iFGF23 Plasma Levels in Transfusion-Dependent β-Thalassemia: Insights into Bone and Iron Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical and Biochemical Data
2.2. FGF23 Assay
2.3. Radiological Data
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farmakis, D.; Porter, J.; Taher, A.; Domenica Cappellini, M.; Angastiniotis, M.; Eleftheriou, A. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. Hemasphere 2022, 6, e732. [Google Scholar] [CrossRef]
- Chauhan, W.; Shoaib, S.; Fatma, R.; Zaka-ur-Rab, Z.; Afzal, M. Beta-thalassemia and the advent of new interventions beyond transfusion and iron chelation. Br. J. Clin. Pharmacol. 2022, 88, 3610–3626. [Google Scholar] [CrossRef]
- Motta, I.; Bou-Fakhredin, R.; Taher, A.T.; Cappellini, M.D. Beta Thalassemia: New Therapeutic Options Beyond Transfusion and Iron Chelation. Drugs 2020, 80, 1053–1063. [Google Scholar] [CrossRef]
- De Sanctis, V.; Eleftheriou, A.; Malaventura, C. Thalassaemia International Federation Study Group on Growth and Endocrine Complications in Thalassaemia. Prevalence of endocrine complications and short stature in patients with thalassaemia major: A multicenter study by the Thalassaemia International Federation (TIF). Pediatr. Endocrinol. Rev. 2004, 2 (Suppl. 2), 249–255. [Google Scholar] [PubMed]
- Casale, M.; Forni, G.L.; Cassinerio, E.; Pasquali, D.; Origa, R.; Serra, M.; Campisi, S.; Peluso, A.; Renni, R.; Cattoni, A.; et al. Risk factors for endocrine complications in transfusion-dependent thalassemia patients on chelation therapy with deferasirox: A risk assessment study from a multi-center nation-wide cohort. Haematologica 2021, 107, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Baldini, M.I.; Del Monte, P.; Gigante, A.; Grandone, A.; Origa, R.; Poggi, M.; Gadda, F.; Lai, R.; Marchetti, M.; et al. Good Clinical Practice of the Italian Society of Thalassemia and Haemoglobinopathies (SITE) for the Management of Endocrine Complications in Patients with Haemoglobinopathies. J. Clin. Med. 2022, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Vasiliu, C.; Trandafir, A.I.; Albu, S.E.; Dumitrascu, M.-C.; Popa, A.; Mehedintu, C.; Petca, R.-C.; Petca, A.; Sandru, F. New Entity-Thalassemic Endocrine Disease: Major Beta-Thalassemia and Endocrine Involvement. Diagnostics 2022, 12, 1921. [Google Scholar] [CrossRef]
- Farmaki, K.; Tzoumari, I.; Pappa, C.; Chouliaras, G.; Berdoukas, V. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br. J. Haematol. 2010, 148, 466–475. [Google Scholar] [CrossRef]
- Ambrosio, M.R.; Cattaneo, C.A.; Gagliardi, I.; Carnevale, A.; Zatelli, M.C. Aetiology, diagnosis and treatment of thalassemia-associated osteoporosis of the adult. J. Endocrinol. Investig. 2025. [Google Scholar] [CrossRef]
- Stefanopoulos, D.; Papaioannou, N.A.; Papavassiliou, A.G.; Mastorakos, G.; Vryonidou, A.; Michou, A.; Dontas, I.A.; Lyritis, G.; Kassi, E.; Tournis, S. A contemporary therapeutic approach to bone disease in beta-thalassemia—A review. J. Frailty Sarcopenia Falls 2018, 3, 13–25. [Google Scholar] [CrossRef]
- Manolopoulos, P.P.; Lavranos, G.; Mamais, I.; Angouridis, A.; Giannakou, K.; Johnson, E.O. Vitamin D and bone health status in beta thalassemia patients—Systematic review. Osteoporos. Int. 2021, 32, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, I.; Celico, M.; Gamberini, M.R.; Pontrelli, M.; Fortini, M.; Carnevale, A.; Napoli, N.; Zatelli, M.C.; Ambrosio, M.R. Efficacy and Safety of Teriparatide in Beta-Thalassemia Major Associated Osteoporosis: A Real-Life Experience. Calcif. Tissue Int. 2022, 111, 56–65. [Google Scholar] [CrossRef] [PubMed]
