Long-Term Evolution of Post-COVID-19 Echocardiographic Parameters in Patients with Chronic Kidney Disease: A Prospective Comparative Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Demographic and Echocardiography Parameters
2.4. Statistical Analysis
- Within-subjects factor: Time (3 levels);
- Between-subjects factor: COVID-19 status (2 levels);
- Dependent variables: LV EF, LV Tei Index, FAC, TAPSE, RVFWLS, RV Tei Index.
3. Results
3.1. Baseline Demographic and Clinical Characteristics
3.2. Baseline Echocardiographic Characteristics
3.3. Cardiac Biomarkers
3.4. Follow-Up Echocardiography
3.5. Correlations Between Cardiac Biomarkers and Echocardiographic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Salvo, G.D.; Sade, L.E.; Pearce, K.; et al. Global Evaluation of Echocardiography in Patients with COVID-19. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, M.; Tudoran, C.; Lazureanu, V.E.; Marinescu, A.R.; Pop, G.N.; Pescariu, A.S.; Enache, A.; Cut, T.G. Alterations of Left Ventricular Function Persisting during Post-Acute COVID-19 in Subjects without Previously Diagnosed Cardiovascular Pathology. J. Pers. Med. 2021, 11, 225. [Google Scholar] [CrossRef]
- Alimi, H.; Bigdelu, L.; Poorzand, H.; Ghaderi, F.; Emadzadeh, M.; Yadollahi, A.; Izadi-Moud, A.; Fazlinezhad, A.; Danesh, M.R. Echocardiographic Assessment of Recovered Patients with Mild COVID-19 Infection: A Case-Control Study. J. Cardiovasc. Echogr. 2024, 34, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Olszanecka, A.; Wojciechowska, W.; Bednarek, A.; Kusak, P.; Wizner, B.; Terlecki, M.; Stolarz-Skrzypek, K.; Klocek, M.; Drożdż, T.; Sładek, K.; et al. Serial Echocardiographic Evaluation of COVID-19 Patients without Prior History of Structural Heart Disease: A 1-Year Follow-up CRACoV-HHS Study. Front. Cardiovasc. Med. 2023, 10, 1230669. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Oz, A.G.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342. [Google Scholar] [CrossRef]
- Landler, N.E.; Olsen, F.J.; Christensen, J.; Bro, S.; Feldt-Rasmussen, B.; Hansen, D.; Kamper, A.-L.; Christoffersen, C.; Ballegaard, E.L.F.; Sørensen, I.M.H.; et al. Associations Between Albuminuria, Estimated GFR and Cardiac Phenotype in a Cohort with Chronic Kidney Disease: The CPH-CKD ECHO Study. J. Card. Fail. 2022, 28, 1615–1627. [Google Scholar] [CrossRef]
- Paoletti, E.; Bellino, D.; Cassottana, P.; Rolla, D.; Cannella, G. Left Ventricular Hypertrophy in Nondiabetic Predialysis CKD. Am. J. Kidney Dis. 2005, 46, 320–327. [Google Scholar] [CrossRef]
- Bansal, N.; Keane, M.; Delafontaine, P.; Dries, D.; Foster, E.; Gadegbeku, C.A.; Go, A.S.; Hamm, L.L.; Kusek, J.W.; Ojo, A.O.; et al. A Longitudinal Study of Left Ventricular Function and Structure from CKD to ESRD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2013, 8, 355–362. [Google Scholar] [CrossRef]
- Cai, Q.-Z.; Lu, X.-Z.; Lu, Y.; Wang, A.Y.-M. Longitudinal Changes of Cardiac Structure and Function in CKD (CASCADE Study). J. Am. Soc. Nephrol. 2014, 25, 1599–1608. [Google Scholar] [CrossRef]
- Tanasa, A.; Tapoi, L.; Ureche, C.; Sascau, R.; Statescu, C.; Covic, A. Left Atrial Strain: A Novel “Biomarker” for Chronic Kidney Disease Patients? Echocardiography 2021, 38, 2077–2082. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Hu, Y.-A.; Chen, Y.-T.; Yu, W.-C.; Lin, C.-C.; Li, S.-Y. Beyond Prevalence: Significance and Differential Impact of Echocardiographic Abnormalities in Dialysis Patients. J. Nephrol. 2024, 37, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J.Ž. Long COVID: A Clinical Update. Lancet 2024, 404, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Filev, R.; Lyubomirova, M.; Bogov, B.; Kalinov, K.; Hristova, J.; Svinarov, D.; Garev, A.; Rostaing, L. Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) for Patients—3-Year Follow-Up of Patients with Chronic Kidney Disease. Biomedicines 2024, 12, 1259. [Google Scholar] [CrossRef]
- Mahalingasivam, V.; Faucon, A.-L.; Sjölander, A.; Bosi, A.; González-Ortiz, A.; Lando, S.; Fu, E.L.; Nitsch, D.; Bruchfeld, A.; Evans, M.; et al. Kidney Function Decline After COVID-19 Infection. JAMA Netw. Open 2024, 7, e2450014. [Google Scholar] [CrossRef]
- Tapoi, L.; Apetrii, M.; Dodi, G.; Nistor, I.; Voroneanu, L.; Siriteanu, L.; Onofriescu, M.; Kanbay, M.; Covic, A. Long-Term Cardio-Vascular Risk Assessment in Chronic Kidney Disease and Kidney Transplanted Patients Following SARS-CoV-2 Disease: Protocol for Multi-Center Observational Match Controlled Trial. BMC Nephrol. 2022, 23, 176. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected: Interim Guidance, 13 March 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://iris.who.int/handle/10665/331446 (accessed on 2 November 2024).
