Frequent Gastrointestinal Cancer Complications in Japanese Patients with Acute or Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention
Abstract
:1. Introduction
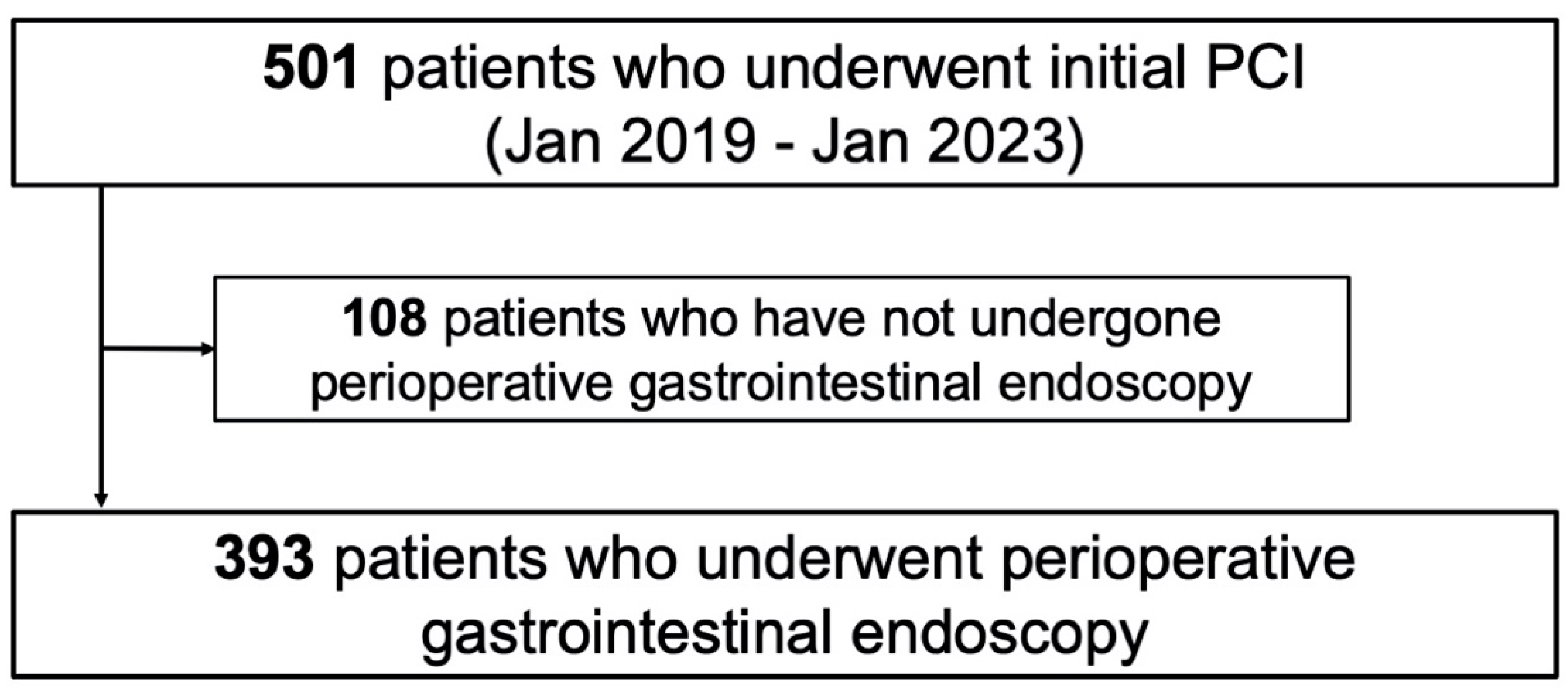
2. Materials and Methods
2.1. Study Protocol and Population
2.2. DAPT Administration and Gastrointestinal Examination
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Association Between Patients with PCI and Malignancy
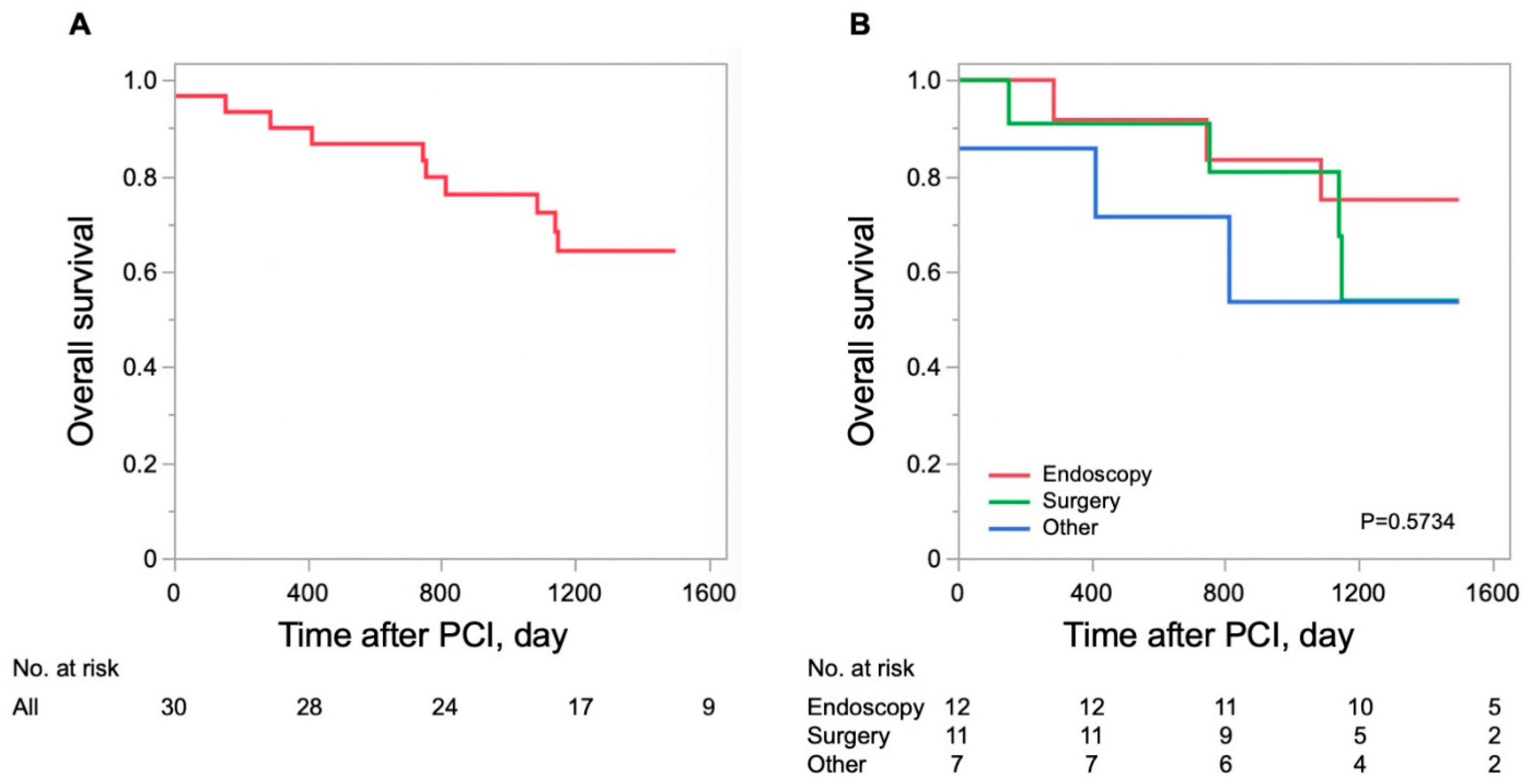
3.3. Survival of Patients with PCI with Gastrointestinal Malignancies
4. Discussion
4.1. Coronary Artery Disease and Gastrointestinal Cancer
4.2. Non-Cardiac Surgery and Coronary Revascularization
4.3. Antiplatelet Therapy and Bleeding Complications
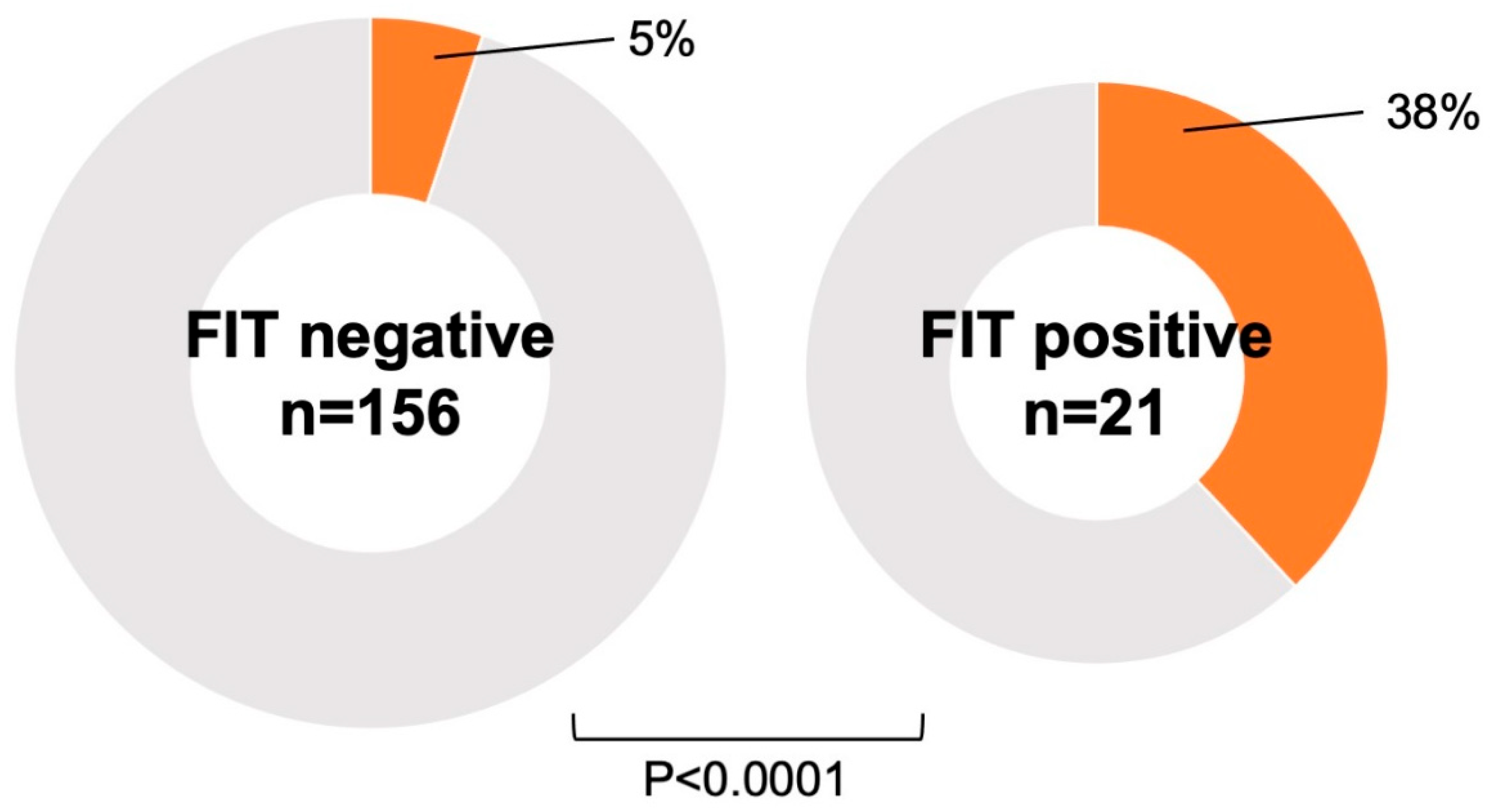
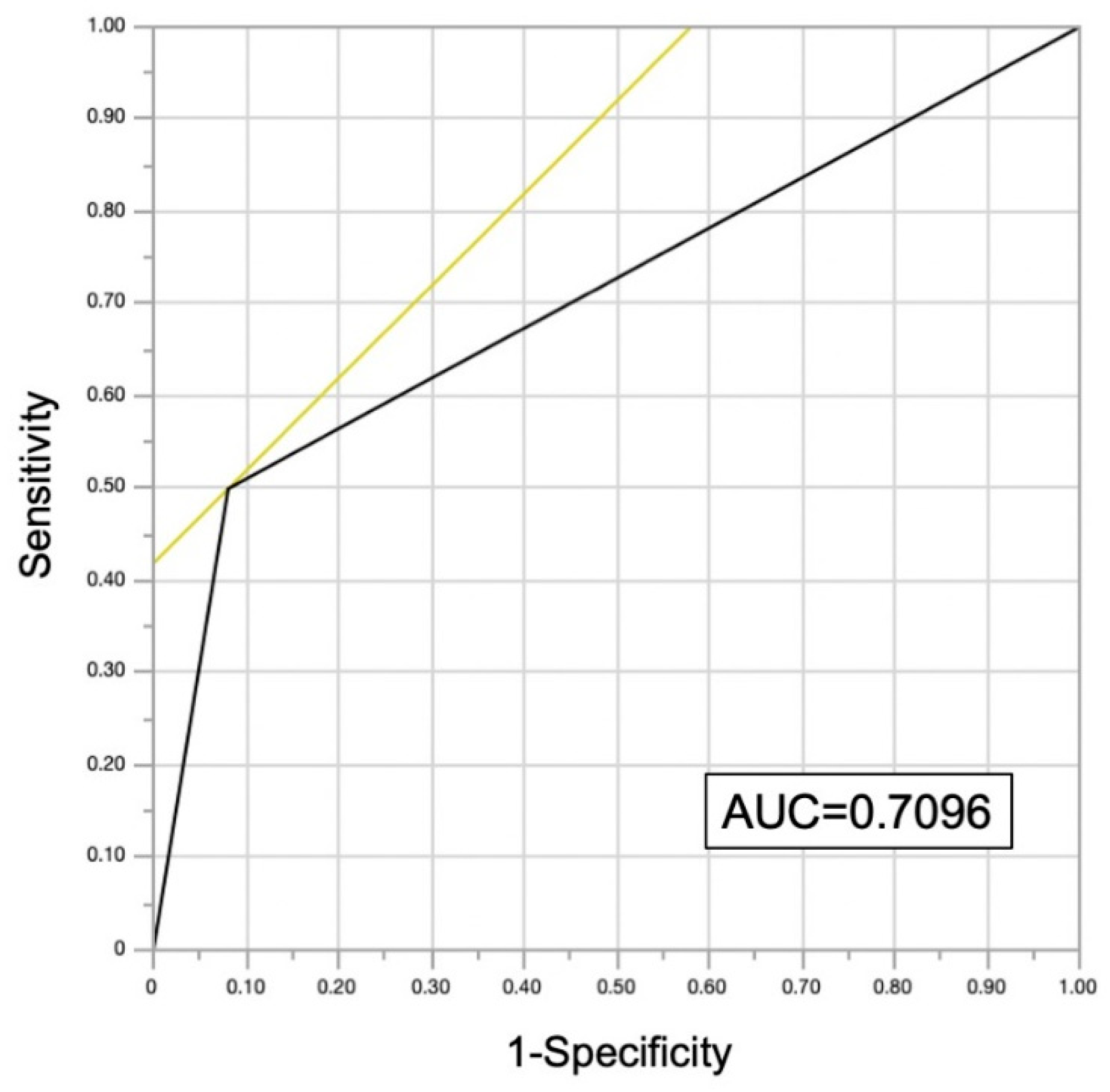
4.4. FIT and Malignancy
4.5. Potential Confounding Factors Associated with Survival of PCI Patients with Malignancy
4.6. Clinical Significance of This Study
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| CCS | chronic coronary syndrome |
| DAPT | dual antiplatelet therapy |
| FIT | fecal immunochemical testing |
| IHD | ischemic heart disease |
| PCI | percutaneous coronary intervention |
References
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Ichim, C.; Pavel, V.; Mester, P.; Schmid, S.; Todor, S.B.; Stoia, O.; Anderco, P.; Kandulski, A.; Müller, M.; Heumann, P.; et al. Assessing Key Factors Influencing Successful Resuscitation Outcomes in Out-of-Hospital Cardiac Arrest (OHCA). J. Clin. Med. 2024, 13, 7399. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, T.; Benedetto, U.; Bacchi-Reggiani, L.; Della Riva, D.; Biondi-Zoccai, G.; Feres, F.; Abizaid, A.; Hong, M.K.; Kim, B.K.; Jang, Y.; et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: A pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015, 385, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Frigoli, E.; Heg, D.; Tijssen, J.; Jüni, P.; Vranckx, P.; Ozaki, Y.; Morice, M.C.; Chevalier, B.; Onuma, Y.; et al. