Abstract
Background/Objectives: Bempedoic acid (BA) is a novel lipid-lowering agent that reduces low-density lipoprotein cholesterol (LDL-c) and cardiovascular events. Limited real-world data on its effectiveness and safety are available. This study aimed to evaluate the utilization and clinical performance of BA in routine clinical practice. Moreover, an explorative pharmacoeconomic analysis was performed. Methods: We prospectively enrolled consecutive patients with dyslipidemia who started 180 mg BA, alone or with 10 mg ezetimibe, across five outpatient clinics in Campania Region, Italy from September to December 2023. Clinical and laboratory assessments, including lipid profile, hepatic function, and creatine phosphokinase levels, were performed at baseline and at least after one month follow-up. Side effects were recorded. Results: 111 patients (age 65 ± 9 years, 61% male) were included. At BA initiation, 70.3% were on maximally tolerated statin dosage and ezetimibe, 16.2% on ezetimibe alone, and 13.5% on PCSK9 inhibitors due to statin intolerance. BA significantly reduced LDL-c serum levels (89.9 ± 33.0 vs. 56 ± 27.6 mg/dL; p < 0.0001), with 46% achieving therapeutic targets. LDL-c decreased by 28% in patients on intensive statins/ezetimibe and by 45% in statin-intolerant patients, with reduced healthcare costs. Side effects were infrequent (10%) and reversible. Adherence was 99%, and persistence 90%. Conclusions: In our clinical pratice, BA was primarily used in high-risk patients with dyslipidemia who failed to reach LDL-c therapeutic target with statins/ezetimibe, and to a lesser extent, in statin-intolerant individuals. BA treatment enabled 54% to reach LDL-c therapeutic target. BA was well tolerated, and showed high adherence and persistence, contributing to cost savings.
1. Introduction
Bempedoic acid (BA) is a novel lipid-lowering agent that exerts its effect by inhibiting the enzyme adenosine triphosphate-citrate lyase (ATC or ACLY), blocking the mevalonate pathway of cholesterol synthesis, upstream of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, which is the target of statins [1]. Its complementary mechanism of action, distinct from statins, allows for additional low-density lipoprotein cholesterol (LDL-c) reduction when combined with other lipid-lowering therapies [1]. Several clinical evidences suggest that a substantial proportion of patients, ranging from 20% to 30%, in various cohorts fail to reach guideline-recommended LDL-c therapeutic targets with statins alone, reinforcing the need for alternative treatments such as BA [2,3]. The improved LDL-c control provided by BA leads to a lower risk of major cardiovascular outcome events; moreover, BA demonstrates a generally favorable safety profile, with the exception of an elevated risk of gout [4,5,6,7]. BA was recently approved in Italy for the treatment of hypercholesterolemia in subjects with statin intolerance or those who are unable to reach the LDL-c therapeutic target despite receiving the maximal tolerated lipid-lowering therapy [8,9]. Few data are available about the clinical performance of BA in real-world settings [10,11]. The aim of this study was to assess the clinical utilization of BA in our clinical practice and to evaluate its clinical performance among real-world patients; moreover, an explorative pharmacoeconomic analysis was performed.
2. Materials and Methods
2.1. Study Population
We included all consecutive patients diagnosed with dyslipidemia, unresponsive to dietary modifications and physical activity, who started 180 mg BA, either as monotherapy or in fixed combination with 10 mg ezetimibe, followed at five outpatient clinics in Campania Region, Italy, from September 2023 to December 2023. Patients with documented dyslipidemia who met clinical criteria for BA therapy (statin intolerance or inadequate LDL-c reduction despite maximal tolerated lipid-lowering therapy) were eligible to be included in the present study. Exclusion criteria were age lower than 18 years, pregnancy or breastfeeding, known hypersensitivity or contraindications to BA, significant hepatic dysfunction (ALT or AST serum levels > 3 × the upper limit of normal), severe renal impairment (eGFR < 30 mL/min/1.73 m2).
