Pruritus and Neuropsychiatric Symptoms Among Patients with Darier Disease—An Overlooked and Interconnected Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection and Analysis
3. Results
3.1. Patient Characteristics
3.2. Disease Characteristics
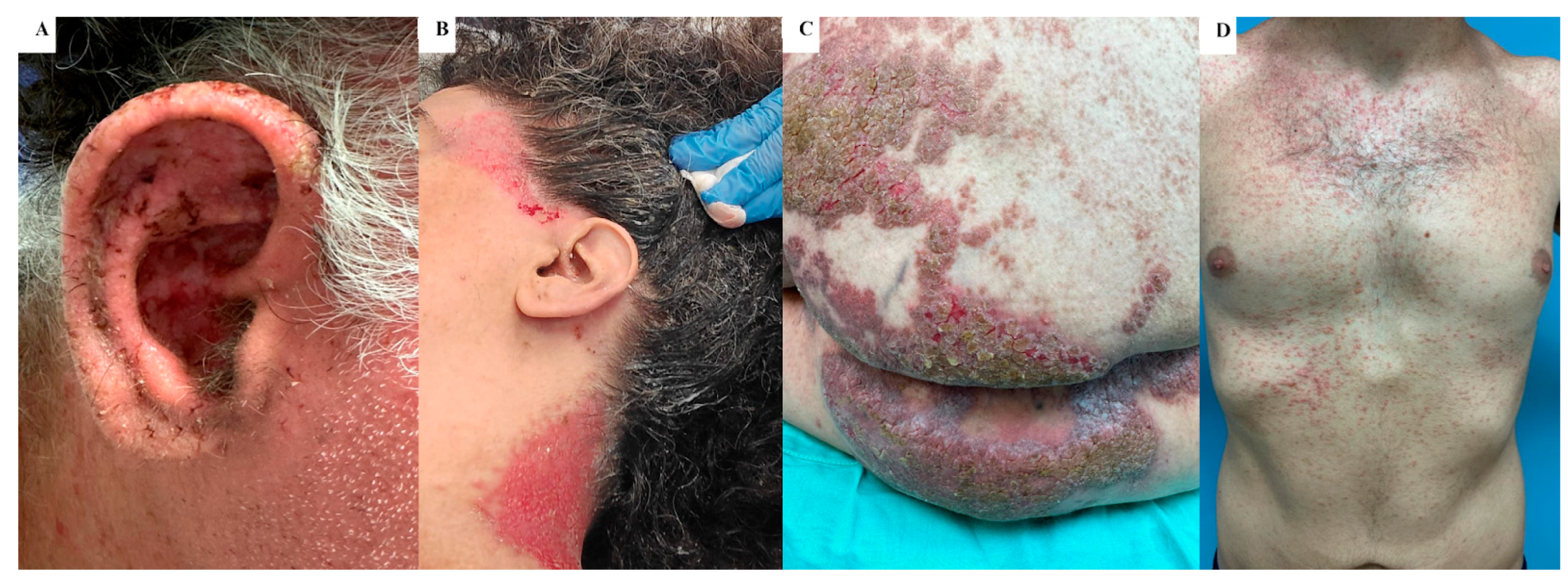
3.3. Outcomes
4. Discussion
4.1. Summary
4.2. Darier Disease and Neuropsychiatric Conditions
4.3. Pruritus and Darier Disease
4.4. Clinical Applications and Treatment of Pruritus in Darier Disease
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takagi, A.; Kamijo, M.; Ikeda, S. Darier disease. J. Dermatol. 2016, 43, 275–279. [Google Scholar] [CrossRef]
- Tu, C.-L.; Bikle, D.D. Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function. Best Pr. Res. Clin. Endocrinol. Metab. 2013, 27, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Kanakpur, S.H.; Caculo, D.U. Rare ocular manifestations in keratosis follicularis (Darier–White disease). Indian J. Ophthalmol. 2017, 65, 874–876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burge, S.M.; Wilkinson, J.D. Darier-White disease: A review of the clinical features in 163 patients. J. Am. Acad. Dermatol. 1992, 27, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Bachar-Wikström, E.; Wikström, J. Darier Disease—A Multi-organ Condition? Acta Derm. Venereol. 2021, 101, adv00430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinikumpu, S.-P.; Jokelainen, J.; Tasanen, K.; Timonen, M.; Huilaja, L. Association between Pruritus and Psychosocial Well-being: A Population-based Study among 6,809 Subjects. Acta Derm. Venereol. 2023, 103, adv00837. [Google Scholar] [CrossRef]
- Xiong, G.; Abu-Hilal, M. Itch and Insomnia in Patients with Prurigo Nodularis and Other Dermatologic Conditions. J. Cutan. Med. Surg. 2024, 28, 591–592. [Google Scholar] [CrossRef]
- Dodiuk-Gad, R.; Cohen-Barak, E.; Khayat, M.; Milo, H.; Amariglio-Diskin, L.; Danial-Faran, N.; Sah, M.; Ziv, M.; Shani-Adir, A.; Amichai, B.; et al. Darier disease in Israel: Combined evaluation of genetic and neuropsychiatric aspects. Br. J. Dermatol. 2015, 174, 562–568. [Google Scholar] [CrossRef]
- Gordon-Smith, K.; Jones, L.; Burge, S.; Munro, C.; Tavadia, S.; Craddock, N. The neuropsychiatric phenotype in Darier disease. Br. J. Dermatol. 2010, 163, 515–522. [Google Scholar] [CrossRef]