- White, K.E.; Evans, W.E.; O’Riordan, J.L.; Speer, M.C.; Econs, M.J.; Lorenz-Depiereux, B.; Grabowski, M.; Meitinger, T.; Strom, T.M. ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef]
- Ho, B.B.; Bergwitz, C. FGF23 signalling and physiology. J. Mol. Endocrinol. 2021, 66, R23–R32. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Engel, J.L.; Wiley, S.E.; Xiao, J.; Gonzalez, D.J.; Nidumanda Appaiah, H.; Koller, A.; Nizet, V.; White, K.E.; Dixon, J.E. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 5520–5525. [Google Scholar] [CrossRef]
- Edmonston, D.; Wolf, M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat. Rev. Nephrol. 2020, 16, 7–19. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. WIREs Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, O.; Imura, A.; Iwano, A.; Freund, J.-N.; Henrissat, B.; Fujimori, T.; Nabeshima, Y. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J. Biol. Chem. 2004, 279, 9777–9784. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.-C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef]
- Cipriani, C.; Minisola, S.; Colangelo, L.; DE Martino, V.; Ferrone, F.; Biamonte, F.; Danese, V.; Sonato, C.; Santori, R.; Occhiuto, M.; et al. FGF23 functions and disease. Minerva Endocrinol. 2022, 47, 437–448. [Google Scholar] [CrossRef]
- Andrukhova, O.; Zeitz, U.; Goetz, R.; Mohammadi, M.; Lanske, B.; Erben, R.G. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 2012, 51, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Slavic, S.; Smorodchenko, A.; Zeitz, U.; Shalhoub, V.; Lanske, B.; Pohl, E.E.; Erben, R.G. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 2014, 6, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 Is a Potent Regulator of Vitamin D Metabolism and Phosphate Homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.K.; Andrukhova, O.; Clinkenbeard, E.L.; White, K.E.; Erben, R.G. Excessive Osteocytic Fgf23 Secretion Contributes to Pyrophosphate Accumulation and Mineralization Defect in Hyp Mice. PLoS Biol. 2016, 14, e1002427. [Google Scholar] [CrossRef]
- Murali, S.K.; Roschger, P.; Zeitz, U.; Klaushofer, K.; Andrukhova, O.; Erben, R.G. FGF23 Regulates Bone Mineralization in a 1,25(OH)2 D3 and Klotho-Independent Manner. J. Bone Miner. Res. 2016, 31, 129–142. [Google Scholar] [CrossRef]
- Coe, L.M.; Madathil, S.V.; Casu, C.; Lanske, B.; Rivella, S.; Sitara, D. FGF-23 Is a Negative Regulator of Prenatal and Postnatal Erythropoiesis. J. Biol. Chem. 2014, 289, 9795–9810. [Google Scholar] [CrossRef]
- Daryadel, A.; Bettoni, C.; Haider, T.; Imenez Silva, P.H.; Schnitzbauer, U.; Pastor-Arroyo, E.M.; Wenger, R.H.; Gassmann, M.; Wagner, C.A. Erythropoietin stimulates fibroblast growth factor 23 (FGF23) in mice and men. Pflugers Arch.-Eur. J. Physiol. 2018, 470, 1569–1582. [Google Scholar] [CrossRef]
- Hanudel, M.R.; Eisenga, M.F.; Rappaport, M.; Chua, K.; Qiao, B.; Jung, G.; Gabayan, V.; Gales, B.; Ramos, G.; De Jong, M.A.; et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol. Dial. Transplant. 2019, 34, 2057–2065. [Google Scholar] [CrossRef]
- Aprile, A.; Raggi, L.; Bolamperti, S.; Villa, I.; Storto, M.; Morello, G.; Marktel, S.; Tripodo, C.; Cappellini, M.D.; Motta, I.; et al. Inhibition of FGF23 is a therapeutic strategy to target hematopoietic stem cell niche defects in β-thalassemia. Sci. Transl. Med. 2023, 15, eabq3679. [Google Scholar] [CrossRef]
- Wolf, M.; Koch, T.A.; Bregman, D.B. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 2013, 28, 1793–1803. [Google Scholar] [CrossRef]