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N. Engl. J. Med. 2021, 385, 1643–1655. [Google Scholar] [CrossRef]
- Watanabe, H.; Domei, T.; Morimoto, T.; Natsuaki, M.; Shiomi, H.; Toyota, T.; Ohya, M.; Suwa, S.; Takagi, K.; Nanasato, M.; et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs. 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 2019, 321, 2414–2427. [Google Scholar] [CrossRef]
- Toyota, T.; Shiomi, H.; Morimoto, T.; Natsuaki, M.; Kimura, T. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: A comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS ONE 2017, 12, e0174502. [Google Scholar] [CrossRef]
- Nikolsky, E.; Stone, G.W.; Kirtane, A.J.; Dangas, G.D.; Lansky, A.J.; McLaurin, B.; Lincoff, A.M.; Feit, F.; Moses, J.W.; Fahy, M.; et al. Gastrointestinal bleeding in patients with acute coronary syndromes: Incidence, predictors, and clinical implications: Analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J. Am. Coll. Cardiol. 2009, 54, 1293–1302. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, D.; Choo, E.H.; Choi, I.J.; Lim, S.; Lee, K.Y.; Hwang, B.H.; Park, M.W.; Lee, J.M.; Park, C.S.; et al. Association Between Bleeding and New Cancer Detection and the Prognosis in Patients With Myocardial Infarction. J. Am. Heart Assoc. 2022, 11, e026588. [Google Scholar] [CrossRef]
- Ueki, Y.; Vogeli, B.; Karagiannis, A.; Zanchin, T.; Zanchin, C.; Rhyner, D.; Otsuka, T.; Praz, F.; Siontis, G.C.M.; Moro, C.; et al. Ischemia and Bleeding in Cancer Patients Undergoing Percutaneous Coronary Intervention. JACC CardioOncol 2019, 1, 145–155. [Google Scholar] [CrossRef]