- Finlay, A.; Khan, G. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Yeshurun, A.; Ziv, M.; Cohen-Barak, E.; Vered, S.; Rozenman, D.; Sah, M.; Khayat, M.; Polyakov, O.; Amichai, B.; Zlotogorski, A.; et al. An Update on the Cutaneous Manifestations of Darier Disease. J. Cutan. Med. Surg. 2021, 25, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Dodiuk-Gad, R.; Cohen-Barak, E.; Ziv, M.; Shani-Adir, A.; Amichai, B.; Zlotogorski, A.; Shalev, S.; Rozenman, D. Health-related quality of life among Darier’s disease patients. J. Eur. Acad. Dermatol. Venereol. 2011, 27, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Dodiuk-Gad, R.; Lerner, M.; Breznitz, Z.; Cohen-Barak, E.; Ziv, M.; Shani-Adir, A.; Amichai, B.; Zlotogorski, A.; Shalev, S.; Rozenman, D. Learning disabilities in Darier’s disease patients. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 314–319. [Google Scholar] [CrossRef]
- Reiter, O.; Leshem, A.; Alexander-Shani, R.; Brandwein, M.; Cohen, Y.; Yeshurun, A.; Ziv, M.; Elinav, E.; Hodak, E.; Dodiuk-Gad, R.P. Bacterial Skin Dysbiosis in Darier Disease. Dermatology 2024, 240, 443–452. [Google Scholar] [CrossRef]
- Sakuntabhai, A.; Burge, S.; Monk, S.; Hovnanian, A. Spectrum of novel ATP2A2 mutations in patients with Darier’s disease. Hum. Mol. Genet. 1999, 8, 1611–1619. [Google Scholar] [CrossRef]
- Barfield, R.L.; Barrett, K.R.; Moon, C.M.; David-Bajar, K. Pruritic linear papules on a 75-year-old woman: A case of localized Darier-White disease. Cutis 2002, 70, 225–228. [Google Scholar]
- Magdaleno-Tapial, J.; Valenzuela-Oñate, C.; Martínez-Domenech, Á.; García-Legaz-Martínez, M.; Sánchez-Carazo, J.L.; Miquel, V.A. A pruriginous eruption on the back, worsening in the summer. Clin. Exp. Dermatol. 2018, 44, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Sartori-Valinotti, J.; Peters, M.; Wieland, C. Segmental type 1 D arier disease: A case series highlighting late-onset disease. Br. J. Dermatol. 2015, 173, 587–589. [Google Scholar] [CrossRef]
- Rogner, D.; Heimerl, L.; Biedermann, T.; Sattler, E.; Zink, A. Unmet Needs in Darier’s Disease from a Patient’s Perspective: Lessons Learnt from the German Registry. Acta Derm. Venereol. 2024, 104, adv19663. [Google Scholar] [CrossRef]
- Amar, Y.; Rogner, D.; Silva, R.L.; Foesel, B.U.; Ud-Dean, M.; Lagkouvardos, I.; Steimle-Grauer, S.A.; Niedermeier, S.; Kublik, S.; Jargosch, M.; et al. Darier’s disease exhibits a unique cutaneous microbial dysbiosis associated with inflammation and body malodour. Microbiome 2023, 11, 162, Erratum in Microbiome 2023, 11, 206. 10.1186/s40168-023-01665-0. [Google Scholar] [CrossRef]