2.2. Study Protocol
This is a multicenter, prospective observational cohort study designed to reflect routine clinical practice. At baseline, all participants underwent a comprehensive evaluation including medical history, physical examination, 12 lead electrocardiogram (ECG) and laboratory assessment; which were repeated at follow-up after an uninterrupted 180 mg BA therapy, alone or in combination with 10 mg ezetimibe (BA + Eze).
The laboratory assessment included total cholesterol (TC), LDL-c, high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, urate, and creatine phosphokinase (CPK) serum levels. Blood tests, collected after an 8-h overnight fast, were performed at baseline, before treatment initiation, and at least one month after the administration of the study drug. A pill count (number of doses taken) was performed. All side effects leading to temporary or definitive suspension were reported. All patients provided written informed consent for data storage and analysis. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and it was approved by the local ethics committee (Ethical Committee of University of Campania “Luigi Vanvitelli”—Monaldi Hospital; ID 23263/2022; approval date: 15 December 2022).
2.3. Study Objectives
The objectives of this study were as follows: (1) to report the clinical utilization of BA in our routine clinical practice; (2) to assess the effectiveness and safety of BA treatment; (3) to evaluate adherence and persistence to BA therapy; (4) to perform an exploratory pharmacoeconomic evaluation of BA use in a real-world setting.
The effectiveness was evaluated based on the reduction in LDL-c serum levels at follow-up among overall population and across patient subgroups. Treatment adherence was measured using the pill count method, calculated as the number of doses taken divided by the number of doses dispensed from the last prescription, multiplied by 100 (%). Persistence to therapy was defined as the absence of any temporary or permanent interruption due to side effects.
2.4. Statistical Analysis
Categorical data were presented as frequency and percentage, while continuous variables were expressed as either median (interquartile range [IQR]) or mean ± SD, depending on their distribution, which was assessed using both the Kolmogorov–Smirnov and the Shapiro–Wilk tests. Differences for categorical variables were assessed by the chi-square test, with the application of Yates correction where appropriate. Either parametric Student’s t-test or nonparametric Mann–Whitney U test and Wilcoxon test were used to compare continuous variables, depending on their distribution. Box plot analysis was performed to visually depict LDL levels within each therapy group at baseline and after BA add-on. A 2-sided p-value < 0.05 was considered statistically significant. All analyses were conducted using SPSS software version 24.0 (SPSS, Chicago, IL, USA), while box plots were generated using RStudio® 2024.09.1 software (RStudio, Boston, MA, USA).
3. Results
111 consecutive patients (age 65 ± 9 years, 61% male) with dyslipidemia not achieving LDL-c therapeutic target with available lipid-lowering therapies were enrolled. The baseline characteristics of the study population are showed in Table 1. According to the European Society of Cardiology Guidelines, 98 patients (88%) were at very high cardiovascular risk, while 13 patients (12%) were at high cardiovascular risk [12].

Table 1.
Baseline clinical features of the study cohort.
At the BA prescription, 78 patients (70.3%) were on maximum tolerated statins dosage and ezetimibe, of whom 53 patients (67.9%) were on 20 mg rosuvastatin (Rosu/Eze group) and 25 (32.1%) were on 40 mg atorvastatin (Ato/Eze group); 18 (16.2%) were on 10 mg ezetimibe alone; 15 patients (13.5%) were on PCSK9i therapy for at least six months. Finally, 67 patients (60.3%) started BA alone therapy, and 44 patients (39.7%) started the fixed combination of BA and ezetimibe.