- Helsingen, L.M.; Vandvik, P.O.; Jodal, H.C.; Agoritsas, T.; Lytvyn, L.; Anderson, J.C.; Auer, R.; Murphy, S.B.; Almadi, M.A.; Corley, D.A.; et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A clinical practice guideline. BMJ 2019, 367, l5515. [Google Scholar] [CrossRef] [PubMed]
- Quintero, E.; Castells, A.; Bujanda, L.; Cubiella, J.; Salas, D.; Lanas, Á.; Andreu, M.; Carballo, F.; Morillas, J.D.; Hernández, C.; et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N. Engl. J. Med. 2012, 366, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Handy, C.E.; Quispe, R.; Pinto, X.; Blaha, M.J.; Blumenthal, R.S.; Michos, E.D.; Lima, J.A.C.; Guallar, E.; Ryu, S.; Cho, J.; et al. Synergistic Opportunities in the Interplay Between Cancer Screening and Cardiovascular Disease Risk Assessment: Together We Are Stronger. Circulation 2018, 138, 727–734. [Google Scholar] [CrossRef]
- Chan, A.O.; Jim, M.H.; Lam, K.F.; Morris, J.S.; Siu, D.C.; Tong, T.; Ng, F.H.; Wong, S.Y.; Hui, W.M.; Chan, C.K.; et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA 2007, 298, 1412–1419. [Google Scholar] [CrossRef]
- Sharma, V.; Aggarwal, A. Helicobacter pylori: Does it add to risk of coronary artery disease. World J. Cardiol. 2015, 7, 19–25. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Zheng, Y.; Cai, H. The causal effect of Helicobacter pylori infection on coronary heart disease is mediated by the body mass index: A Mendelian randomization study. Sci. Rep. 2024, 14, 1688. [Google Scholar] [CrossRef]
- Wilson, S.H.; Fasseas, P.; Orford, J.L.; Lennon, R.J.; Horlocker, T.; Charnoff, N.E.; Melby, S.; Berger, P.B. Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting. J. Am. Coll. Cardiol. 2003, 42, 234–240. [Google Scholar] [CrossRef]