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Shafiabadi Hassani, N.; Shojaee, A.; Khodaprast, Z.; Sepahvandi, R.; Shahrestanaki, E.; Rastad, H. Echocardiographic Features of Cardiac Injury Related to COVID-19 and Their Prognostic Value: A Systematic Review. J. Intensive Care Med. 2021, 36, 500–508. [Google Scholar] [CrossRef]
- Skulstad, H.; Cosyns, B.; Popescu, B.A.; Galderisi, M.; Salvo, G.D.; Donal, E.; Petersen, S.; Gimelli, A.; Haugaa, K.H.; Muraru, D.; et al. COVID-19 Pandemic and Cardiac Imaging: EACVI Recommendations on Precautions, Indications, Prioritization, and Protection for Patients and Healthcare Personnel. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 592–598. [Google Scholar] [CrossRef]
- Kaya, H.; Asoglu, R.; Afsin, A.; Tibilli, H.; Kurt, E.; Kafadar, S.; Gulacti, U.; Kafadar, H. Evaluation of Myocardial Performance Index in Patients with COVID-19: An Echocardiographic Follow-up Study. Rev. Port. Cardiol. 2022, 41, 455–461. [Google Scholar] [CrossRef]
- Tryfou, E.S.; Kostakou, P.M.; Chasikidis, C.G.; Kostopoulos, V.S.; Serafetinidis, I.I.; Ferdianaki, E.K.; Mihas, C.; Olympios, C.D.; Kouris, N.T. Biventricular Myocardial Function in COVID-19 Recovered Patients Assessed by Speckle Tracking Echocardiography: A Prospective Cohort Echocardiography Study. Int. J. Cardiovasc. Imaging 2021, 38, 995. [Google Scholar] [CrossRef]
- Kunal, S.; Gupta, M.D.; Faizuddin, M.; Mp, G.; Bansal, A.; Batra, V.; Safal; Muduli, S.; Yusuf, J. Serial Evaluation of Biventricular Function in COVID-19 Recovered Patients Using Speckle Tracking Echocardiography. Indian Heart J. 2024, 76, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Baruch, G.; Rothschild, E.; Sadon, S.; Szekely, Y.; Lichter, Y.; Kaplan, A.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; et al. Evolution of Right and Left Ventricle Routine and Speckle-Tracking Echocardiography in Patients Recovering from Coronavirus Disease 2019: A Longitudinal Study. Eur. Heart J. Cardiovasc. Imaging 2021, 23, jeab190. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.S.; Bui, Q.M.; King, K.; DeMaria, A.; Daniels, L.B. Subclinical Left Ventricular Dysfunction in COVID-19. IJC Heart Vasc. 2021, 34, 100770. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Su, C.