- Imel, E.A.; Peacock, M.; Gray, A.K.; Padgett, L.R.; Hui, S.L.; Econs, M.J. Iron Modifies Plasma FGF23 Differently in Autosomal Dominant Hypophosphatemic Rickets and Healthy Humans. J. Clin. Endocrinol. Metab. 2011, 96, 3541–3549. [Google Scholar] [CrossRef]
- Farrow, E.G.; Yu, X.; Summers, L.J.; Davis, S.I.; Fleet, J.C.; Allen, M.R.; Robling, A.G.; Stayrook, K.R.; Jideonwo, V.; Magers, M.J.; et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc. Natl. Acad. Sci. USA 2011, 108, E1146–E1155. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Asaad, S.; Econs, M.; Rubin, J.E. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018, 2018:bcr-2017-222851. [Google Scholar] [CrossRef] [PubMed]
- Urbina, T.; Belkhir, R.; Rossi, G.; Carbonnel, F.; Pavy, S.; Collins, M.; Mariette, X.; Seror, R. Iron Supplementation–Induced Phosphaturic Osteomalacia: FGF23 is the Culprit. J. Bone Miner. Res. 2018, 33, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Bishay, R.H.; Ganda, K.; Seibel, M.J. Long-term iron polymaltose infusions associated with hypophosphataemic osteomalacia: A report of two cases and review of the literature. Ther. Adv. Endocrinol. 2017, 8, 14–19. [Google Scholar] [CrossRef]
- Fukao, W.; Hasuike, Y.; Yamakawa, T.; Toyoda, K.; Aichi, M.; Masachika, S.; Kantou, M.; Takahishi, S.; Iwasaki, T.; Yahiro, M.; et al. Oral Versus Intravenous Iron Supplementation for the Treatment of Iron Deficiency Anemia in Patients on Maintenance Hemodialysis—Effect on Fibroblast Growth Factor-23 Metabolism. J. Ren. Nutr. 2018, 28, 270–277. [Google Scholar] [CrossRef]
- Girelli, D.; Nemeth, E.; Swinkels, D.W. Hepcidin in the diagnosis of iron disorders. Blood 2016, 127, 2809–2813. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016, 89, 135–146. [Google Scholar] [CrossRef]
- Hanudel, M.R.; Chua, K.; Rappaport, M.; Gabayan, V.; Valore, E.; Goltzman, D.; Ganz, T.; Nemeth, E.; Salusky, I.B. Effects of dietary iron intake and chronic kidney disease on fibroblast growth factor 23 metabolism in wild-type and hepcidin knockout mice. Am. J. Physiol.-Ren. Physiol. 2016, 311, F1369–F1377. [Google Scholar] [CrossRef]
- Higashimoto, Y.; Tanaka, K.; Matsui, T.; Sakaguchi, T.; Yamagishi, S.I.; Motomiya, Y. Fibroblast Growth Factor 23 Contributes to Regulation of Hepcidin/Ferroportin Axis. Austin. J. Pharmacol. Ther. 2020, 8(1), 1118. [Google Scholar] [CrossRef]
- Tangngam, H.; Mahachoklertwattana, P.; Poomthavorn, P.; Chuansumrit, A.; Sirachainan, N.; Chailurkit, L.; Khlairit, P. Under-recognized Hypoparathyroidism in Thalassemia. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 324. [Google Scholar] [CrossRef]
- Stefanopoulos, D.; Nasiri-Ansari, N.; Dontas, I.; Vryonidou, A.; Galanos, A.; Psaridi, L.; Fatouros, I.G.; Mastorakos, G.; Papavassiliou, A.G.; Kassi, E.; et al. Fibroblast Growth Factor 23 (FGF23) and Klotho Protein in Beta-Thalassemia. Horm. Metab. Res. 2020, 52, 194–201. [Google Scholar] [CrossRef]
- Shah, F.; Maggio, A. Blood transfusion. In Guidelines for the Management of Transfusion-Dependent Β-Thalassaemia (TDT), 5th ed.; Taher, A.T., Farmakis, D., Porter, J.B., Cappellini, M.D., Musallam, K.M., Eds.; Thalassaemia International Federation: Nicosia, Cyprus, 2025. [Google Scholar]
- Forni, G.L.; De Franceschi, L.; Lisi, R.; Vassanelli, A.; Marson, P.; Ostuni, A.; Gigante, A. Le Strategie Trasfusionali Nelle Emoglobinopatie. Buone Pratiche SITE-SIMTI-SIdEM 2024. Available online: https://www.site-italia.org/storage/site/article/pdf/117/1-LE%20STRATEGIE%20TRASFUSIONALI%20NELLE%20EMOGLOBINOPATIE.pdf (accessed on 7 March 2025).
- The Jamovi Project (2023). jamovi. (Version 2.4) [Computer Software]. Available online: https://www.jamovi.org (accessed on 7 March 2025).