3.1. Follow-Up
At a median follow-up of 60 days (IQR 44.5–60 days), 10 patients (9.0%) discontinued BA therapy due to musculoskeletal complaints (n = 4, 3.6%), gastrointestinal complaints (n = 3, 2.7%), or hyperuricemia (n = 3, 2.7%). The study population cohort exhibited a statistically significant reduction in LDL-c serum level (89.9 ± 33.0 vs. 56 ± 27.6 mg/dL; p < 0.0001), TC (155.5 ± 36.7 vs. 124.3- ± 31.1 mg/dL; p < 0.0001), and non-HDL cholesterol (97.0 ± 41.6 vs. 80.2- ± 28.0 mg/dL; p < 0.0001). While no significant variations were observed in TG (127.3 ± 57.4 vs. 119.3 ± 39.0 mg/dL; p = 0.7) and HDL-c (51.1 ± 16.9 vs. 44.1 ± 11.4 mg/dL; p = 0.1) plasma levels. A statistically significant increase with no clinical relevance was shown in AST (33.2 ± 10.5 vs. 39.2 ± 14.4 UI/dL; p = 0.002), ALT (36.7 ± 13.2 vs. 40.3 ± 16.3 UI/dL; p = 0.06), and uric acid (5.1 ± 1.4 vs. 5.4 ± 1.3 mg/dL; p = 0.014) serum levels. CPK serum levels (141.3 ± 147.7 vs. 136.8 ± 87.7 UI/dL; p = 0.7) remained stable over time. No temporary interruptions were reported throughout the study. The adherence rate to the therapy regimen reached 99%, demonstrating high treatment compliance.
3.2. Subgroup Analysis
In Rosu/Eze group (n = 53), adding BA therapy resulted in a 29.8% reduction in LDL-c (77.8 ± 31.0 vs. 54.6 ± 25.9 mg/dL; p < 0.0001) (Figure 1), with 60.4% patients (n = 32) achieving the appropriate LDL-c therapeutic target for their risk level. Similarly, in the Ato/Eze group (n = 25), adding BA therapy resulted in a 26.3% reduction in LDL-c (77.5 ± 33.2 vs. 57.1 ± 24.7 mg/dL; p < 0.019) (Figure 1), with 36% patients (n = 9) achieving the appropriate LDL-c therapeutic target for their risk level. Moreover, the add-on therapy with BA allowed 53% of patients on high-intensity statins and ezetimibe at both high and very high CV risk to achieve their LDL-c therapeutic target. Among patients on PCSK9i therapy (n = 15), the use of BA/Eze fixed combination therapy resulted in a 50% LDL-c reduction (93.0 ± 26.1 vs. 46.9 mg/dL ± 26.2 mg/dL; p < 0.0001) (Figure 1), with 60% of them (n = 9) reaching the LDL-c therapeutic target at follow-up. Finally, in patients on ezetimibe monotherapy (n = 18), adding BA resulted in a 42.0% reduction in LDL-c (83.9 ± 21.8 vs. 48.6 ± 20.4 mg/dL; p < 0.0001), with 56.5% of them (n = 10) reaching the LDL-c therapeutic target.
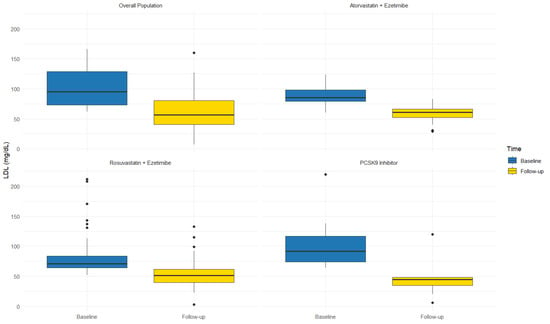
Figure 1.
LDL levels across different lipid-lowering therapies at baseline and after bempedoic acid add-on.
3.3. Pharmacoeconomic Analysis
We evaluated two treatment scenarios for 78 patients who had not achieved their LDL-c therapeutic target with background therapy and were treated with BA as an add-on therapy. In Scenario 1 (real world) we calculated the annual treatment cost by determining the number of patients reaching their target with 12 months of ezetimibe + statins + BA (n = 41). For the patients who did not achieve their target (n = 37), we considered the cost of one month of ezetimibe + statins + BA, followed by 12 months of treatment with ezetimibe + statins + PCSK9i. In Scenario 2 (pre-BA era), we calculated the cost assuming all 78 patients would have been treated with ezetimibe + statins + PCSK9i for the entire year. Prices considered include those published on the web platform of Campania Regional Society for the healthcare service (SO.RE.SA) [13], the Italian Medicine Agency (AIFA) transparency lists [14] for first and second-level treatments, and the prices published in the Official Gazette of the Italian Republic [15] for BA.