-T.; Sung, J.-M.; Wang, S.P.H.; Su, Y.-R.; Yang, C.-S.; Tsai, L.-M.; Chen, J.-H.; Tsai, W.-C. Association of Left Ventricular Longitudinal Strain with Mortality among Stable Hemodialysis Patients with Preserved Left Ventricular Ejection Fraction. Clin. J. Am. Soc. Nephrol. 2013, 8, 1564–1574. [Google Scholar] [CrossRef]
- Sulemane, S.; Panoulas, V.F.; Bratsas, A.; Grapsa, J.; Brown, E.A.; Nihoyannopoulos, P. Subclinical Markers of Cardiovascular Disease Predict Adverse Outcomes in Chronic Kidney Disease Patients with Normal Left Ventricular Ejection Fraction. Int. J. Cardiovasc. Imaging 2017, 33, 687–698. [Google Scholar] [CrossRef]
- Caiado, L.D.C.; Azevedo, N.C.; Azevedo, R.R.C.; Caiado, B.R. Cardiac Involvement in Patients Recovered from COVID-19 Identified Using Left Ventricular Longitudinal Strain. J. Echocardiogr. 2022, 20, 51–56. [Google Scholar] [CrossRef]
- Vernooij, R.W.M.; van Ommen, A.-M.L.N.; Valstar, G.B.; Cramer, M.J.; Teske, A.J.; Menken, R.; Hofstra, L.; Rutten, F.H.; Bots, M.L.; den Ruijter, H.M.; et al. Association of Mild Kidney Dysfunction with Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction. ESC Heart Fail. 2024, 11, 315–326. [Google Scholar] [CrossRef]
- Porras, C.P.; Dal Canto, E.; van Ommen, A.-M.L.; Handoko, M.L.; Haitjema, S.; de Groot, M.C.H.; Bots, M.L.; Verhaar, M.C.; Vernooij, R.W.M. Left Ventricular Diastolic Dysfunction across Levels of Kidney Function: A Cross-Sectional Study Based on Routine Clinical Practice Data. J. Clin. Med. 2024, 13, 5313. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, Z.; Meulendijks, E.R.; Baylan, U.; Waas, I.S.E.; Bugiani, M.; Tuinman, P.R.; Fronczek, J.; Heunks, L.M.A.; de Groot, J.R.; et al. Atrial Inflammation and Microvascular Thrombogenicity Are Increased in Deceased COVID-19 Patients. Cardiovasc. Pathol. 2023, 64, 107524. [Google Scholar] [CrossRef]
- Bokhari, S.R.; Mansur, A.; Khan Assir, M.Z.; Ittifaq, A.; Sarwar, S. Echocardiographic Evaluation of Left Atrial Volume Index in Patients with Chronic Kidney Disease. Saudi J. Kidney Dis. Transpl. 2020, 31, 750–758. [Google Scholar] [CrossRef]
