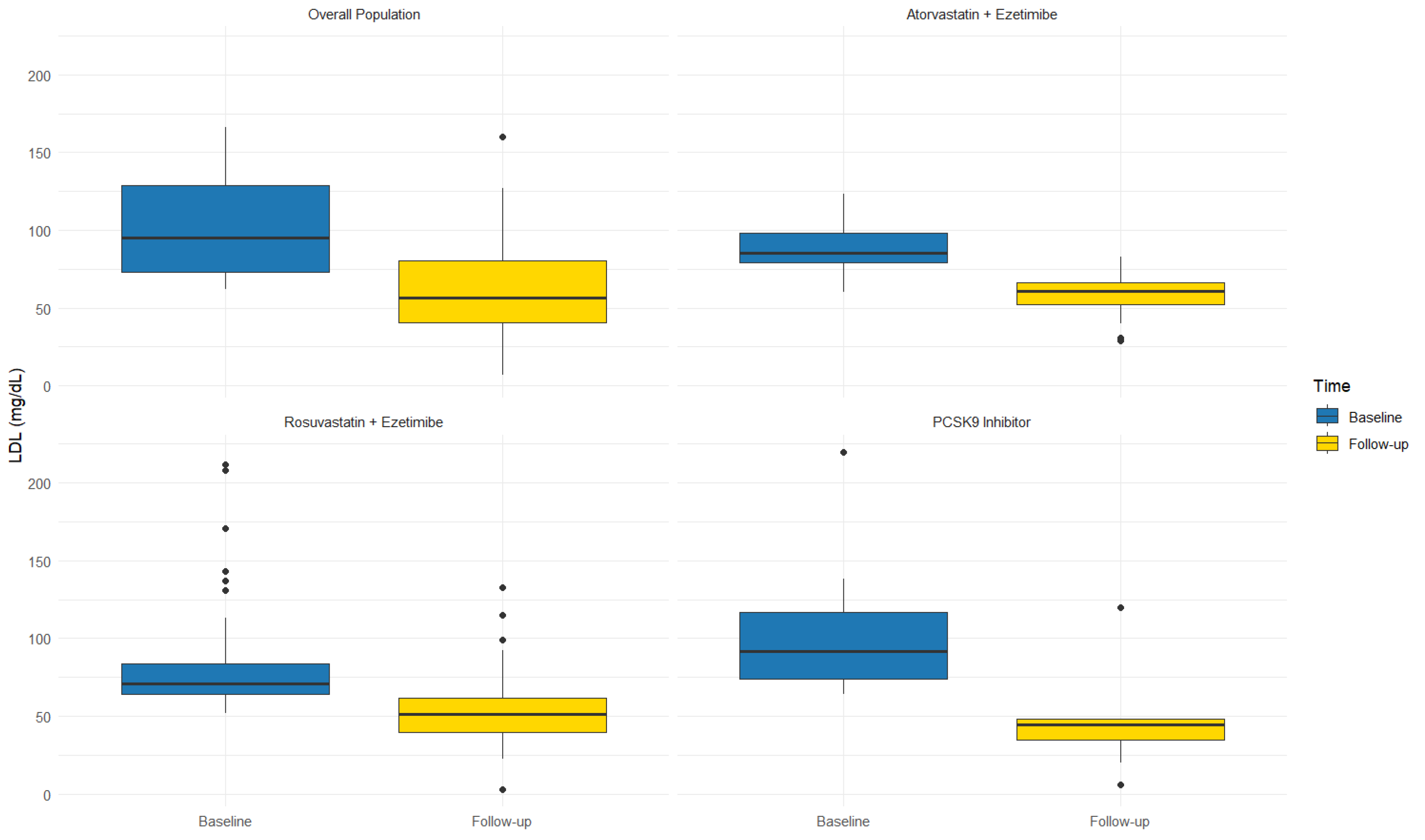
3.1. Follow-Up
3.2. Subgroup Analysis
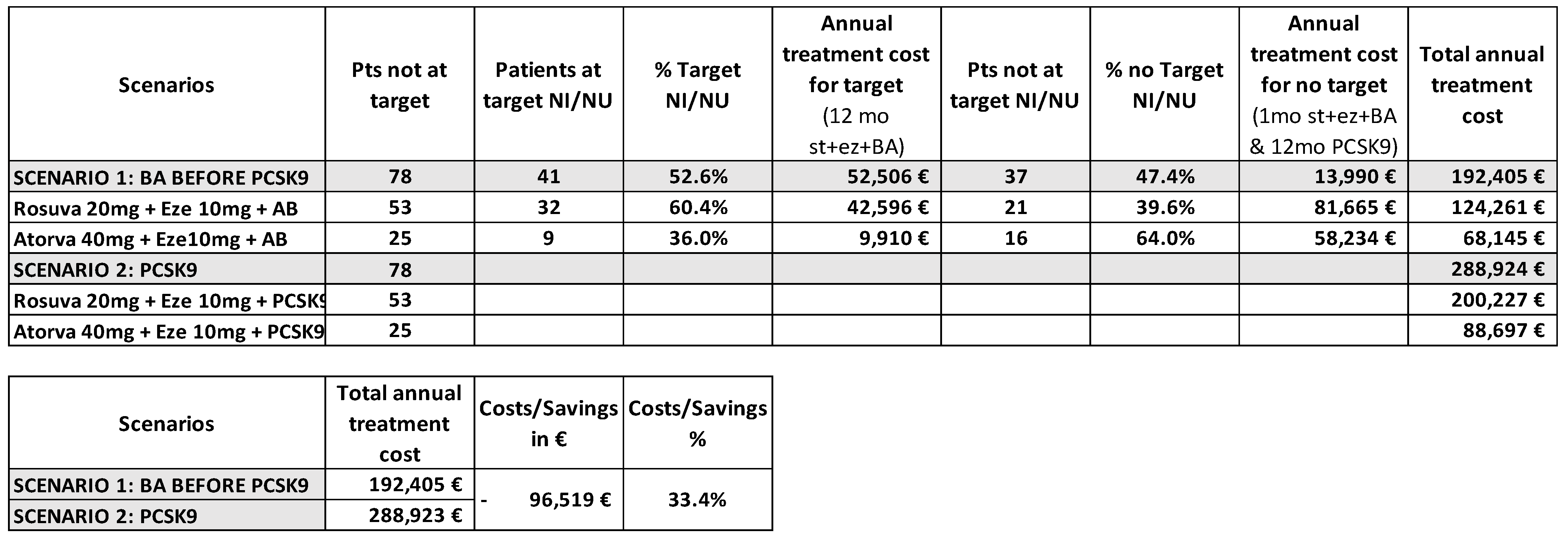
3.3. Pharmacoeconomic Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinkosky, S.L.; Newton, R.S.; Day, E.A.; Ford, R.J.; Lhotak, S.; Austin, R.C.; Lalwani, N.D. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat. Commun. 2016, 7, 13457. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Pintó, X.; Escobar, C.; Varona, J.F.; Gámez, J.M. Real-World Attainment of Low-Density Lipoprotein Cholesterol Goals in Patients at High Risk of Cardiovascular Disease Treated with High-Intensity Statins: The TERESA Study. J. Clin. Med. 2023, 12, 3187. [Google Scholar] [CrossRef] [PubMed]
- American College of Cardiology. ASCVD Patients Face Barriers Reaching Guideline-Recommended Cholesterol Goals. Available online: https://www.acc.org/about-acc/press-releases/2019/11/13/15/38/ascvd-patients-face-barriers-reaching-guideline-recommended-cholesterol-goals (accessed on 20 February 2025).
- Goldberg, A.C.; Leiter, L.A.; Stroes, E.S.; Baum, S.J.; Hanselman, J.C.; Bloedon, L.T.; Duell, P.B. Effect of bempedoic acid vs. placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: The CLEAR Wisdom randomized clinical trial. JAMA 2019, 322, 1780–1788. [Google