- Tokushige, A.; Shiomi, H.; Morimoto, T.; Furukawa, Y.; Nakagawa, Y.; Kadota, K.; Iwabuchi, M.; Shizuta, S.; Tada, T.; Tazaki, J.; et al. Incidence and outcome of surgical procedures after coronary bare-metal and drug-eluting stent implantation: A report from the CREDO-Kyoto PCI/CABG registry cohort-2. Circ. Cardiovasc. Interv. 2012, 5, 237–246. [Google Scholar] [CrossRef]
- Koshy, A.N.; Ha, F.J.; Gow, P.J.; Han, H.C.; Amirul-Islam, F.M.; Lim, H.S.; Teh, A.W.; Farouque, O. Computed tomographic coronary angiography in risk stratification prior to non-cardiac surgery: A systematic review and meta-analysis. Heart 2019, 105, 1335–1342. [Google Scholar] [CrossRef]
- Eagle, K.A.; Coley, C.M.; Newell, J.B.; Brewster, D.C.; Darling, R.C.; Strauss, H.W.; Guiney, T.E.; Boucher, C.A. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann. Intern. Med. 1989, 110, 859–866. [Google Scholar] [CrossRef]
- McFalls, E.O.; Ward, H.B.; Moritz, T.E.; Goldman, S.; Krupski, W.C.; Littooy, F.; Pierpont, G.; Santilli, S.; Rapp, J.; Hattler, B.; et al. Coronary-artery revascularization before elective major vascular surgery. N. Engl. J. Med. 2004, 351, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Moritz, T.E.; Ward, H.B.; Pierpont, G.; Goldman, S.; Larsen, G.C.; Littooy, F.; Krupski, W.; Thottapurathu, L.; Reda, D.J.; et al. Usefulness of revascularization of patients with multivessel coronary artery disease before elective vascular surgery for abdominal aortic and peripheral occlusive disease. Am. J. Cardiol. 2008, 102, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.Y.; Lawrence, H.P.; Wong, D.T. The effects of prophylactic coronary revascularization or medical management on patient outcomes after noncardiac surgery--a meta-analysis. Can. J. Anaesth. 2007, 54, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Livhits, M.; Ko, C.Y.; Leonardi, M.J.; Zingmond, D.S.; Gibbons, M.M.; de Virgilio, C. Risk of surgery following recent myocardial infarction. Ann. Surg. 2011, 253, 857–864. [Google Scholar] [CrossRef]