- Zochios, V.; Parhar, K.; Tunnicliffe, W.; Roscoe, A.; Gao, F. The Right Ventricle in ARDS. Chest 2017, 152, 181–193. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, F.M.A.; Vos, J.L.; Koop, Y.; van Dijk, A.P.J.; Duijnhouwer, A.L.; de Mast, Q.; van de Veerdonk, F.L.; Bosch, F.; Kok, B.; Netea, M.G.; et al. Cardiac Function in Relation to Myocardial Injury in Hospitalised Patients with COVID-19. Neth. Heart J. 2020, 28, 410. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.; Singh, S.; Garfield, B.; Morosin, M.; Surkova, E.; Mandalia, M.S.; Dias, B.; Androulakis, E.; Price, L.C.; McCabe, C.; et al. Right Ventricular Dysfunction in Critically Ill COVID-19 ARDS. Int. J. Cardiol. 2020, 327, 251. [Google Scholar] [CrossRef]
- Dini, F.L.; Demmer, R.T.; Simioniuc, A.; Morrone, D.; Donati, F.; Guarini, G.; Orsini, E.; Caravelli, P.; Marzilli, M.; Colombo, P.C. Right Ventricular Dysfunction Is Associated with Chronic Kidney Disease and Predicts Survival in Patients with Chronic Systolic Heart Failure. Eur. J. Heart Fail. 2012, 14, 287–294. [Google Scholar] [CrossRef]
- Husain-Syed, F.; DiFrancesco, M.F.; Deo, R.; Barr, R.G.; Scialla, J.J.; Bluemke, D.A.; Kronmal, R.A.; Lima, J.A.C.; Praestgaard, A.; Tracy, R.P.; et al. Associations between eGFR and Albuminuria with Right Ventricular Measures: The MESA-Right Ventricle Study. Clin. Kidney J. 2023, 16, 1508–1520. [Google Scholar] [CrossRef]
- Tamulėnaitė, E.; Žvirblytė, R.; Ereminienė, R.; Žiginskienė, E.; Ereminienė, E. Changes of Left and Right Ventricle Mechanics and Function in Patients with End-Stage Renal Disease Undergoing Haemodialysis. Medicina 2018, 54. [Google Scholar] [CrossRef]
- Martha, J.W.; Pranata, R.; Wibowo, A.; Lim, M.A. Tricuspid Annular Plane Systolic Excursion (TAPSE) Measured by Echocardiography and Mortality in COVID-19: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 105, 351. [Google Scholar] [CrossRef]
- Ingec, M.; Oguz, E.G.; Yildirim, T.; Ulas, T.; Horoz, M. The Effect of Hemodialysis on Cardiac Enzyme Levels and Echocardiographic Parameters. Int. J. Artif. Organs 2014, 37, 513–520. [Google Scholar] [CrossRef]
- Jacobs, L.H.; van de Kerkhof, J.J.; Mingels, A.M.; Passos, V.L.; Kleijnen, V.W.; Mazairac, A.H.; van der Sande, F.M.; Wodzig, W.K.; Konings, C.J.; Leunissen, K.M.; et al. Inflammation, Overhydration and Cardiac Biomarkers in Haemodialysis Patients: A Longitudinal Study. Nephrol. Dial. Transplant. 2010, 25, 243–248. [Google Scholar] [CrossRef]