Scholar] [CrossRef]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N. Engl. J. Med. 2023, 388, 1353–1364. [Google Scholar] [CrossRef]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M.; CLEAR Harmony Trial. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef]
- Filippo, O.; D’Ascenzo, F.; Iannaccone, M.; Bertaina, M.; Leone, A.; Borzillo, I.; Ravetti, E.; Solano, A.; Pagliassotto, I.; Nebiolo, M.; et al. Safety and efficacy of bempedoic acid: A systematic review and meta-analysis of randomised controlled trials. Cardiovasc. Diabetol. 2023, 22, 324. [Google Scholar] [CrossRef] [PubMed]
- NILEMDO Determina n.20/2023 del 13 Gennaio 2023. Gazzetta Ufficiale della Repubblica Italiana 2023, The OJ 22 of 27-1-2023. Available online: https://www.gazzettaufficiale.it/atto/vediMenuHTML?atto.dataPubblicazioneGazzetta=2023-01-27&atto.codiceRedazionale=23A00339&tipoSerie=serie_generale&tipoVigenza=originario (accessed on 13 February 2025).
- NUSTENDI Determina n.21/2023 del 13 Gennaio 2023. Gazzetta Ufficiale della Repubblica Italiana 2023, The OJ 22 of 27-1-2023. Available online: https://www.gazzettaufficiale.it/atto/vediMenuHTML?atto.dataPubblicazioneGazzetta=2023-01-27&atto.codiceRedazionale=23A00340&tipoSerie=serie_generale&tipoVigenza=originario (accessed on 13 February 2025).