- Souberbielle, J.-C.; Prié, D.; Piketty, M.-L.; Rothenbuhler, A.; Delanaye, P.; Chanson, P.; Cavalier, E. Evaluation of a New Fully Automated Assay for Plasma Intact FGF23. Calcif. Tissue Int. 2017, 101, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Hidaka, N.; Kato, H. The pathophysiology of hypophosphatemia. Best Pract. Res. Clin. Endocrinol. Metab. 2024, 38, 101851. [Google Scholar] [CrossRef]
- Rivoira, M.A.; Peralta López, M.E.; Areco, V.; Díaz De Barboza, G.; Dionisi, M.P.; Tolosa De Talamoni, N. Emerging concepts on the FGF23 regulation and activity. Mol. Cell. Biochem. 2025, 480, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R. Biochemical markers of bone turnover. Clin. Chim. Acta 2001, 313, 95–105. [Google Scholar] [CrossRef]
- Yang, W.-P.; Chang, H.-H.; Li, H.-Y.; Lai, Y.-C.; Huang, T.-Y.; Tsai, K.-S.; Lin, K.-H.; Lin, D.-T.; Jou, S.-T.; Lu, M.-Y.; et al. Iron Overload Associated Endocrine Dysfunction Leading to Lower Bone Mineral Density in Thalassemia Major. J. Clin. Endocrinol. Metab. 2020, 105, e1015–e1024. [Google Scholar] [CrossRef]
- Bouksila, M.; Mrad, M.; Kaabachi, W.; Kalai, E.; Smaoui, W.; Rekik, S.; Krir, A.; Issaoui, N.; Hamzaoui, K.; Sahli, H.; et al. Correlation of Fgf23 and Balp with Bone Mineral Density in Hemodialysis Patients. J. Med. Biochem. 2019, 38, 418–426. [Google Scholar] [CrossRef]
- Pellegrino, F.; Zatelli, M.C.; Bondanelli, M.; Carnevale, A.; Cittanti, C.; Fortini, M.; Gamberini, M.R.; Giganti, M.; Ambrosio, M.R. Dual-energy X-ray absorptiometry pitfalls in Thalassemia Major. Endocrine 2019, 65, 469–482. [Google Scholar] [CrossRef]
- Lucioni, E.; Pellegrino, F.; Remor, D.; Cossu, A.; Niero, D.; Longo, F.; Zatelli, M.C.; Giganti, M.; Carnevale, A.; Ambrosio, M.R. Bone densitometry in Thalassemia major: A closer look at pitfalls and operator-related errors in a 10-year follow-up population. Radiol. Med. 2024, 129, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Prideaux, M.; Wijenayaka, A.R.; Yang, D.; Ormsby, R.T.; Bonewald, L.F.; Atkins, G.J. Sclerostin Directly