- Fourzali, K.; Yosipovitch, G. Genodermatoses with itch as a prominent feature. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Seli, D.; Ellis, K.T.; Goldust, M.; Shah, K.; Hu, R.; Zhou, J.; McNiff, J.M.; Choate, K.A. Association of Somatic ATP2A2 Damaging Variants with Grover Disease. JAMA Dermatol. 2023, 159, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Curman, P.; Jebril, W.; Larsson, H.; Bachar-Wikstrom, E.; Cederlöf, M.; Wikstrom, J.D. Darier disease is associated with neurodegenerative disorders and epilepsy. Sci. Rep. 2024, 14, 7109. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Ishiwata, M.; Weitemier, A.Z.; Shoji, H.; Monai, H.; Miyamoto, H.; Yamakawa, K.; Miyakawa, T.; McHugh, T.J.; Kato, T. Brain-specific heterozygous loss-of-function of ATP2A2, endoplasmic reticulum Ca2+ pump responsible for Darier’s disease, causes behavioral abnormalities and a hyper-dopaminergic state. Hum. Mol. Genet. 2021, 30, 1762–1772. [Google Scholar] [CrossRef]
- Curman, P.; Jebril, W.; Larsson, H.; Bachar-Wikström, E.; Cederlöf, M.; Wikström, J.D. Increased risk of depression and anxiety in individuals with Darier disease. Br. J. Dermatol. 2024, 191, 462–463. [Google Scholar] [CrossRef]
- Cederlöf, M.; E Bergen, S.; Långström, N.; Larsson, H.; Boman, M.; Craddock, N.; Östberg, P.; Lundström, S.; Sjölander, A.; Nordlind, K.; et al. The association between Darier disease, bipolar disorder, and schizophrenia revisited: A population-based family study. Bipolar Disord. 2014, 17, 340–344. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Rosenberg, S.S.; Spitzer, N.C. Calcium Signaling in Neuronal Development. Cold Spring Harb. Perspect. Biol. 2011, 3, a004259. [Google Scholar] [CrossRef]
- Spitzer, N.C.; Gu, X.; Olson, E. Action potentials, calcium transients and the control of differentiation of excitable cells. Curr. Opin. Neurobiol. 1994, 4, 70–77. [Google Scholar] [CrossRef]
- Gomez, T.M.; Spitzer, N.C. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature 1999, 397, 350–355. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Wei, H. Calcium Dysregulation in Alzheimer’s Disease: A Target for New Drug Development. J. Alzheimers Dis Parkinsonism. 2017, 7, 374. [Google Scholar] [CrossRef]
- Zaichick, S.V.; McGrath, K.M.; Caraveo, G. The role of Ca2+ signaling in Parkinson’s disease. Dis. Model. Mech. 2017, 10, 519–535. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Calcium signalling and psychiatric disease: Bipolar disorder and schizophrenia. Cell Tissue Res. 2014, 357, 477–492. [Google Scholar] [CrossRef]