- Laveborn, E.; Lindmark, K.; Skagerlind, M.; Stegmayr, B. NT-proBNP and Troponin T Levels Differ after Haemodialysis with a Low versus High Flux Membrane. Int. J. Artif. Organs 2015, 38, 69–75. [Google Scholar] [CrossRef]
- Gangji, A.S.; Brimble, K.S.; Margetts, P.J. Association between Markers of Inflammation, Fibrosis and Hypervolemia in Peritoneal Dialysis Patients. Blood Purif. 2009, 28, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Stegmayr, B.; Brännström, T.; Forsberg, U.; Jonson, P.; Stegmayr, C.; Hultdin, J. Microbubbles of Air May Occur in the Organs of Hemodialysis Patients. ASAIO J. 2012, 58, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Chaikijurajai, T.; Choles, H.R.; Tang, W.H.W. Do Natriuretic Peptide Measurements Provide Insights into Management of End-Stage Renal Disease Patients Undergoing Dialysis? Curr. Heart Fail. Rep. 2020, 17, 449–456. [Google Scholar] [CrossRef]
- Takase, H.; Dohi, Y. Kidney Function Crucially Affects B-Type Natriuretic Peptide (BNP), N-Terminal proBNP and Their Relationship. Eur. J. Clin. Investig. 2014, 44, 303–308. [Google Scholar] [CrossRef]
- Jafri, L.; Kashif, W.; Tai, J.; Siddiqui, I.; Azam, I.; Shahzad, H.; Ghani, F. B-Type Natriuretic Peptide versus Amino Terminal pro-B Type Natriuretic Peptide: Selecting the Optimal Heart Failure Marker in Patients with Impaired Kidney Function. BMC Nephrol. 2013, 14, 117. [Google Scholar] [CrossRef]
| All Patients n = 206 | Age (Years) | Male (%) | Smoker (%) | HTN (%) | DM (%) | Afib (%) | HF (%) | CAD(%) | Pulmonary Disease (%) |
|---|---|---|---|---|---|---|---|---|---|
| COVID-19 n = 121 | 58.87 ± 15.27 | 52.1 | 6.6 | 82.6 | 33.1 | 20.7 | 47.9 | 32.2 | 6.6 |
| Control n = 85 | 54.25 ± 15.53 | 55.3 | 16.5 | 75.3 | 17.6 | 11.8 | 29.4 | 15.3 | 5.9 |
| p = 0.04 | p = 0.64 | p = 0.02 | p = 0.19 | p = 0.01 | p = 0.09 | p = 0.01 | p = 0.01 | p = 0.83 | |
| HD patients n = 134 | |||||||||
| COVID-19 n = 79 | 62.72 ± 14.45 | 48.1 | 7.6 | 82.3 | 38.0 | 27.8 | 55.7 | 39.2 | 8.9 |
| Control n = 55 | 59.93 ± 62.72 | 50.9 | 21.8 | 76.4 | 20.0 | 18.2 | 38.2 | 20.0 | 5.5 |
| p = 0.22 | p = 0.74 | p = 0.01 | p = 0.40 | p = 0.02 | p = 0.19 | p = 0.04 | p = 0.01 | p = 0.46 |