Stimulates Osteocyte Synthesis of Fibroblast Growth Factor-23. Calcif. Tissue Int. 2021, 109, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, D.; Hartley, I.R.; De Castro Diaz, L.F.; Theng, E.; Li, X.; Gafni, R.I.; Collins, M.T. PTH and FGF23 Exert Interdependent Effects on Renal Phosphate Handling: Evidence from Patients with Hypoparathyroidism and Hyperphosphatemic Familial Tumoral Calcinosis Treated with Synthetic Human PTH 1–34. J. Bone Miner. Res. 2020, 37, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Saki, F.; Salehifar, A.; Kassaee, S.R.; Omrani, G.R. Association of vitamin D and FGF23 with serum ferritin in hypoparathyroid thalassemia: A case control study. BMC Nephrol. 2020, 21, 482. [Google Scholar] [CrossRef]
- Kattamis, A. Renal function abnormalities and deferasirox. Lancet Child Adolesc. Health 2019, 3, 2–3. [Google Scholar] [CrossRef]
- Yui, J.C.; Geara, A.; Sayani, F. Deferasirox-associated Fanconi syndrome in adult patients with transfusional iron overload. Vox Sanguinis 2021, 116, 793–797. [Google Scholar] [CrossRef]
- Dee, C.M.A.; Cheuk, D.K.L.; Ha, S.; Chiang, A.K.; Chan, G.C. Incidence of deferasirox-associated renal tubular dysfunction in children and young adults with beta-thalassaemia. Br. J. Haematol. 2014, 167, 434–436. [Google Scholar] [CrossRef]
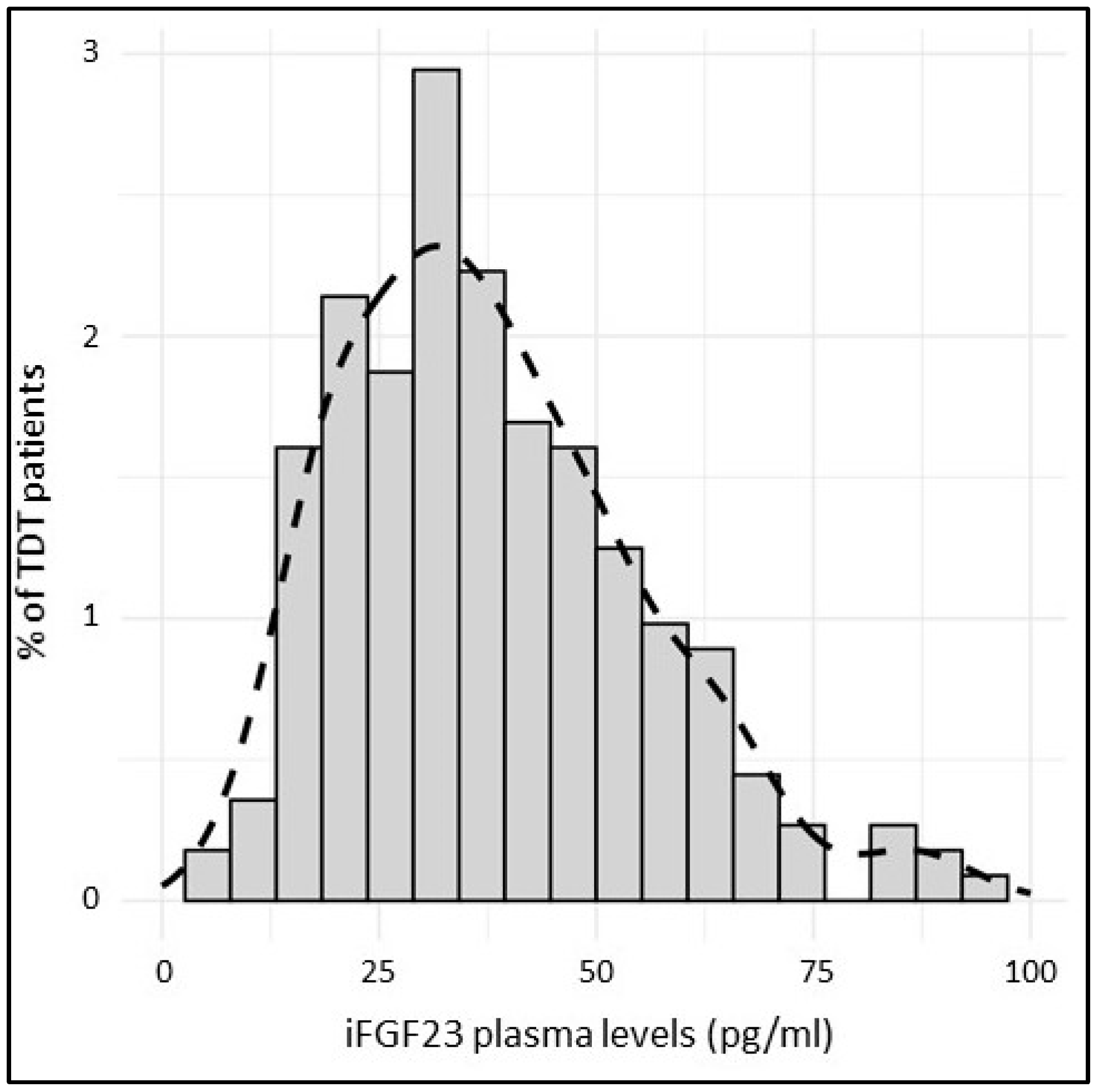
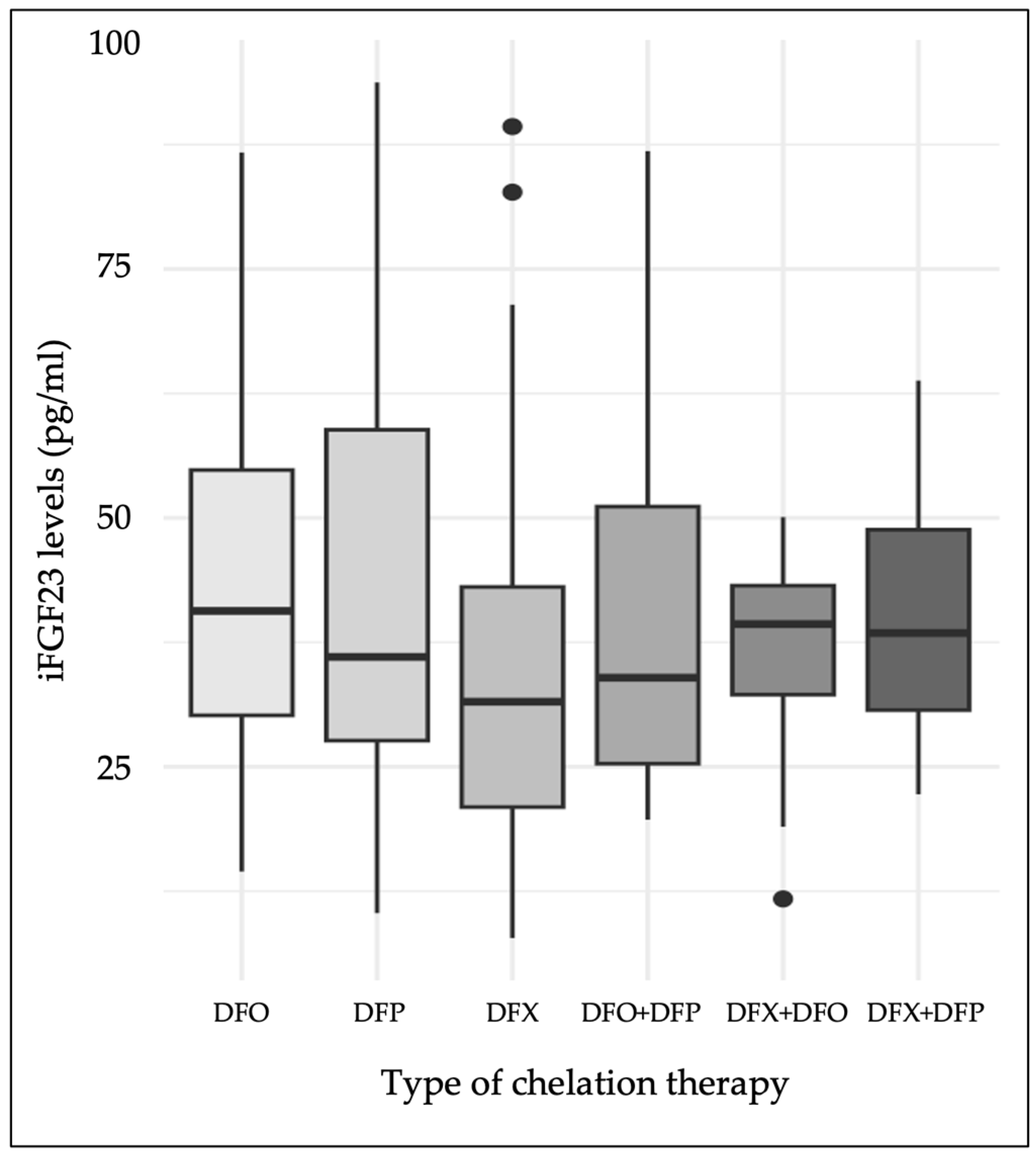
| Clinical Data | ||
|---|---|---|
| N | % | |
| Sex: | ||
| Females | 117 | 54.9% |
| Males | 96 | 45.1% |
| Splenectomy: | ||
| Yes | 136 | 63.8% |
| No | 77 | 36.2% |
| Chelation therapy: | ||
| DFX | 87 | 40.8% |
| DFO | 46 | 21.6% |
| DFP | 39 | 18.3% |
| DFX + DFO | 13 | 6.1% |
| DFX + DFP | 8 | 3.8% |
| DFO + DFP | 20 | 9.4% |
| Laboratory and Radiological Data | ||
|---|---|---|
| Median | IQR | |
| iFGF23 (pg/mL) | 36.0 | [24.9, 48.8] |
| Age (years) | 50.0 | [44.0, 54.0] |
| Age at transfusion initiation (months) | 12.0 | [6.00, 36.0] |
| BMI (kg/m2) | 22.4 | [20.4, 24.6] |
| eGFR (mL/min) | 91.8 | [74.8, 112] |
| 24 h urinary proteins (mg/die) | 160 | [105, 234] |