- Plein, H.; Berk, M.; Eppel, S.; Butkow, N. Augmented platelet calcium uptake in response to serotonin stimulation in patients with major depression measured using Mn2+ influx and 45Ca2+ uptake. Life Sci. 1999, 66, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Pourtavakoli, A.; Ghafouri-Fard, S. Calcium signaling in neurodevelopment and pathophysiology of autism spectrum disorders. Mol. Biol. Rep. 2022, 49, 10811–10823. [Google Scholar] [CrossRef] [PubMed]
- Ambur, A.; Zaidi, A.; Dunn, C.; Nathoo, R. Impaired calcium signalling and neuropsychiatric disorders in Darier disease: An exploratory review. Exp. Dermatol. 2022, 31, 1302–1310. [Google Scholar] [CrossRef]
- Soares, G.B.; Mahmoud, O.; Yosipovitch, G.; Mochizuki, H. The mind–skin connection: A narrative review exploring the link between inflammatory skin diseases and psychological stress. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 821–834. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef]
- Ahn, J.-W.; Kim, S.-E.; Kim, D.-Y.; Jeong, I.; Kim, S.; Chung, S.; Lee, S.E. Cav3.2 T-Type Calcium Channel Mediates Acute Itch and Contributes to Chronic Itch and Inflammation in Experimental Atopic Dermatitis. J. Investig. Dermatol. 2023, 144, 612–620.e6. [Google Scholar] [CrossRef]
- Mahmoud, O.; Oladipo, O.; Mahmoud, R.H.; Yosipovitch, G. Itch: From the skin to the brain—Peripheral and central neural sensitization in chronic itch. Front. Mol. Neurosci. 2023, 16, 1272230. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Barry, D.M.; Liu, X.-Y.; Yin, S.; Munanairi, A.; Meng, Q.-T.; Cheng, W.; Mo, P.; Wan, L.; Liu, S.-B.; et al. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci. Signal. 2016, 9, ra71. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Bernhard, J.D. Clinical practice. Chronic pruritus. N. Engl. J. Med. 2013, 368, 1625–1634. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2013, 133, 448–460.e7. [Google Scholar] [CrossRef] [PubMed]
- Momose, A.; Kudo, S.; Sato, M.; Saito, H.; Nagai, K.; Katabira, Y.; Funyu, T. Calcium ions are abnormally distributed in the skin of haemodialysis patients with uraemic pruritus. Nephrol. Dial. Transplant. 2004, 19, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Hernandez, L.; Yosipovitch, G.; Sadato, N.; Kakigi, R. The Amygdala Network for Processing Itch in Human Brains. Acta Derm. Venereol. 2020, 100, adv00345. [Google Scholar] [CrossRef]
- Mack, M.R.; Kim, B.S. The Itch–Scratch Cycle: A Neuroimmune Perspective. Trends Immunol. 2018, 39, 980–991. [Google Scholar] [CrossRef]