| Echocardiographic Parameter | COVID-19 Group N= 121 | CONTROL Group N = 85 | p Value |
|---|---|---|---|
| Left ventricle | |||
| IVS (mm) | 12.14 ± 2.22 | 12.48 ± 2.29 | 0.23 |
| LVPW (mm) | 11.71 ± 2.18 | 12.47 ± 2.16 | 0.01 |
| LVEDD (mm) | 46.73 ± 7.36 | 44.69 ± 5.92 | 0.03 |
| LVESD (mm) | 30.77 ± 7.25 | 27.01 ± 5.26 | <0.01 |
| LV EDV (ml) | 112.03 ± 49.88 | 92.50 ± 33.57 | 0.01 |
| LV ESV (ml) | 51.37 ± 34.74 | 39.72 ± 18.96 | 0.01 |
| LV EF (%) | 55.52 ± 10.15 | 57.36 ± 8.77 | 0.19 |
| 57 (IQR 50.75–62.25) | 58 (IQR 53–63) | ||
| LV GLS (%) | −16.86 ± 4.44 | −17.72 ± 2.81 | 0.12 |
| −18.00 (IQR (−20.00, −16.00) | −18.5 (IQR −20.875, −16.125) | ||
| LV Tei index | 0.60 ± 0.16 | 0.50 ± 0.12 | <0.01 |
| Right ventricle | |||
| TAPSE (mm) | 21.61 ± 4.81 | 22.17 ± 4.64 | 0.56 |
| 21 (IQR 18–24) | 21 (IQR 18–24) | ||
| FAC (%) | 40.56 ± 10.82 | 45.02 ±8.06 | 0.01 |
| RVFWLS (%) | −18.01 ± 5.27 | −20.46 ± 2.93 | 0.01 |
| −18 (IQR −21, −15) | −21.00 (IQR −23.50, −18.50) | ||
| Tricuspid S’ velocity (cm/s) | 13.12 ± 2.91 | 12.76 ± 2.93 | 0.38 |
| RV Tei Index | 0.60 ± 0.17 | 0.54 ± 0.16 | 0.01 |
| PAAT (ms) | 96.10 ± 26.17 | 100.40 ± 25.56 | 0.24 |
| TR Vmax (m/s) | 2.16 ± 0.72 | 2.12 ± 0.52 | 0.65 |
| PASP (mmHg) | 26.31 ± 14.30 | 23.74 ± 10.33 | 0.37 |
| Proximal RVOT (mm) | 34.57 ± 5.00 | 32.94 ± 4.49 | 0.01 |
| RV basal diameter (mm) | 36.58 ± 7.18 | 33.72 ± 6.03 | 0.01 |
| RV mid diameter (mm) | 29.28 ± 7.26 | 25.31 ± 5.95 | <0.01 |
| RV longitudinal diameter mm) | 69.91 ± 10.18 | 69.76 ± 8.80 | 0.91 |
| RV free wall thickness (mm) | 5.96 ± 1.44 | 5.39 ± 1.51 | 0.01 |
| Inferior vena cava (mm) | 16.29 ± 3.41 | 14.79 ± 3.03 | 0.01 |
| Diastolic function | |||
| LAVI (mL/m2) | 43.17 ± 17.84 | 39.07 ± 16.22 | 0.06 |
| DTE (ms) | 196.85 ± 51.41 | 195.33 ± 53.77 | 0.84 |
| E-wave velocity (cm/s) | 86.08 ± 29.57 | 80.10 ± 26.20 | 0.18 |
| A-wave velocity (cm/s) | 87.26 ± 24.25 | 87.64 ± 28.51 | 0.62 |
| E/A | 0.94 ± 0.37 | 0.92 ± 0.41 | 0.56 |
| medial e′ velocity (cm/s) | 6.98 ± 2.08 | 6.97 ± 2.28 | 0.84 |
| lateral e′ velocity (cm/s) | 9.69 ± 3.17 | 9.73 ± 3.21 | 0.92 |
| E/e′ | 11.19 ± 4.29 | 10.68 ± 4.89 | 0.24 |
| Biomarkers | COVID-19 Group N = 88 | CONTROL Group N = 75 | p Value |