| 24 h urinary creatinine (g/die) | 1.00 | [0.800, 1.30] |
| 24 h urinary calcium (mg/die) | 291 | [202, 403] |
| 24 h urinary phosphate (g/die) | 0.700 | [0.500, 0.900] |
| ALP (U/L) | 75.5 | [61.0, 96.0] |
| Urea (mg/dL) | 42.0 | [35.0, 50.0] |
| Creatinine (mg/dL) | 0.735 | [0.610, 0.880] |
| Calcium (mg/dL) | 9.50 | [9.10, 9.80] |
| Phosphate (mg/dL) | 3.65 | [3.20, 4.10] |
| Serum iron (μg/dL) | 231 | [206, 267] |
| Magnesium (mg/dL) | 2.15 | [1.99, 2.31] |
| Epo (mUI/mL) | 49.9 | [29.1, 73.1] |
| PTH (pg/mL) | 26.0 | [19.0, 37.0] |
| 25-OH vitamin D (ng/mL) | 29.8 | [22.3, 36.1] |
| BAP (μg/L) | 16.9 | [12.7, 23.6] |
| Ctx (ng/mL) | 0.237 | [0.146, 0.405] |
| Osteocalcin (ng/mL) | 18.7 | [15.0, 22.1] |
| IGF1 (ng/mL) | 79.7 | [57.0, 99.8] |
| TSH (μU/mL) | 2.16 | [1.56, 2.89] |
| Zinc (μg/mL) | 86.0 | [77.0, 95.5] |
| Transferrin (mg/dL) | 167 | [151, 180] |
| sTfR (mg/L) | 3.25 | [2.33, 4.25] |
| Ferritin (ng/mL) | 536 | [339, 950] |
| MRI parameters: | ||
| Heart T2* (ms) | 43.0 | [39.0, 45.0] |
| Liver T2* (ms) | 8.57 | [4.24, 15.8] |
| LIC | 3.21 | [1.81, 6.49] |
| DXA parameters: | ||
| LS BMD | 0.800 | [0.732, 0.894] |
| F BMD | 0.719 | [0.640, 0.818] |
| FN BMD | 0.609 | [0.534, 0.684] |
| Predictor | Estimate (β) | Standard Error | 95% CI | p-Value |
|---|---|---|---|---|
| Sex (males–females) | 3.52 | 2.37 | [−1.14, 8.19] | 0.138 |
| Age † | 0.48 | 0.13 | [0.23, 0.73] | <0.001 |
| Age at transfusion initiation † | 0.021 | 0.007 | [0.007, 0.035] | 0.004 |
| BMI † | 0.94 | 0.37 | [0.22, 1.66] | 0.011 |
| Splenectomy † | 4.95 | 2.44 | [0.14, 9.76] | 0.044 |
| Phosphate † | 5.81 | 1.6 | [2.66, 8.97] | <0.001 |
| Calcium † | 4.86 | 2.17 | [0.57, 9.14] | 0.027 |
| BAP † | −0.24 | 0.092 | [−0.42, −0.062] | 0.009 |
| Osteocalcin † | −0.39 | 0.15 | [−0.69, −0.092] | 0.011 |
| Ctx | −7.14 | 4.26 | [−15.5, 1.26] | 0.095 |
| ALP | −0.067 | 0.035 | [−0.14, 0.002] | 0.057 |
| PTH | 0.039 | 0.079 | [−0.12, 0.2] | 0.627 |
| 25-OH vitamin D | 0.16 | 0.11 | [−0.065, 0.38] | 0.165 |