- Hawn, M.T.; Graham, L.A.; Richman, J.S.; Itani, K.M.; Henderson, W.G.; Maddox, T.M. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. Jama 2013, 310, 1462–1472. [Google Scholar] [CrossRef]
- Holcomb, C.N.; Hollis, R.H.; Graham, L.A.; Richman, J.S.; Valle, J.A.; Itani, K.M.; Maddox, T.M.; Hawn, M.T. Association of Coronary Stent Indication With Postoperative Outcomes Following Noncardiac Surgery. JAMA Surg. 2016, 151, 462–469. [Google Scholar] [CrossRef]
- Tokushige, A.; Shiomi, H.; Morimoto, T.; Ono, K.; Furukawa, Y.; Nakagawa, Y.; Kadota, K.; Iwabuchi, M.; Shizuta, S.; Tada, T.; et al. Influence of initial acute myocardial infarction presentation on the outcome of surgical procedures after coronary stent implantation: A report from the CREDO-Kyoto PCI/CABG Registry Cohort-2. Cardiovasc. Interv. Ther. 2013, 28, 45–55. [Google Scholar] [CrossRef]
- Livhits, M.; Gibbons, M.M.; de Virgilio, C.; O’Connell, J.B.; Leonardi, M.J.; Ko, C.Y.; Zingmond, D.S. Coronary revascularization after myocardial infarction can reduce risks of noncardiac surgery. J. Am. Coll. Surg. 2011, 212, 1018–1026. [Google Scholar] [CrossRef]
- Pedersen, F.; Butrymovich, V.; Kelbæk, H.; Wachtell, K.; Helqvist, S.; Kastrup, J.; Holmvang, L.; Clemmensen, P.; Engstrøm, T.; Grande, P.; et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J. Am. Coll. Cardiol. 2014, 64, 2101–2108. [Google Scholar] [CrossRef]
- Leon, M.B.; Baim, D.S.; Popma, J.J.; Gordon, P.C.; Cutlip, D.E.; Ho, K.K.; Giambartolomei, A.; Diver, D.J.; Lasorda, D.M.; Williams, D.O.; et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N. Engl. J. Med. 1998, 339, 1665–1671. [Google Scholar] [CrossRef]