- Chen, W.-Z.; Shen, T.-Y.; Wang, M.; Yuan, L.; Wang, L.-H.; Ding, W.-Q.; Shi, X.-X.; Wang, X.-F.; Bo, B.-S.; Liang, Z.-F.; et al. An atlas of itch-associated neural dynamics in the mouse brain. Cell Rep. 2023, 42, 113304. [Google Scholar] [CrossRef]
- Schielein, L.; Ziehfreund, S.; Biedermann, T.; Zink, A. ‘When you do not feel comfortable in your skin, you cannot get out of it’; a qualitative interview study exploring psychosocial impact and coping strategies among patients with prurigo nodularis. Br. J. Dermatol. 2024, 191, 845–846. [Google Scholar] [CrossRef]
- Patel, T.; Ishiuji, Y.; Yosipovitch, G. Nocturnal itch: Why do we itch at night? Acta Derm. Venereol. 2007, 87, 295–298. [Google Scholar] [CrossRef]
- Lavery, M.J.; Stull, C.; Kinney, M.O.; Yosipovitch, G. Nocturnal Pruritus: The Battle for a Peaceful Night’s Sleep. Int. J. Mol. Sci. 2016, 17, 425. [Google Scholar] [CrossRef] [PubMed]
- Cavieres-Lepe, J.; Ewer, J. Reciprocal Relationship Between Calcium Signaling and Circadian Clocks: Implications for Calcium Homeostasis, Clock Function, and Therapeutics. Front. Mol. Neurosci. 2021, 14, 666673. [Google Scholar] [CrossRef]
- Krist, A.H.; Tong, S.T.; A Aycock, R.; Longo, D.R. Engaging Patients in Decision-Making and Behavior Change to Promote Prevention. Stud. Health Technol. Inform. 2017, 240, 284–302. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, N.; Gašić, A.; Šitum, M.; Majda, V.; Kolić, M. A Case of Segmental Darier Disease. Acta Dermatovenerol Croat. 2022, 30, 201–202. [Google Scholar] [PubMed]
- Does, A.V.; Ju, T.; Mohsin, N.; Chopra, D.; Yosipovitch, G. How to get rid of itching. Pharmacol. Ther. 2023, 243, 108355. [Google Scholar] [CrossRef]
- Ettinger, M.; Burner, T.; Sharma, A.; Chang, Y.-T.; Lackner, A.; Prompsy, P.; Deli, I.M.; Traxler, J.; Wahl, G.; Altrichter, S.; et al. Th17-associated cytokines IL-17 and IL-23 in inflamed skin of Darier disease patients as potential therapeutic targets. Nat. Commun. 2023, 14, 7470. [Google Scholar] [CrossRef]
- Boehmer, D.; Eyerich, K.; Darsow, U.; Biedermann, T.; Zink, A. Variable response to low-dose naltrexone in patients with Darier disease: A case series. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 950–953. [Google Scholar] [CrossRef]
- Mahmoud, O.; Soares, G.B.; Yosipovitch, G. Transient Receptor Potential Channels and Itch. Int. J. Mol. Sci. 2022, 24, 420. [Google Scholar] [CrossRef]