| Magnesium † | −11.7 | 3.97 | [−19.6, −3.92] | 0.003 |
| Zinc | −0.079 | 0.06 | [−0.19, 0.03] | 0.164 |
| IGF1 † | −0.094 | 0.031 | [−0.155, −0.033] | 0.003 |
| TSH | 0.96 | 0.66 | [−0.34, 2.25] | 0.147 |
| 24 h urinary proteins † | −0.024 | 0.009 | [−0.042, −0.005] | 0.01 |
| 24 h urinary calcium † | −0.026 | 0.008 | [−0.042, −0.009] | 0.003 |
| 24 h urinary phosphate | −3.89 | 2.27 | [−8.37, 0.59] | 0.089 |
| 24 h urinary creatinine | 2.91 | 3.38 | [−3.76, 9.58] | 0.39 |
| eGFR | 0.052 | 0.039 | [−0.025, 0.13] | 0.185 |
| Creatinine | 7.43 | 6.34 | [−5.06, 19.9] | 0.242 |
| Urea | 0.177 | 0.098 | [−0.017, 0.371] | 0.073 |
| Epo | 0.007 | 0.013 | [−0.019, 0.033] | 0.594 |
| Transferrin | 0.09 | 0.05 | [−0.01, 0.19] | 0.09 |
| sTfR † | 2.35 | 0.87 | [0.65, 4.06] | 0.007 |
| Ferritin | −0.0009 | 0.002 | [−0.004, 0.002] | 0.555 |
| Serum iron | −0.046 | 0.023 | [−0.092, 0.0003] | 0.052 |
| Chelation therapy: | ||||
| DFP-DFX † | 7.57 | 3.27 | [1.31, 14.2] | 0.022 |
| DFO-DFX † | 9.26 | 3.09 | [3.16, 15.4] | 0.003 |
| DFO + DFP-DFX | 6.52 | 4.21 | [−1.78, 14.8] | 0.123 |
| DFX + DFO-DFX | 1.87 | 5.05 | [−8.08, 11.8] | 0.711 |
| DFX + DFP-DFX | 6.59 | 6.27 | [−5.77, 19] | 0.294 |
| MRI parameters: | ||||
| LIC | −0.073 | 0.22 | [−0.52, 0.37] | 0.746 |
| Liver T2* | −0.264 | 0.16 | [−0.59, 0.06] | 0.11 |
| Heart T2* | 0.039 | 0.14 | [−0.24, 0.32] | 0.782 |
| DXA scan | ||||
| LS-BMD | 16 | 8.47 | [−0.71, 32.7] | 0.06 |
| F-BMD | 14.4 | 8.98 | [−3.27, 32.2] | 0.11 |
| FN-BMD | 9.87 | 10.9 | [−11.6, 31.3] | 0.37 |
| Predictor | Estimate (β) | Standard Error | 95% CI | p-Value |
|---|---|---|---|---|
| Age | 0.245 | 0.224 | [−0.199, 0.689] | 0.276 |
| Age at transfusion initiation * | 0.022 | 0.009 | [0.004, 0.040] | 0.015 |
| BMI | 0.467 | 0.451 | [−0.424, 1.359] | 0.302 |
| Splenectomy | −2.851 | 3.077 | [−8.938, 3.237] | 0.356 |
| eGFR | 0.004 | 0.055 | [−0.106, 0.114] | 0.944 |
| Calcium * | 7.283 | 2.919 | [1.508, 13.058] | 0.014 |
| Phosphate * | 5.402 | 1.835 | [1.771, 9.034] | 0.004 |
| Magnesium | −6.875 | 4.439 | [−15.657, 1.908] | 0.124 |