In Scenario 1 the use of BA as an add-on therapy before introducing PCSK9i resulted in a total annual treatment cost of €192,405. In Scenario 2, where all 78 patients would have been treated with PCSK9i for the entire year, the total annual cost was €288,924. The triple oral lipid lowering therapy, before introducing PCSK9i, therefore yielded savings of €96,519, representing a 33.4% reduction compared to Scenario 2 (Figure 2).
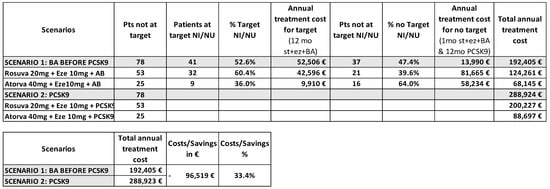
Figure 2.
Pharmacoeconomic analysis of BA treatment in patients not achieving their LDL-c therapeutic target with background therapy. Scenario 1 describes the real-world setting; Scenario 2 describes the optimal approach in pre-BA era.
4. Discussion
The key findings of our study are the following: in our clinical practice, BA was mainly prescribed to patients with dyslipidemia who were not achieving LDL-c therapeutic target despite maximum tolerated statin dosage and ezetimibe treatment, and to one-third of the patients with statin intolerance. BA resulted in a 28% reduction in LDL-c serum levels for patients on intensive statins and ezetimibe, and a 45% reduction for statin-intolerant patients. Among the overall population, 54% of patients receiving BA achieved the LDL-c therapeutic target after at least one month of treatment, accompanied by a reduction in regional healthcare spending. BA was safe, well-tolerated, and demonstrated high adherence and persistence.
While few and contrasting data are available about the effectiveness and safety of BA in real-world settings, none of these studies have been conducted in European countries [10,11], and no data are yet available on the utilization of BA in Italian clinical practices.
In a retrospective study [10] including 73 American patients with increased risk for atherosclerotic events and statin intolerance, BA was linked to clinically significant LDL-c reduction (LDL-c −36.7%, −31%, and −20.3% at ≤3, 6, and 12 months, respectively). However, a significant incidence of emergent adverse events (32.8%), primarily musculoskeletal complaints, led to drug discontinuation, was reported. In a real-world open-label study [11] including 122 Indian patients with acute coronary syndrome, the combination of 40 mg rosuvastatin, ezetimibe, and BA, initiated at hospital admission, resulted in a 60.6% reduction in LDL-c at the 6-week follow-up. Five patients developed gout, leading to the discontinuation of BA.
Our data align with previous real-world studies [10,11], indicating that the degree of LDL-c lowering with BA may exceed those reported in phase III randomized control trials (RCTs) [4,5,6]. A pooled analysis of four RCTs evaluating BA in patients with atherosclerotic cardiovascular disease and/or heterozygous familial hypercholesterolemia showed a LDL-c reduction of 17.8% for patients on statins and 24.5% for statin-intolerant patients after 12 weeks of treatment [16]. Several factors may explain these discrepancies: first, the heterogeneity in LDL-c lowering with BA may be influenced by genetic variations in the ACLY gene [17], as well as polymorphisms in other genes involved in cholesterol homeostasis [18]; second, the inter-patient variability in BA pharmacokinetics [19]; finally, differences in patient adherence to diet, lifestyle interventions, and medications, all of which contribute to variability in LDL-c levels [20]. In terms of safety, we observed a lower percentage of adverse events leading to treatment discontinuation compared to previous real-world studies. Our results also differ from those reported in RCTs [21]. Myalgia was the most common side effect, followed by gastrointestinal complaints, which in our study were mainly due to the combination of ezetimibe and BA [22]. Increased uric acid serum levels did not lead to gout, and the levels normalized upon BA discontinuation. The once-daily administration and favorable safety profile of BA contributed to high patient adherence and persistence to treatment, higher than those reported for other lipid-lowering therapies [23,24]. However, we recommend appropriate laboratory and clinical assessment in order to early detect potential side effects and to optimize treatment persistence. In our clinical practice, BA was mainly prescribed to patients at high and very high cardiovascular risk who did not achieve the LDL-c therapeutic target despite maximally tolerated statins dosage and ezetimibe. Among them, BA led to a 28% reduction in serum LDL-c levels and led 53% of patients to achieve the therapeutic LDL-c c target for their risk level.