|---|---|---|---|
| Baseline | |||
| TnI (pg/mL) | 53.02 ± 60.42 | 14.15 ± 14.70 | 0.01 |
| NT-proBNP (ng/mL) | 4.69 ± 6.16 | 2.69 ± 3.11 | 0.37 |
| 6 months | |||
| TnI (pg/mL) | 37.82 ± 45.44 | 12.86 ± 15.17 | <0.01 |
| NT-proBNP (ng/mL) | 10.61 ± 37.72 | 5.56 ± 21.81 | <0.01 |
| 12 months | |||
| TnI (pg/mL) | 15.84 ± 15.26 | 17.00 ± 16.19 | 0.21 |
| NT-proBNP (ng/mL) | 7.39 ± 32.95 | 3.74 ± 21.07 | 0.01 |
| Echocardiographic Parameter | COVID-19 Group N = 88 | CONTROL Group N = 75 | p Value |
|---|---|---|---|
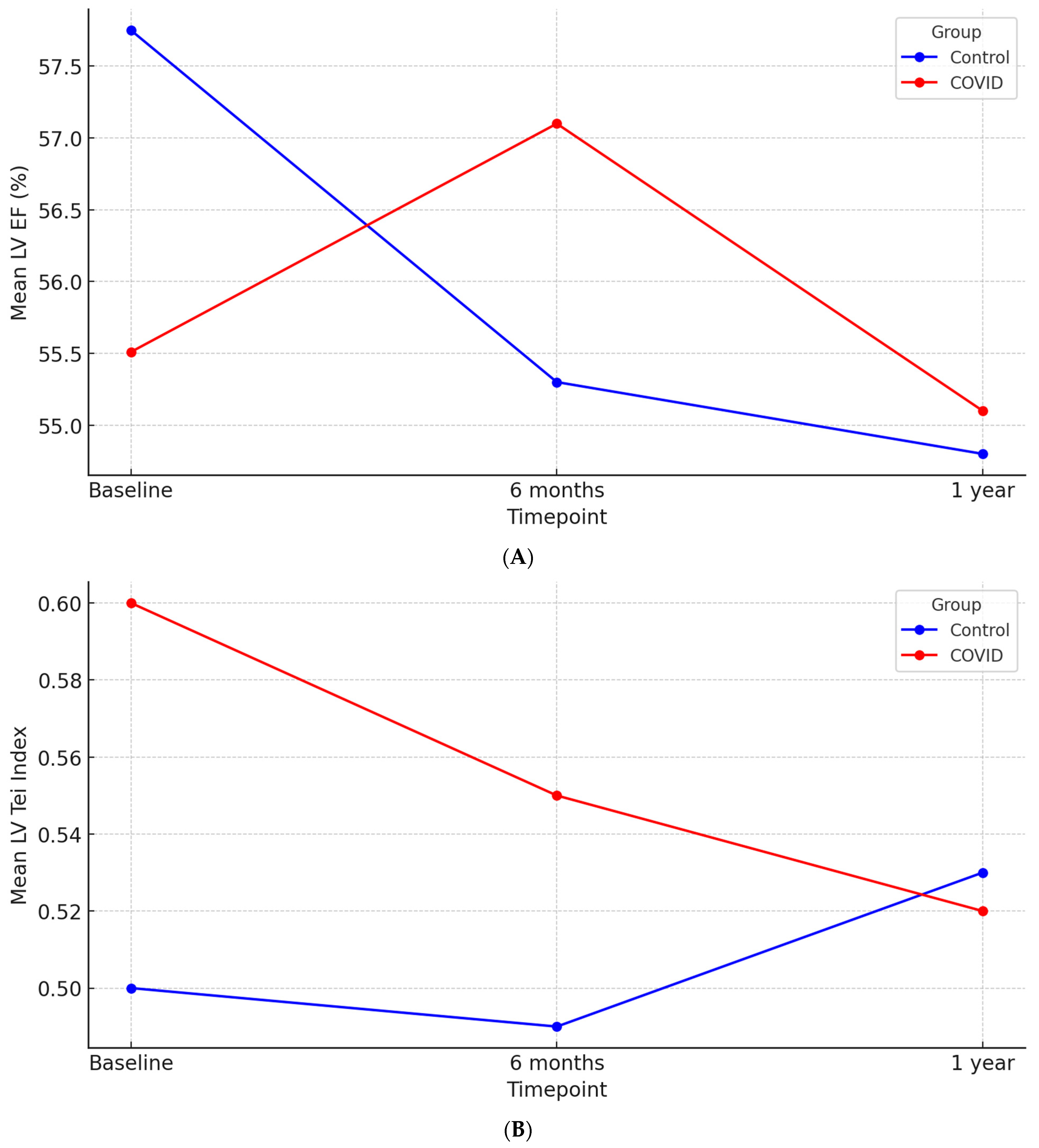
| LVEF (%) | 55.51 ± 10.23 | 57.74 ± 8.87 | 0.11 |
| 57 (IQR 50.50–63.50) | 59 (IQR 53.85–64.15) | ||
| LV GLS (%) | −17.18 ± 4.13 | −18.04 ± 2.69 | 0.14 |
| −18.00 (IQR (−19.94, −16.06) | −19.00 (IQR −21.00, −17.00) | ||
| LV Tei index | 0.59 ± 0.16 | 0.50 ± 0.11 | <0.01 |
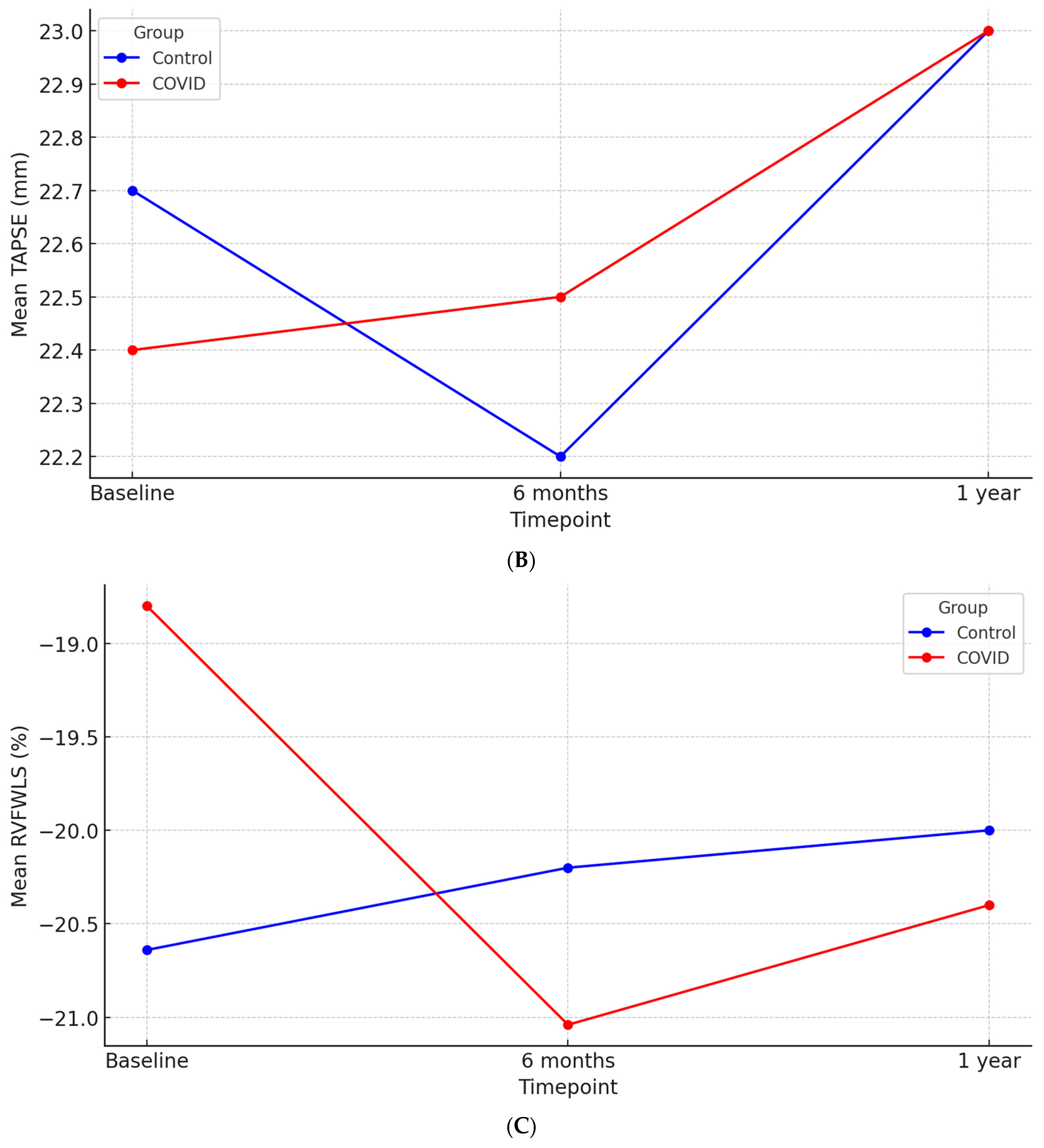
| TAPSE (mm) | 22.40 ± 4.48 | 22.69 ± 4.49 | 0.86 |
| 21.5 (IQR 18.5–24.5) | 22 (IQR 18.5–25.5) | ||
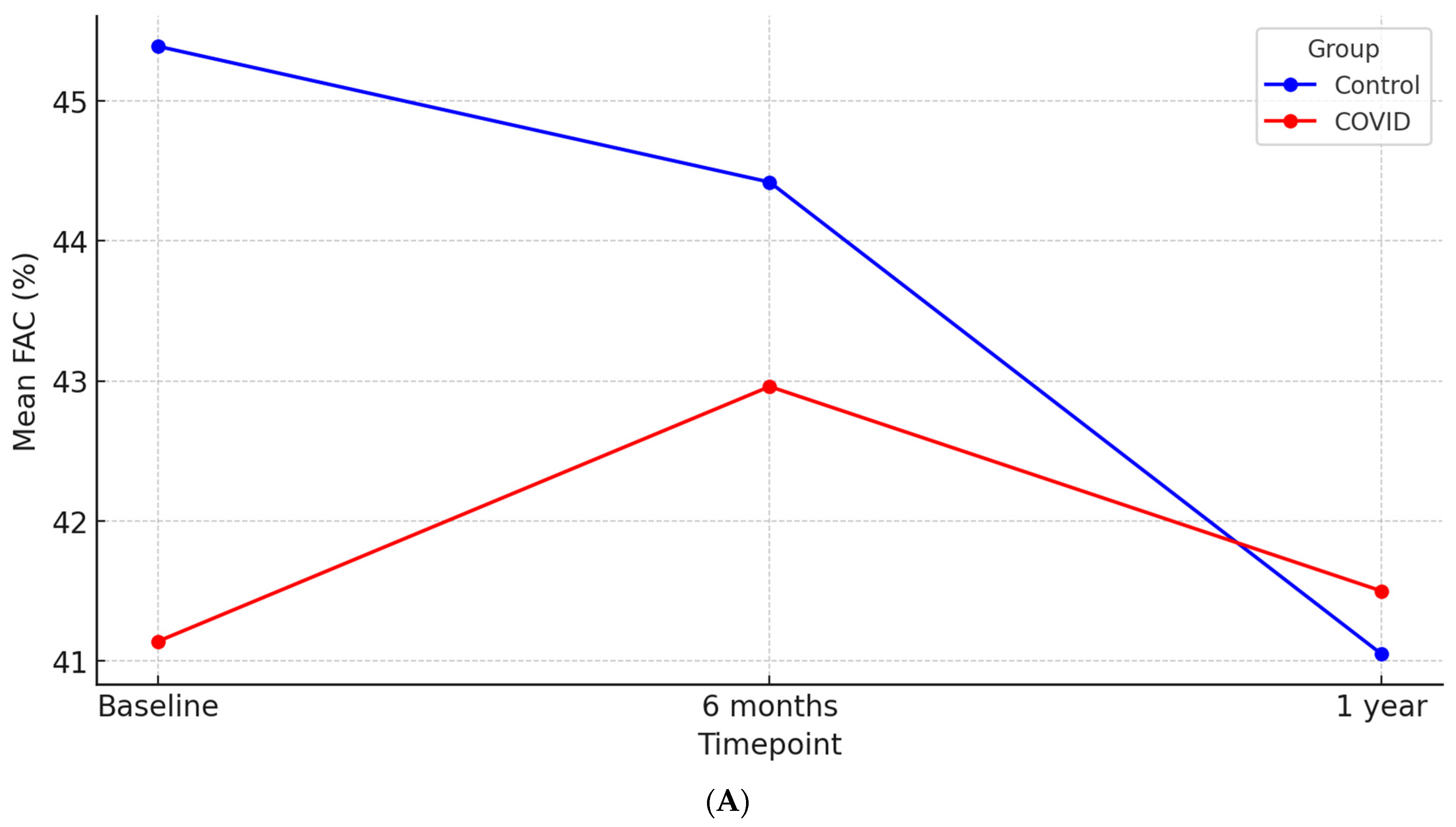
| FAC (%) | 41.14 ± 10.90 | 45.38 ± 8.08 | 0.01 |
| RVFWLS (%) | −18.80 ± 4.47 | −20.64 ± 2.94 | 0.01 |
| −18.60 (IQR −20.60,−16.60) | −21.00 (IQR −23.50,−18.50) | ||
| Tricuspid S’ velocity (cm/s) | 13.51 ± 2.82 | 12.88 ± 2.77 | 0.16 |
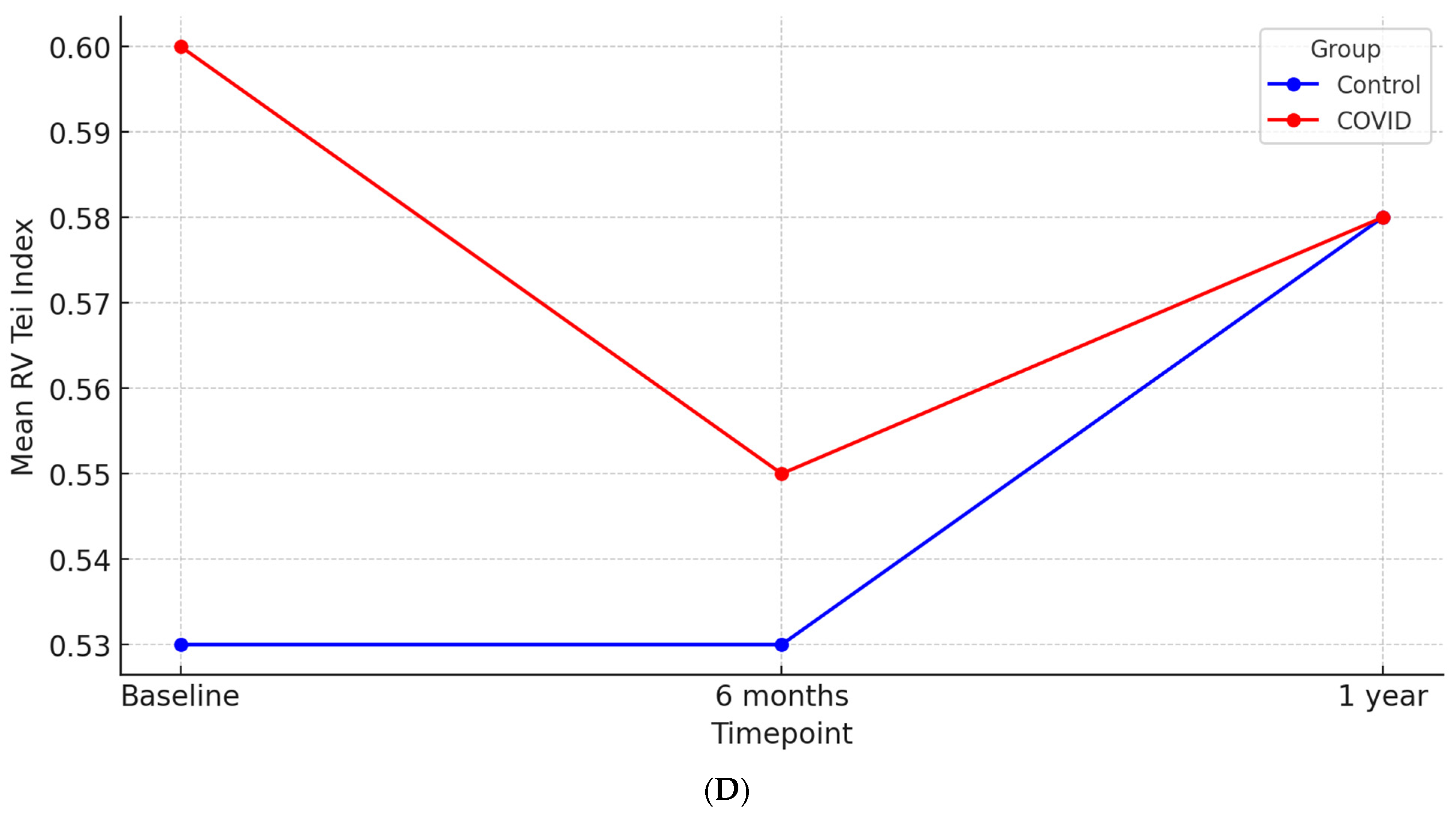
| RV Tei Index | 0.59 ± 0.18 | 0.53 ± 0.15 | 0.01 |
| Echocardiographic Parameter | COVID-19 Group (N = 88) 6 Months | CONTROL Group (N = 75) 6 Months | p Value | COVID-19 Group (N = 88) 12 Months | CONTROL Group (N = 75) 12 Months | p Value |
|---|---|---|---|---|---|---|
| LVEF (%) | 57.07 ± 9.77 | 55.34 ± 7.70 | 0.16 | 55.11 ± 9.12 | 54.85 ± 7.70 | 0.50 |
| 57.5 (IQR 51.5–63.50) | 54.5 (IQR 50.05–58.95) | 56.45 (IQR 50.95–61.95) | 55 (IQR 50.35–59.65) | |||
| LV GLS (%) | −18.47 ± 3.79 | −18.61 ± 2.93 | 0.82 | −16.76 ± 3.62 | −17.53 ± 3.04 | 0.36 |
| LV Tei index | 0.59 ± 0.15 | 0.49 ± 0.10 | 0.01 | 0.51 ± 0.13 | 0.53 ± 0.11 | 0.29 |
| TAPSE (mm) | 22.51 ± 4.02 | 22.20 ± 4.21 | 0.64 | 22.51± 4.28 | 23.12 ± 4.01 | 0.35 |
| FAC (%) | 42.95 ± 9.92 | 44.53 ±9.28 | 0.29 | 41.49 ± 10.08 | 41.05 ±8.94 | 0.76 |
| RVFWLS (%) | −21.03 ± 3.73 | −20.15 ± 7.72 | 0.67 | −20.41 ± 4.44 | −20.01 ± 8.19 | 0.65 |