| Osteocalcin * | −0.697 | 0.241 | [−1.173, −0.221] | 0.004 |
| BAP | 0.146 | 0.138 | [−0.127, 0.420] | 0.291 |
| sTfR | 1.711 | 1.229 | [−0.719, 4.142] | 0.166 |
| IGF1 | −0.061 | 0.047 | [−0.153, 0.032] | 0.196 |
| 24 h urinary proteins | −0.017 | 0.010 | [−0.038, 0.004] | 0.103 |
| 24 h urinary calcium | −0.012 | 0.009 | [−0.030, 0.006] | 0.186 |
| Chelation therapy: | ||||
| DFP-DFX | 5.639 | 3.759 | [−1.800, 13.077] | 0.136 |
| DFO-DFX * | 7.935 | 3.953 | [0.114, 15.757] | 0.047 |
| DFO + DFP-DFX * | 13.716 | 5.172 | [3.483, 23.949] | 0.009 |
| DFX + DFO-DFX | −0.502 | 5.902 | [−12.181, 11.176] | 0.932 |
| DFX + DFP-DFX | 4.774 | 6.314 | [−7.720, 17.268] | 0.451 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobbo, A.; Longo, F.; Cattaneo, C.A.; Verrienti, M.; Marzi, G.; Chamekh, F.; Culcasi, M.; Cossu, A.; Zatelli, M.C.; Ambrosio, M.R. iFGF23 Plasma Levels in Transfusion-Dependent β-Thalassemia: Insights into Bone and Iron Metabolism. J. Clin. Med. 2025, 14, 1834. https://doi.org/10.3390/jcm14061834
Gobbo A, Longo F, Cattaneo CA, Verrienti M, Marzi G, Chamekh F, Culcasi M, Cossu A, Zatelli MC, Ambrosio MR. iFGF23 Plasma Levels in Transfusion-Dependent β-Thalassemia: Insights into Bone and Iron Metabolism. Journal of Clinical Medicine. 2025; 14(6):1834. https://doi.org/10.3390/jcm14061834
Chicago/Turabian StyleGobbo, Alberto, Filomena Longo, Camilla Alice Cattaneo, Martina Verrienti, Gianluca Marzi, Fatima Chamekh, Martina Culcasi, Alberto Cossu, Maria Chiara Zatelli, and Maria Rosaria Ambrosio. 2025. "iFGF23 Plasma Levels in Transfusion-Dependent β-Thalassemia: Insights into Bone and Iron Metabolism" Journal of Clinical Medicine 14, no. 6: 1834. https://doi.org/10.3390/jcm14061834
APA StyleGobbo, A., Longo, F., Cattaneo, C. A., Verrienti, M., Marzi, G., Chamekh, F., Culcasi, M., Cossu, A., Zatelli, M. C., & Ambrosio, M. R. (2025). iFGF23 Plasma Levels in Transfusion-Dependent β-Thalassemia: Insights into Bone and Iron Metabolism. Journal of Clinical Medicine, 14(6), 1834. https://doi.org/10.3390/jcm14061834