- Mastorino, L.; Rosset, F.; Gelato, F.; Ortoncelli, M.; Cavaliere, G.; Quaglino, P.; Ribero, S. Chronic Pruritus in Atopic Patients Treated with Dupilumab: Real Life Response and Related Parameters in 354 Patients. Pharmaceuticals 2022, 15, 883. [Google Scholar] [CrossRef]
- Holt, S.; Saju, S.; Miller, R. A case of Darier disease with perioral cutaneous cobblestoning treated with dupilumab. JAAD Case Rep. 2024, 49, 68–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schut, C.; Mollanazar, N.; Kupfer, J.; Gieler, U.; Yosipovitch, G. Psychological Interventions in the Treatment of Chronic Itch. Acta Derm. Venereol. 2016, 96, 157–161. [Google Scholar] [CrossRef] [PubMed]
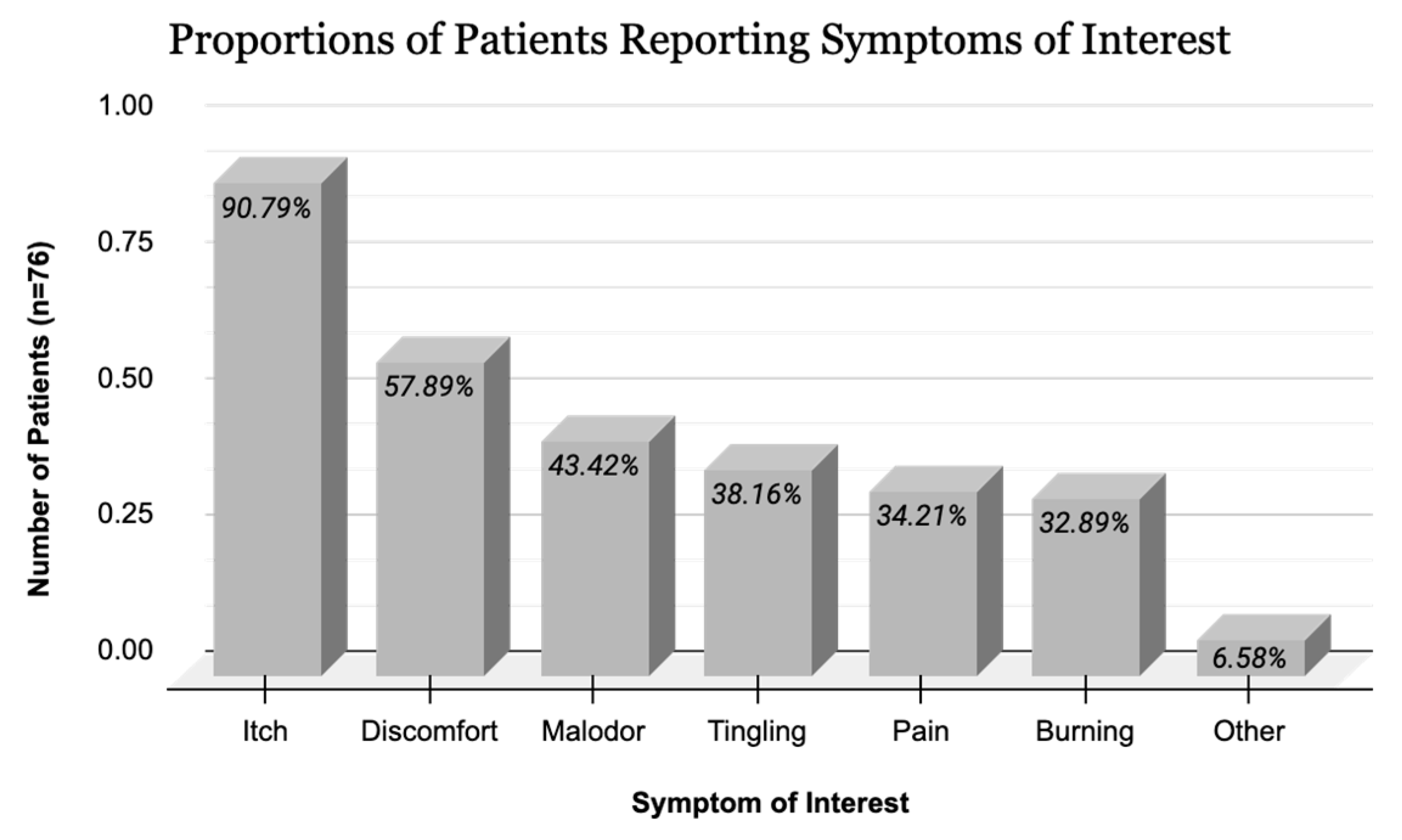
| Disease Severity (n = 75) | p Value 1 | |||
|---|---|---|---|---|
| Mild n = 25 N (%) | Moderate n = 37 N (%) | Severe n = 13 N (%) | ||
| Itch | 21 (84.0) | 36 (97.3) | 12 (92.3) | 0.175 |
| Tingling | 9 (36.0) | 16 (43.2) | 3 (23.1) | 0.427 |
| Pain | 5 (20.0) | 14 (37.8) | 7 (53.9) | 0.098 |
| Discomfort | 11 (44.0) | 25 (67.6) | 7 (53.9) | 0.177 |
| Malodor | 3 (12.0) | 21 (56.8) | 9 (69.2) | <0.001 * |
| Burning | 6 (24.0) | 17 (46.0) | 2 (15.4) | 0.064 |
| %BSA Affected (n = 71) | p Value 1 | |||||
|---|---|---|---|---|---|---|
| N | Mean | STD | Median | |||
| Itch | No | 6 | 12.8 | 15.6 | 7.0 | 0.109 |
| Yes | 66 | 21.2 | 14.6 | 17.0 | ||
| Tingling | No | 47 | 20.8 | 16.3 | 17.0 | 0.753 |
| Yes | 25 | 19.8 | 11.5 | 18.0 | ||
| Pain | No | 47 | 17.8 | 12.5 | 15.0 | 0.080 |
| Yes | 25 | 25.6 | 17.4 | 20.0 | ||
| Discomfort | No | 32 | 18.6 | 16.5 | 15.0 | 0.086 |
| Yes | 40 | 22.1 | 13.2 | 20.0 | ||
| Malodor | No | 39 | 14.0 | 10.1 | 12.0 | <0.001 * |
| Yes | 33 | 28.1 | 15.8 | 20.0 | ||
| Burning | No | 48 | 19.7 | 15.8 | 15.0 | 0.198 |
| Patients Who Reported Itch, Tingling, or Pain in the CRF | DLQI Symptom Score, on a Scale of 1 to 4 | p Value 1 | ||
|---|---|---|---|---|
| Mean | STD | Median | ||
| No (n = 6) | 1.5 | 0.6 | 1.5 | 0.028 * |
| Yes (n = 68) | 2.4 | 1.0 | 2.0 | |
| Itch | Tingling | Pain | Discomfort | Malodor | Burning | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No N = 7 N (%) | Yes N = 69 N (%) | p Value 1 | No N = 47 N (%) | Yes N = 29 N (%) | p Value 1 | No N = 50 N (%) | Yes N = 26 N (%) | p Value 1 | No N = 32 N (%) | Yes N = 44 N (%) | p Value 1 | No N = 43 N (%) | Yes N = 33 N (%) | p Value 1 | No N = 51 N (%) | Yes N = 25 N (%) | p Value 1 | |
| Anxiety (yes) | 1 (14.3) | 17 (25.0) | 1.000 | 7 (15.2) | 11 (37.9) | 0.025 | 10 (20.0) | 8 (32.0) | 0.251 | 4 (12.9) | 14 (31.8) | 0.059 | 9 (21.4) | 9 (27.3) | 0.556 | 10 (20.0) | 8 (32.0) | 0.251 |