- Hallas, J.; Dall, M.; Andries, A.; Andersen, B.S.; Aalykke, C.; Hansen, J.M.; Andersen, M.; Lassen, A.T. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: Population based case-control study. BMJ 2006, 333, 726. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation 2019, 140, 240–261. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.; Morimoto, T.; Yamaji, K.; Watanabe, H.; Yoshikawa, Y.; Shiomi, H.; Nakagawa, Y.; Furukawa, Y.; Kadota, K.; Ando, K.; et al. Prediction of Thrombotic and Bleeding Events After Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J. Am. Heart Assoc. 2018, 7, e008708. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Watanabe, H.; Morimoto, T.; Yoshikawa, Y.; Shiomi, H.; Shizuta, S.; Ono, K.; Yamaji, K.; Soga, Y.; Hyodo, M.; et al. Impact of Baseline Thrombocytopenia on Bleeding and Mortality After Percutaneous Coronary Intervention. Am. J. Cardiol. 2018, 121, 1304–1314. [Google Scholar] [CrossRef]
- Mabe, K.; Inoue, K.; Kamada, T.; Kato, K.; Kato, M.; Haruma, K. Endoscopic screening for gastric cancer in Japan: Current status and future perspectives. Dig. Endosc. 2022, 34, 412–419. [Google Scholar] [CrossRef]
- Rossini, R.; Musumeci, G.; Visconti, L.O.; Bramucci, E.; Castiglioni, B.; De Servi, S.; Lettieri, C.; Lettino, M.; Piccaluga, E.; Savonitto, S.; et al. Perioperative management of antiplatelet therapy in patients with coronary stents undergoing cardiac and non-cardiac surgery: A consensus document from Italian cardiological, surgical and anaesthesiological societies. EuroIntervention 2014, 10, 38–46. [Google Scholar] [CrossRef]
- Yashima, K.; Shabana, M.; Kurumi, H.; Kawaguchi, K.; Isomoto, H. Gastric Cancer Screening in Japan: A Narrative Review. J. Clin. Med. 2022, 11, 4337. [Google Scholar] [CrossRef]