Recently, Lincoff et al. showed that cardiovascular risk reduction with BA was comparable to that of statins for a given absolute reduction of LDL-c lowering [25]. Because we observed a mean reduction in LDL-c of 33.9 mg/dL (0.876 mmol/L), by using the 2010 Cholesterol Treatment Trialists’ Collaboration meta-analysis [26], we can estimate a relative reduction risk in major cardiovascular events of 19% yearly.
This approach, based on international recommendations [27,28], allowed us to avoid prescribing high-cost lipid-lowering therapies for half of the study population, potentially reducing healthcare spending in the Campania region. Our findings demonstrate a potential cost savings of 33.4% by incorporating BA as a preliminary step in the treatment regimen before resorting to more expensive PCSK9i.
Limitations
This study has some limitations. First, the small cohort of patients from a single region in Italy, which may limit the generalizability of our findings. Second, the short follow-up period, which may restrict our ability to evaluate long-term effectiveness and safety outcomes. Third, the lack of control group, consequently we could not assess the placebo effect or perform statistical adjustments for potential confounders. Despite these limitations, our study provides valuable insights into the clinical utilization, performance, adherence, and cost-effectiveness of BA in routine practice. Larger European studies with longer follow-up observation time are currently lacking.
5. Conclusions
In a real-world setting, BA was mainly prescribed to patients with dyslipidemia at heightened CV risk who had not achieved LDL-c therapeutic targe despite using the maximum tolerated statins dosage and ezetimibe, and to one-third of the patients enrolled due to statin intolerance. BA led to a 28% reduction in LDL-c in patients on high-intensity statins, and a 45% reduction in statin-intolerant patients. Approximately 54% of patients treated with BA reached their LDL-c therapeutic target. Our findings demonstrate a potential cost savings of 33.4% incorporating BA as a preliminary step in the treatment regimen before more expensive drugs. BA was safe, well-tolerated, and characterized by high adherence and persistence.
Author Contributions
Conceptualization, V.R. and G.R.; investigation, V.R., G.R., A.P., A.D.F., M.C., R.F., G.C., A.C., G.N., F.S.d.U., G.I. and L.A.; writing—original draft preparation, V.R. and G.R.; writing—review and editing, V.R., G.R., A.P., A.D.F., M.C., R.F., G.C., A.C., F.S.d.U., G.N., G.I. and L.A.; formal analysis: A.D.F. and A.C.; supervision, V.R., G.R. and L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical Committee of University of Campania “Luigi Vanvitelli”—Monaldi Hospital; ID 23263/2022; approval date: 15 December 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pinkosky, S.L.; Newton, R.S.; Day, E.A.; Ford, R.J.; Lhotak, S.; Austin, R.C.; Lalwani, N.D. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat. Commun. 2016, 7, 13457. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Pintó, X.; Escobar, C.; Varona, J.F.; Gámez, J.M. Real-World Attainment of Low-Density Lipoprotein Cholesterol Goals in Patients at High Risk of Cardiovascular Disease Treated with High-Intensity Statins: The TERESA Study. J. Clin. Med. 2023, 12, 3187. [Google Scholar] [CrossRef] [PubMed]
- American College of Cardiology. ASCVD Patients Face Barriers Reaching Guideline-Recommended Cholesterol Goals. Available online: https://www.acc.org/about-acc/press-releases/2019/11/13/15/38/ascvd-patients-face-barriers-reaching-guideline-recommended-cholesterol-goals (accessed on 20 February 2025).