- Warden, B.A.; Cardiology, B.A.; Purnell, J.Q.; Duell, P.B.; Fazio, S. Real-world utilization of bempedoic acid in an academic preventive cardiology practice. J. Clin. Lipidol. 2022, 16, 94–103. [Google Scholar] [CrossRef]
- Mahajan, K.; Puri, R.; Duell, P.B.; Dutta, D.; Yadav, R.; Kumar, S.; Sharma, J.B.; Patel, P.; Dsouza, S.; Gupta, A.; et al. Rapid achievement of low-density lipoprotein cholesterol goals within 1 month after acute coronary syndrome during combination therapy with rosuvastatin, ezetimibe and bempedoic acid: Initial experience from the LAI-REACT study. J. Clin. Lipidol. 2024, 18, e867–e872. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- So.Re.Sa. Anagrafe Farmaci ed Emoderivati. Available online: https://www.soresa.it/pa/Pagine/Anagrafe/Farmaci-Emoderivati.aspx (accessed on 13 February 2025).
- Gazzetta Ufficiale della Repubblica Italiana. Decreto Pubblicato il 27 Gennaio 2023. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2023-01-27&atto.codiceRedazionale=23A00340 (accessed on 13 February 2025).
- Agenzia Italiana del Farmaco (AIFA). Lista Farmaci Equivalenti per Principio Attivo, 15 Gennaio 2025. Available online: https://www.aifa.gov.it/documents/20142/2693710/Lista_farmaci_equivalenti_Principio_Attivo_15.01.2025.pdf (accessed on 13 February 2025).
- Banach, M.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Leiter, L.A.; Mancini, G.B.J.; Ray, K.K.; Flaim, J.; Ye, Z.; Catapano, A.L. Association of Bempedoic Acid Administration with Atherogenic Lipid Levels in Phase 3 Randomized Clinical Trials of Patients with Hypercholesterolemia. JAMA Cardiol. 2020, 5, 1–12. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Xu, S.; Shen, A.Z. ATP-Citrate Lyase (ACLY) in Lipid Metabolism and Atherosclerosis: An Updated Review. Prog. Lipid Res. 2020, 77, 101006. [Google Scholar] [CrossRef]
- Karlson, B.W.; Wiklund, O.; Palmer, M.K.; Nicholls, S.J.; Lundman, P.; Barter, P.J. Variability of Low-Density Lipoprotein Cholesterol Response with Different Doses of Atorvastatin, Rosuvastatin, and Simvastatin: Results from VOYAGER. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 212–217. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Amore, B.M.; Bockbrader, H.; Crass, R.L.; Chapel, S.; Sasiela, W.J.; Emery, M.G. Population Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Modeling of Bempedoic Acid and Low-Density Lipoprotein Cholesterol in Healthy Subjects and Patients with Dyslipidemia. J. Pharmacokinet. Pharmacodyn. 2023, 50, 351–364. [Google Scholar] [CrossRef]
- Dudum, R.; Juraschek, S.P.; Appel, L.J. Dose-Dependent Effects of Lifestyle Interventions on Blood Lipid Levels: Results from the PREMIER Trial. Patient Educ. Couns. 2019, 102, 1882–1891. [Google Scholar] [CrossRef]
- Bays, H.E.; Banach, M.; Catapano, A.L.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Ballantyne, C.M. Bempedoic Acid Safety Analysis: Pooled Data from Four Phase 3 Trials. J. Clin. Lipidol. 2020, 14, 649–659.e6. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, S.; Du, H.; Li, J.; Khan, S.U.; Aertgeerts, B.; Guyatt, G.; Hao, Q.; Bekkering, G.; Li, L.; et al. Safety of Ezetimibe in Lipid-Lowering Treatment: Systematic Review and Meta-Analysis of Randomized Controlled Trials and Cohort Studies. BMJ Med. 2022, 1, e000134. [Google Scholar] [CrossRef]
- Mazhar, F.; Hjemdahl, P.; Clase, C.M.; Johnell, K.; Jernberg, T.; Carrero, J.J. Lipid-lowering treatment intensity, persistence, adherence and goal attainment in patients with coronary heart disease. Am. Heart J. 2022, 251, 78–90. [Google Scholar] [CrossRef]