- Toyoshima, O.; Yamaji, Y.; Nishizawa, T.; Yoshida, S.; Yamada, T.; Kurokawa, K.; Obata, M.; Kondo, R.; Toba, M.; Koike, K. Priority stratification for colonoscopy based on two-sample faecal immunochemical test screening: Results from a cross-sectional study at an endoscopy clinic in Japan. BMJ Open 2021, 11, e046055. [Google Scholar] [CrossRef]
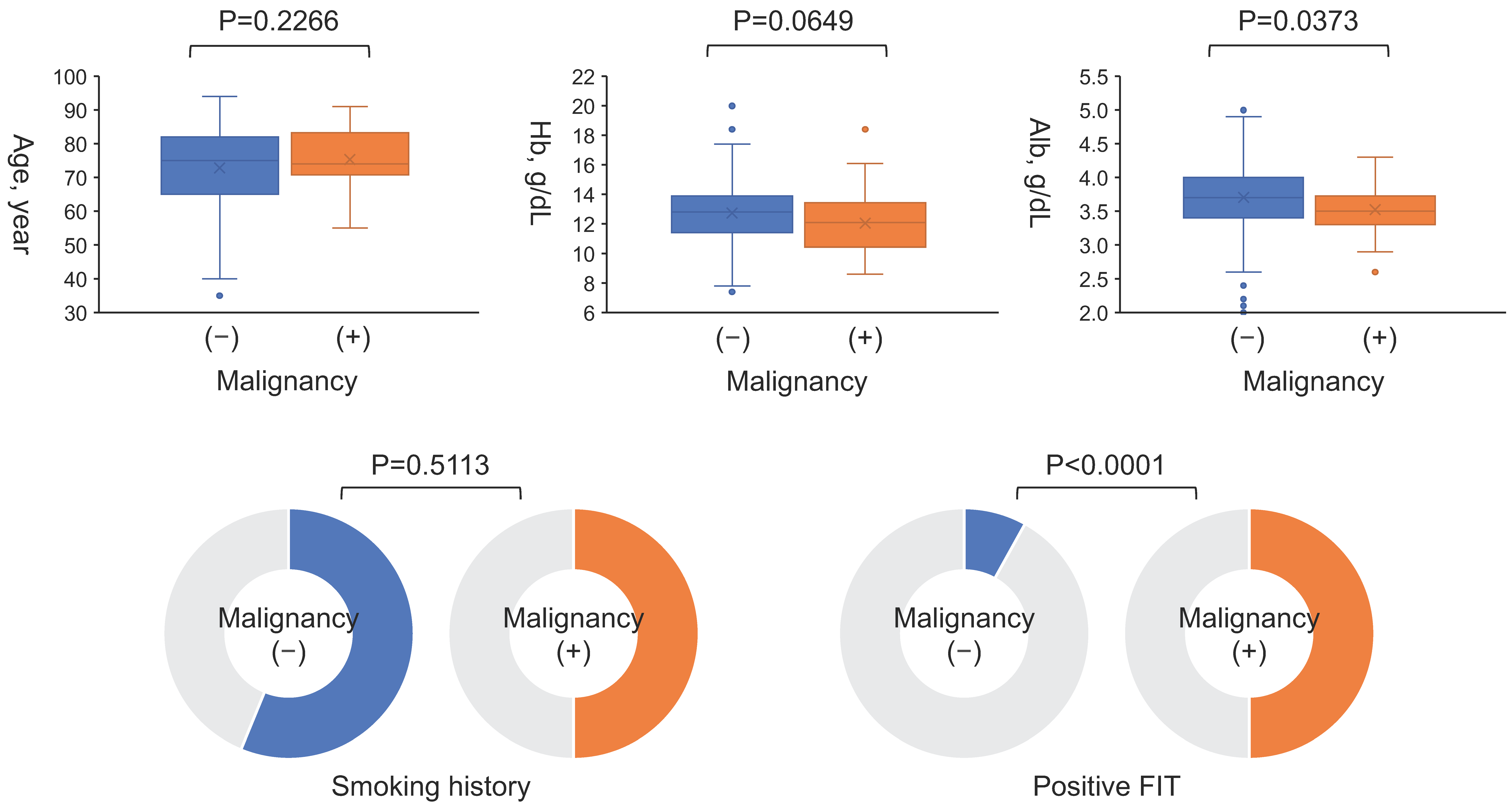
| Variables | All (n = 393) | Malignancy (n = 30) | No Malignancy (n = 363) | p |
|---|---|---|---|---|
| Age, year | 75 (66–82) | 74 (71–83) | 75 (65–82) | 0.2266 |
| Male, n (%) | 277 (70) | 23 (77) | 254 (70) | 0.4398 |
| Body surface area, m2 | 1.64 ± 0.19 | 1.61 ± 0.18 | 1.64 ± 0.19 | 0.3254 |
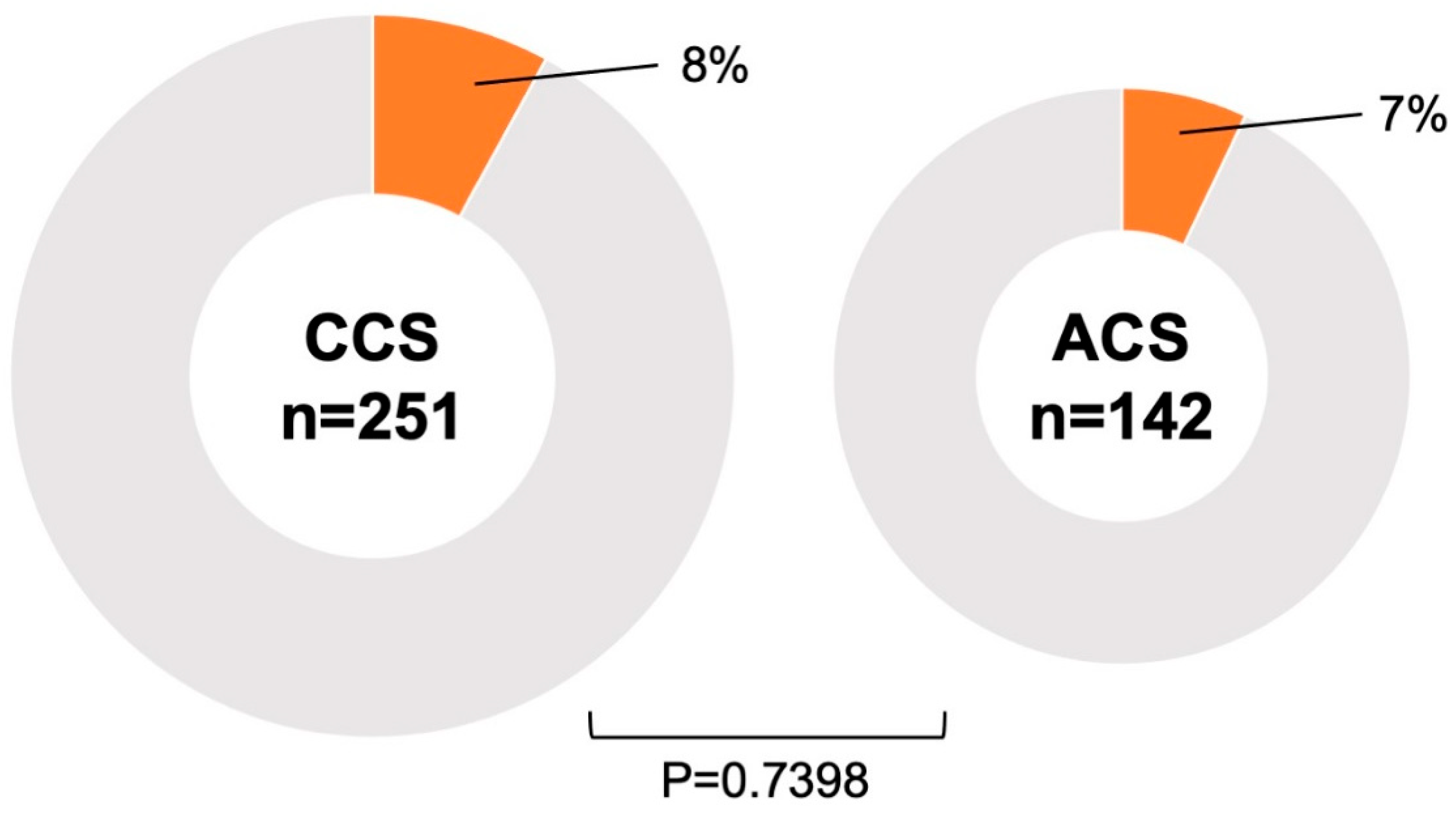
| Acute coronary syndrome, n (%) | 142 (36) | 10 (33) | 132 (36) | 0.7398 |
| Heart failure, n (%) | 89 (23) | 9 (30) | 80 (22) | 0.3167 |
| Hypertension, n (%) | 299 (76) | 24 (80) | 275 (76) | 0.6006 |
| Diabetes mellitus, n (%) | 159 (40) | 12 (40) | 147 (40) | 0.9576 |
| Dyslipidemia, n (%) | 227 (58) | 14 (47) | 213 (59) | 0.2005 |
| Atrial fibrillation/flutter, n (%) | 68 (17) | 7 (23) | 61 (17) | 0.3636 |
| Smoking, n (%) | 219 (56) | 15 (50) | 204 (56) | 0.5113 |
| Hemoglobin, g/dL | 12.7 ± 1.9 | 12.1 ± 2.2 | 12.7 ± 1.9 | 0.0649 |
| Albumin, g/dL | 3.7 (3.4–4.0) | 3.5 (3.3–3.7) | 3.7 (3.4–4.0) | 0.0373 |
| Sodium, mEq/L | 141 (139–143) | 141 (139–142) | 141 (139–143) | 0.0951 |
| eGFR, mL/min/1.73 m2 | 58.9 (45.7–72.8) | 53.1 (41.6–70.7) | 59.1 (46.0–73.1) | 0.4676 |
| BNP, pg/mL | 54.6 (21.7–154.3) | 79.6 (39.9–190.0) | 53.5 (21.5–154.0) | 0.5312 |
| Malignancy by Organ | Stage | Treatment |
|---|---|---|
| Colorectal cancer, n = 18 | Early, n = 11 | Endoscopy, n = 7 Surgery, n = 3 Supportive, n = 1 |
| Advanced, n = 6 | Surgery, n = 6 | |
| Unknown, n = 1 | - | |
| Gastric cancer, n = 8 | Early, n = 6 | Endoscopy, n = 3 Surgery, n = 1 Supportive, n = 2 |