- Goldberg, A.C.; Leiter, L.A.; Stroes, E.S.; Baum, S.J.; Hanselman, J.C.; Bloedon, L.T.; Duell, P.B. Effect of bempedoic acid vs. placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: The CLEAR Wisdom randomized clinical trial. JAMA 2019, 322, 1780–1788. [Google Scholar] [CrossRef]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N. Engl. J. Med. 2023, 388, 1353–1364. [Google Scholar] [CrossRef]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M.; CLEAR Harmony Trial. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef]
- Filippo, O.; D’Ascenzo, F.; Iannaccone, M.; Bertaina, M.; Leone, A.; Borzillo, I.; Ravetti, E.; Solano, A.; Pagliassotto, I.; Nebiolo, M.; et al. Safety and efficacy of bempedoic acid: A systematic review and meta-analysis of randomised controlled trials. Cardiovasc. Diabetol. 2023, 22, 324. [Google Scholar] [CrossRef] [PubMed]
- NILEMDO Determina n.20/2023 del 13 Gennaio 2023. Gazzetta Ufficiale della Repubblica Italiana 2023, The OJ 22 of 27-1-2023. Available online: https://www.gazzettaufficiale.it/atto/vediMenuHTML?atto.dataPubblicazioneGazzetta=2023-01-27&atto.codiceRedazionale=23A00339&tipoSerie=serie_generale&tipoVigenza=originario (accessed on 13 February 2025).
- NUSTENDI Determina n.21/2023 del 13 Gennaio 2023. Gazzetta Ufficiale della Repubblica Italiana 2023, The OJ 22 of 27-1-2023. Available online: https://www.gazzettaufficiale.it/atto/vediMenuHTML?atto.dataPubblicazioneGazzetta=2023-01-27&atto.codiceRedazionale=23A00340&tipoSerie=serie_generale&tipoVigenza=originario (accessed on 13 February 2025).
- Warden, B.A.; Cardiology, B.A.; Purnell, J.Q.; Duell, P.B.; Fazio, S. Real-world utilization of bempedoic acid in an academic preventive cardiology practice. J. Clin. Lipidol. 2022, 16, 94–103. [Google Scholar] [CrossRef]
- Mahajan, K.; Puri, R.; Duell, P.B.; Dutta, D.; Yadav, R.; Kumar, S.; Sharma, J.B.; Patel, P.; Dsouza, S.; Gupta, A.; et al. Rapid achievement of low-density lipoprotein cholesterol goals within 1 month after acute coronary syndrome during combination therapy with rosuvastatin, ezetimibe and bempedoic acid: Initial experience from the LAI-REACT study. J. Clin. Lipidol. 2024, 18, e867–e872. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- So.Re.Sa. Anagrafe Farmaci ed Emoderivati. Available online: https://www.soresa.it/pa/Pagine/Anagrafe/Farmaci-Emoderivati.aspx (accessed on 13 February 2025).
- Gazzetta Ufficiale della Repubblica Italiana. Decreto Pubblicato il 27 Gennaio 2023. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2023-01-27&atto.codiceRedazionale=23A00340 (accessed on 13 February 2025).
- Agenzia Italiana del Farmaco (AIFA). Lista Farmaci Equivalenti per Principio Attivo, 15 Gennaio 2025. Available online: https://www.aifa.gov.it/documents/20142/2693710/Lista_farmaci_equivalenti_Principio_Attivo_15.01.2025.pdf (accessed on 13 February 2025).