- Caso, V.M.; Sperlongano, S.; Liccardo, B.; Romeo, E.; Padula, S.; Arenga, F.; D’Andrea, A.; Caso, P.; Golino, P.; Nigro, G.; et al. The impact of the COVID-19 outbreak on patients’ adherence to PCSK9 inhibitors therapy. J. Clin. Med. 2022, 11, 475. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Ray, K.K.; Sasiela, W.J.; Haddad, T.; Nicholls, S.J.; Li, N.; Cho, L.; Mason, D.; Libby, P.; Goodman, S.G.; et al. Comparative cardiovascular benefits of bempedoic acid and statin drugs. J. Am. Coll. Cardiol. 2024, 84, 152–162. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Banach, M.; Penson, P.E.; Farnier, M.; Fras, Z.; Latkovskis, G.; Laufs, U.; Paneni, F.; Parini, P.; Pirro, M.; Reiner, Ž.; et al. Bempedoic acid in the management of lipid disorders and cardiovascular risk. 2023 position paper of the International Lipid Expert Panel (ILEP). Prog. Cardiovasc. Dis. 2023, 79, 2–11. [Google Scholar] [CrossRef]
- Banach, M.; Reiner, Ž.; Surma, S.; Bajraktari, G.; Bielecka-Dabrowa, A.; Bunc, M.; Bytyçi, I.; Ceska, R.; Cicero, A.F.G.; Dudek, D.; et al. 2024 recommendations on the optimal use of lipid-lowering therapy in established atherosclerotic cardiovascular disease and following acute coronary syndromes: A position paper of the International Lipid Expert Panel (ILEP). Drugs 2024, 84, 1541–1577. [Google Scholar] [CrossRef]
| Characteristics | n = 111 |
|---|---|
| Age (yrs), mean ± SD | 65 ± 9 |
| Gender (male), n (%) | 68 (61.2) |
| BMI (kg/m2) mean ± SD | 27.1 ± 2.3 |
| Smokers, n (%) | 34 (30.6) |
| Hypertension, n (%) | 92 (82.8) |
| Chronic coronary syndrome, n (%) | 74 (66.7) |
| Recent ACS (<1 years), n (%) | 10 (9.0) |
| Heart failure with reduced EF, n (%) | 2 (1.8) |
| Peripheral artery disease, n (%) | 25 (22.5) |
| Previous stroke/TIA, n (%) | 2 (1.8) |
| CKD, n (%) | 8 (7.2) |
| COPD, n (%) | 22 (19.8) |
| Type 2 diabetes mellitus, n (%) | 22 (19.8) |
| Type 1 diabetes mellitus, n (%) | 11 (9.9) |
| Charles Comorbidity Index, median [IQR] | 4.0 [3.0–5.0] |
| EF (%), mean ± SD | 55 ± 7 |
| eGFR (mL/min), mean ± SD | 88 ± 28 |
| Drug Treatment | |
| ACE-i/ARB, n (%) | 96 (86.4%) |
| Beta-blockers, n (%) | 33 (75%) |
| Diuretics, n (%) | 34 (29.7%) |
| Statins, n (%) | 78 (70.3%) |
| Ezetimibe, n (%) | 96 (86.5%) |
| PCSK9i, n (%) | 15 (13.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, V.; Ratti, G.; Parrella, A.; De Falco, A.; Crisci, M.; Franco, R.; Covetti, G.; Caturano, A.; Napolitano, G.; Scotto di Uccio, F.; et al. Clinical Utilization and Performance of Bempedoic Acid in an Italian Real-World Setting: Insight from Campania Region. J. Clin. Med. 2025, 14, 1839. https://doi.org/10.3390/jcm14061839
Russo V, Ratti G, Parrella A, De Falco A, Crisci M, Franco R, Covetti G, Caturano A, Napolitano G, Scotto di Uccio F, et al. Clinical Utilization and Performance of Bempedoic Acid in an Italian Real-World Setting: Insight from Campania Region. Journal of Clinical Medicine. 2025; 14(6):1839. https://doi.org/10.3390/jcm14061839
Chicago/Turabian StyleRusso, Vincenzo, Gennaro Ratti, Antonio Parrella, Aldo De Falco, Mario Crisci, Riccardo Franco, Giuseppe Covetti, Alfredo Caturano, Giovanni Napolitano, Fortunato Scotto di Uccio, and et al. 2025. "Clinical Utilization and Performance of Bempedoic Acid in an Italian Real-World Setting: Insight from Campania Region" Journal of Clinical Medicine 14, no. 6: 1839. https://doi.org/10.3390/jcm14061839
APA StyleRusso, V., Ratti, G., Parrella, A., De Falco, A., Crisci, M., Franco, R., Covetti, G., Caturano, A., Napolitano, G., Scotto di Uccio, F., Izzo, G., & Argenziano, L. (2025). Clinical Utilization and Performance of Bempedoic Acid in an Italian Real-World Setting: Insight from Campania Region. Journal of Clinical Medicine, 14(6), 1839. https://doi.org/10.3390/jcm14061839