| Depression (yes) | 1 (14.3) | 22 (32.8) | 0.424 | 12 (26.7) | 11 (37.9) | 0.307 | 14 (28.6) | 9 (36.0) | 0.514 | 7 (23.3) | 16 (36.4) | 0.234 | 10 (24.4) | 13 (39.4) | 0.166 | 12 (24.5) | 11 (44.0) | 0.086 |
| Suicidal ideation (yes) | 1 (14.3) | 20 (29.4) | 0.665 | 12 (26.7) | 9 (31.0) | 0.642 | 14 (28.6) | 7 (28.0) | 1.000 | 8 (25.8) | 13 (29.6) | 0.723 | 9 (21.4) | 12 (36.4) | 0.153 | 13 (26.0) | 8 (32.0) | 0.585 |
| Suicide attempt (yes) | 1 (14.3) | 4 (5.9) | 0.396 | 1 (2.2) | 4 (13.8) | 0.070 | 2 (4.0) | 3 (12.0) | 0.326 | 1 (3.2) | 4 (9.1) | 0.397 | 1 (2.4) | 4 (12.1) | 0.163 | 3 (6.0) | 2 (8.0) | 1.000 |
| Seen a psychiatrist (yes) | 0 (0.0) | 15 (21.7) | 0.333 | 8 (17.0) | 7 (24.1) | 0.449 | 8 (16.0) | 7 (26.9) | 0.256 | 4 (12.5) | 11 (25.0) | 0.176 | 6 (14.0) | 9 (27.3) | 0.148 | 7 (13.7) | 8 (32.0) | 0.072 |
| Taken psychiatric medication (yes) | 0 (0.0) | 18 (26.1) | 0.188 | 11 (23.4) | 7 (24.1) | 0.942 | 11 (22.0) | 7 (26.9) | 0.632 | 5 (15.6) | 13 (29.6) | 0.159 | 7 (16.3) | 11 (33.3) | 0.083 | 8 (15.7) | 10 (40.0) | 0.019 ** |
| Previously diagnosed with other psychiatric disorders * (yes) | 1 (14.3) | 24 (36.4) | 0.410 | 14 (30.4) | 11 (40.7) | 0.370 | 14 (29.2) | 11 (44.0) | 0.205 | 9 (30.0) | 16 (37.2) | 0.523 | 12 (28.6) | 13 (41.9) | 0.234 | 13 (26.5) | 12 (50.0) | 0.047 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, G.; Sachdeva, M.; Yosipovitch, G.; Ziv, M.; Dodiuk-Gad, R.P. Pruritus and Neuropsychiatric Symptoms Among Patients with Darier Disease—An Overlooked and Interconnected Challenge. J. Clin. Med. 2025, 14, 1818. https://doi.org/10.3390/jcm14061818
Xiong G, Sachdeva M, Yosipovitch G, Ziv M, Dodiuk-Gad RP. Pruritus and Neuropsychiatric Symptoms Among Patients with Darier Disease—An Overlooked and Interconnected Challenge. Journal of Clinical Medicine. 2025; 14(6):1818. https://doi.org/10.3390/jcm14061818
Chicago/Turabian StyleXiong, Grace, Muskaan Sachdeva, Gil Yosipovitch, Michael Ziv, and Roni P. Dodiuk-Gad. 2025. "Pruritus and Neuropsychiatric Symptoms Among Patients with Darier Disease—An Overlooked and Interconnected Challenge" Journal of Clinical Medicine 14, no. 6: 1818. https://doi.org/10.3390/jcm14061818
APA StyleXiong, G., Sachdeva, M., Yosipovitch, G., Ziv, M., & Dodiuk-Gad, R. P. (2025). Pruritus and Neuropsychiatric Symptoms Among Patients with Darier Disease—An Overlooked and Interconnected Challenge. Journal of Clinical Medicine, 14(6), 1818. https://doi.org/10.3390/jcm14061818