- Banach, M.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Leiter, L.A.; Mancini, G.B.J.; Ray, K.K.; Flaim, J.; Ye, Z.; Catapano, A.L. Association of Bempedoic Acid Administration with Atherogenic Lipid Levels in Phase 3 Randomized Clinical Trials of Patients with Hypercholesterolemia. JAMA Cardiol. 2020, 5, 1–12. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Xu, S.; Shen, A.Z. ATP-Citrate Lyase (ACLY) in Lipid Metabolism and Atherosclerosis: An Updated Review. Prog. Lipid Res. 2020, 77, 101006. [Google Scholar] [CrossRef]
- Karlson, B.W.; Wiklund, O.; Palmer, M.K.; Nicholls, S.J.; Lundman, P.; Barter, P.J. Variability of Low-Density Lipoprotein Cholesterol Response with Different Doses of Atorvastatin, Rosuvastatin, and Simvastatin: Results from VOYAGER. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 212–217. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Amore, B.M.; Bockbrader, H.; Crass, R.L.; Chapel, S.; Sasiela, W.J.; Emery, M.G. Population Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Modeling of Bempedoic Acid and Low-Density Lipoprotein Cholesterol in Healthy Subjects and Patients with Dyslipidemia. J. Pharmacokinet. Pharmacodyn. 2023, 50, 351–364. [Google Scholar] [CrossRef]
- Dudum, R.; Juraschek, S.P.; Appel, L.J. Dose-Dependent Effects of Lifestyle Interventions on Blood Lipid Levels: Results from the PREMIER Trial. Patient Educ. Couns. 2019, 102, 1882–1891. [Google Scholar] [CrossRef]
- Bays, H.E.; Banach, M.; Catapano, A.L.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Ballantyne, C.M. Bempedoic Acid Safety Analysis: Pooled Data from Four Phase 3 Trials. J. Clin. Lipidol. 2020, 14, 649–659.e6. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, S.; Du, H.; Li, J.; Khan, S.U.; Aertgeerts, B.; Guyatt, G.; Hao, Q.; Bekkering, G.; Li, L.; et al. Safety of Ezetimibe in Lipid-Lowering Treatment: Systematic Review and Meta-Analysis of Randomized Controlled Trials and Cohort Studies. BMJ Med. 2022, 1, e000134. [Google Scholar] [CrossRef]
- Mazhar, F.; Hjemdahl, P.; Clase, C.M.; Johnell, K.; Jernberg, T.; Carrero, J.J. Lipid-lowering treatment intensity, persistence, adherence and goal attainment in patients with coronary heart disease. Am. Heart J. 2022, 251, 78–90. [Google Scholar] [CrossRef]
- Caso, V.M.; Sperlongano, S.; Liccardo, B.; Romeo, E.; Padula, S.; Arenga, F.; D’Andrea, A.; Caso, P.; Golino, P.; Nigro, G.; et al. The impact of the COVID-19 outbreak on patients’ adherence to PCSK9 inhibitors therapy. J. Clin. Med. 2022, 11, 475. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Ray, K.K.; Sasiela, W.J.; Haddad, T.; Nicholls, S.J.; Li, N.; Cho, L.; Mason, D.; Libby, P.; Goodman, S.G.; et al. Comparative cardiovascular benefits of bempedoic acid and statin drugs. J. Am. Coll. Cardiol. 2024, 84, 152–162. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Banach, M.; Penson, P.E.; Farnier, M.; Fras, Z.; Latkovskis, G.; Laufs, U.; Paneni, F.; Parini, P.; Pirro, M.; Reiner, Ž.; et al. Bempedoic acid in the management of lipid disorders and cardiovascular risk. 2023 position paper of the International Lipid Expert Panel (ILEP). Prog. Cardiovasc. Dis. 2023, 79, 2–11. [Google Scholar] [CrossRef]
- Banach, M.; Reiner, Ž.; Surma, S.; Bajraktari, G.; Bielecka-Dabrowa, A.; Bunc, M.; Bytyçi, I.; Ceska, R.; Cicero, A.F.G.; Dudek, D.; et al. 2024 recommendations on the optimal use of lipid-lowering therapy in established atherosclerotic cardiovascular disease and following acute coronary syndromes: A position paper of the International Lipid Expert Panel (ILEP). Drugs 2024, 84, 1541–1577. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).