| −21.00 (IQR −23.08, −18.93) | −21.00 (IQR −22.5, −19.5) | −21 (IQR −23.5, −18.5 | −21 (IQR −23.25, −18.75) | |||
| Tricuspid S’ velocity (cm/s) | 12.78 ± 2.94 | 12.70 ± 3.27 | 0.86 | 12.30 ± 2.95 | 12.76 ± 3.17 | 0.34 |
| RV Tei Index | 0.54 ± 0.14 | 0.53 ± 0.14 | 0.38 | 0.58 ± 0.19 | 0.58 ± 0.16 | 0.87 |
| PAAT (ms) | 102.18 ± 26.00 | 110.16 ± 24.21 | 0.04 | 98.27 ± 21.62 | 102.29 ± 22.91 | 0.22 |
| Proximal RVOT (mm) | 33.73 ± 4.31 | 31.58 ± 4.23 | 0.01 | 33.06 ± 4.18 | 31.24 ± 4.90 | 0.01 |
| LAVI (ml/m2) | 40.73 ± 14.65 | 34.71 ± 12.64 | 0.01 | 40.26 ± 20.71 | 33.40 ± 13.25 | 0.01 |
| E/e’ | 11.15 ± 3.51 | 10.38 ± 4.15 | 0.07 | 11.38 ± 4.16 | 10.78 ± 4.48 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliu, L.; Diaconu, A.; Onofriescu, M.; Dodi, G.; Covic, A.; Avanu, A.E.; Voroneanu, L.; Vasiliu, V.; Kanbay, M.; Sascău, R.A.; et al. Long-Term Evolution of Post-COVID-19 Echocardiographic Parameters in Patients with Chronic Kidney Disease: A Prospective Comparative Observational Study. J. Clin. Med. 2025, 14, 1823. https://doi.org/10.3390/jcm14061823
Vasiliu L, Diaconu A, Onofriescu M, Dodi G, Covic A, Avanu AE, Voroneanu L, Vasiliu V, Kanbay M, Sascău RA, et al. Long-Term Evolution of Post-COVID-19 Echocardiographic Parameters in Patients with Chronic Kidney Disease: A Prospective Comparative Observational Study. Journal of Clinical Medicine. 2025; 14(6):1823. https://doi.org/10.3390/jcm14061823
Chicago/Turabian StyleVasiliu, Laura, Anca Diaconu, Mihai Onofriescu, Gianina Dodi, Alexandra Covic, Alexandra E. Avanu, Luminita Voroneanu, Vlad Vasiliu, Mehmet Kanbay, Radu A. Sascău, and et al. 2025. "Long-Term Evolution of Post-COVID-19 Echocardiographic Parameters in Patients with Chronic Kidney Disease: A Prospective Comparative Observational Study" Journal of Clinical Medicine 14, no. 6: 1823. https://doi.org/10.3390/jcm14061823
APA StyleVasiliu, L., Diaconu, A., Onofriescu, M., Dodi, G., Covic, A., Avanu, A. E., Voroneanu, L., Vasiliu, V., Kanbay, M., Sascău, R. A., Stătescu, C., & Covic, A. C. (2025). Long-Term Evolution of Post-COVID-19 Echocardiographic Parameters in Patients with Chronic Kidney Disease: A Prospective Comparative Observational Study. Journal of Clinical Medicine, 14(6), 1823. https://doi.org/10.3390/jcm14061823