| Advanced, n = 2 | Surgery, n = 1 Chemotherapy, n = 1 | |
| Esophageal cancer, n = 2 | Advanced, n = 2 | Endoscopy, n = 1 Chemoradiation, n = 1 |
| Laryngeal cancer, n = 1 | Unknown, n = 1 | - |
| Overlap of gastric and colorectal cancer, n = 1 | Early, n = 1 | Endoscopy for both, n = 1 |
| Variables | OR (95% CI) | p |
|---|---|---|
| Age | 1.00 (0.94–1.06) | 0.9338 |
| Albumin | 1.19 (0.32–4.34) | 0.7960 |
| FIT | 12.36 (3.51–43.5) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiba, Y.; Imagawa, S.; Takahashi, Y.; Kubo, K.; Otsuka, K.; Shimazu, K.; Anzai, T.; Yonezawa, K.; Kato, M.; Anzai, T. Frequent Gastrointestinal Cancer Complications in Japanese Patients with Acute or Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2025, 14, 1807. https://doi.org/10.3390/jcm14061807
Chiba Y, Imagawa S, Takahashi Y, Kubo K, Otsuka K, Shimazu K, Anzai T, Yonezawa K, Kato M, Anzai T. Frequent Gastrointestinal Cancer Complications in Japanese Patients with Acute or Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2025; 14(6):1807. https://doi.org/10.3390/jcm14061807
Chicago/Turabian StyleChiba, Yasuyuki, Shogo Imagawa, Yuki Takahashi, Kimitoshi Kubo, Kenta Otsuka, Kyo Shimazu, Teisuke Anzai, Kazuya Yonezawa, Mototsugu Kato, and Toshihisa Anzai. 2025. "Frequent Gastrointestinal Cancer Complications in Japanese Patients with Acute or Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention" Journal of Clinical Medicine 14, no. 6: 1807. https://doi.org/10.3390/jcm14061807
APA StyleChiba, Y., Imagawa, S., Takahashi, Y., Kubo, K., Otsuka, K., Shimazu, K., Anzai, T., Yonezawa, K., Kato, M., & Anzai, T. (2025). Frequent Gastrointestinal Cancer Complications in Japanese Patients with Acute or Chronic Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine, 14(6), 1807. https://doi.org/10.3390/jcm14061807





