Epicardial Ligation of the Left Atrial Appendage in Octogenarians: Safety and Long-Term Efficacy
Abstract
1. Introduction
2. Method
2.1. Patient Selection
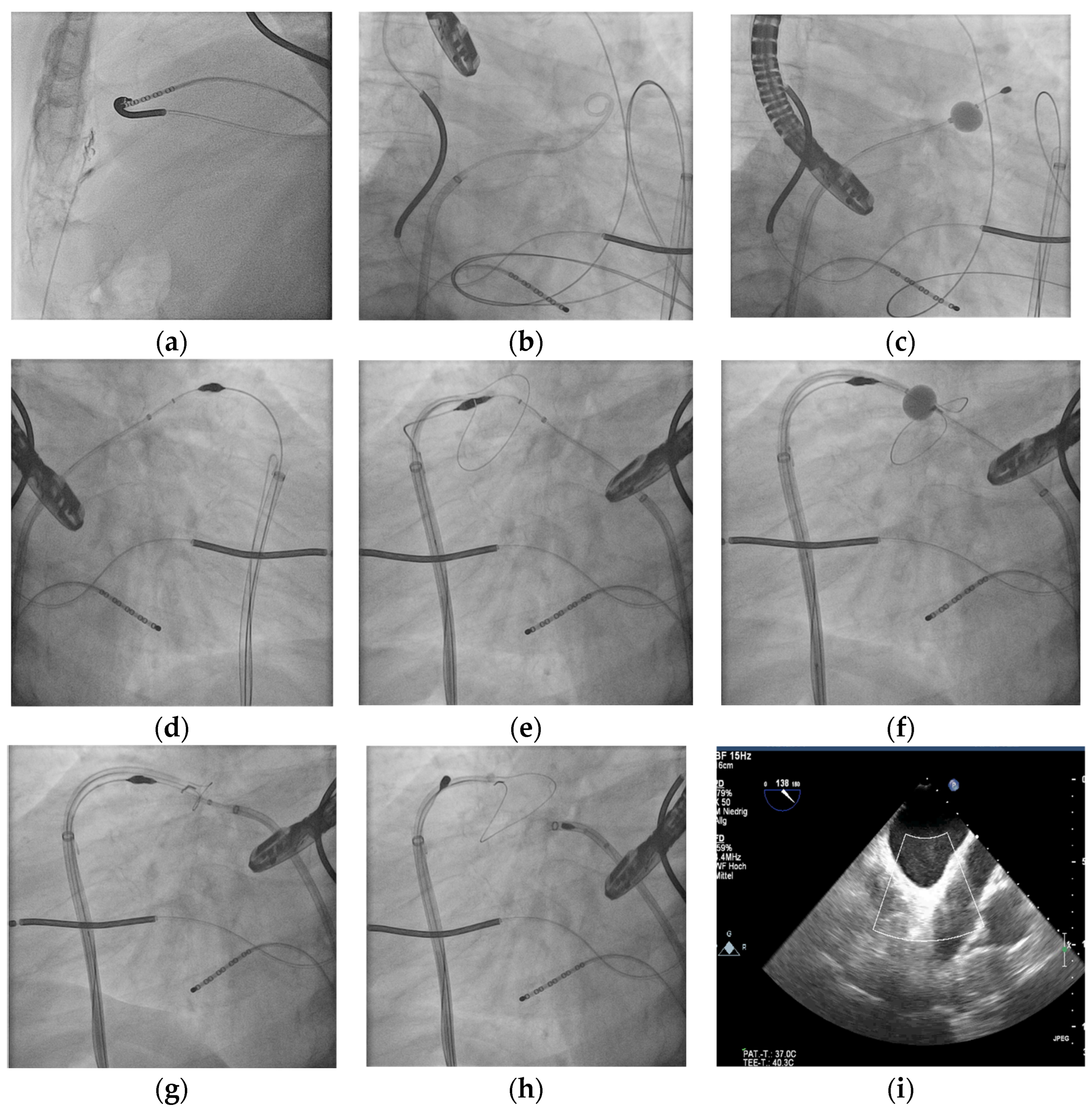
2.2. Procedure
2.3. Follow-Up
3. Results
3.1. Procedure
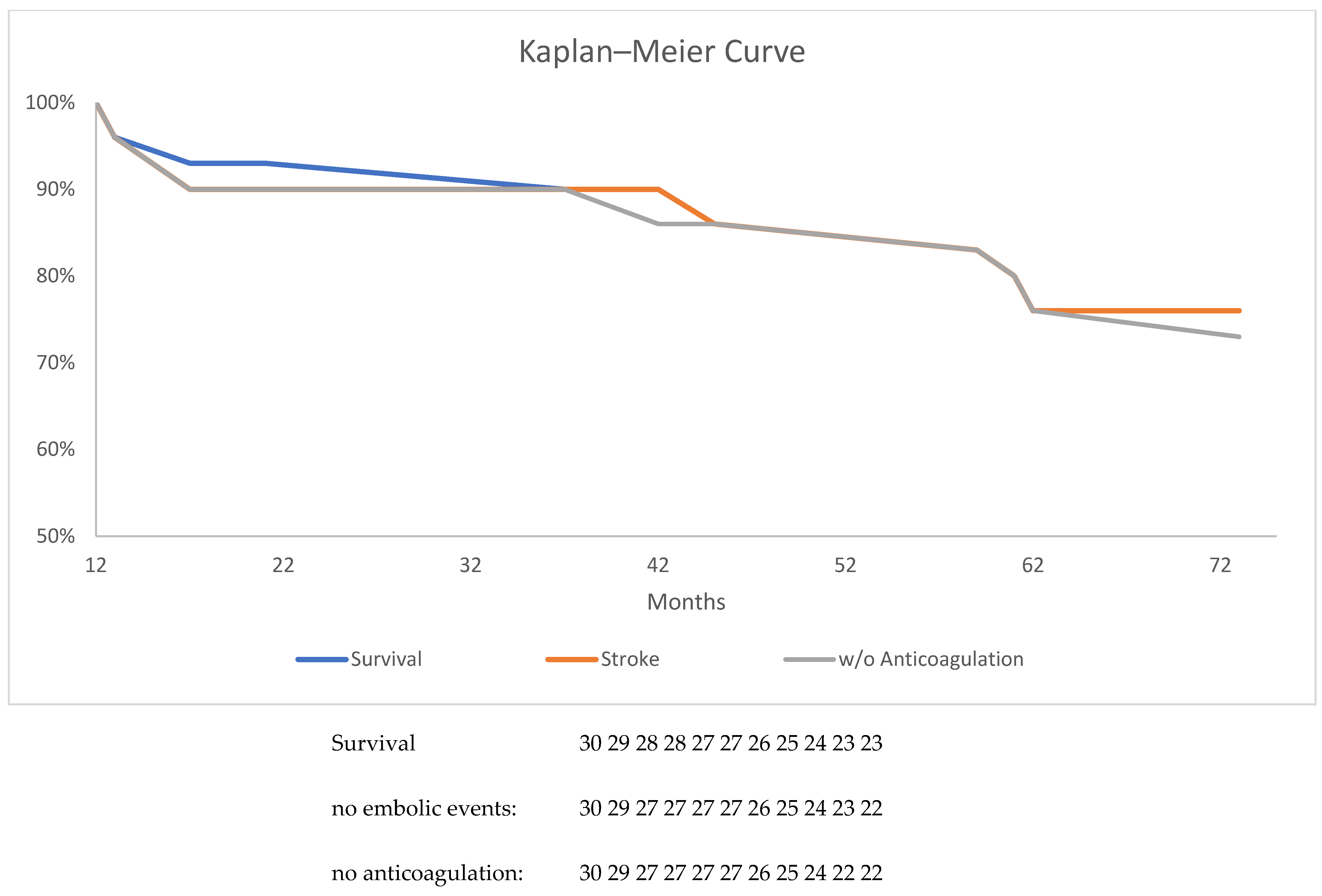
3.2. TOE and Clinical FUP
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| LAA | left atrial appendage |
| FUP | follow-up |
| TOE | transesophageal echocardiogram |
| NOAK | novel oral anticoagulation |
| PDL | peridevice leak |
| ICU | intensive care unit |
| SD | standard deviation |
References
- Volgman, A.S.; Nair, G.; Lyubarova, R.; Merchant, F.M.; Mason, P.; Curtis, A.B.; Wenger, N.K.; Aggarwal, N.T.; Kirkpatrick, J.N.; Benjamin, E.J.; et al. Management of Atrial Fibrillation in Patients 75 Years and Older: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.K.; Buchbinder, M.; Neuzil, P.; Huber, K.; Whisenant, B.; Kar, S.; Swarup, V.; et al. Percutaneous left atrial appendage closure vs. warfarin for atrial fibrillation: A randomized clinical trial. JAMA 2014, 312, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Boersma, L.V.; Schmidt, B.; Betts, T.R.; Sievert, H.; Tamburino, C.; Teiger, E.; Pokushalov, E.; Kische, S.; Schmitz, T.; Stein, K.M.; et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: Peri-procedural outcomes from the EWOLUTION registry. Eur. Heart J. 2016, 37, 2465–2474. [Google Scholar] [CrossRef]
- Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; Nielsen-Kudsk, J.E.; Cruz-Gonzalez, I.; et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: Multicenter experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016, 11, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Nelson, M.R.; Woods, R.L.; Lockery, J.E.; Wolfe, R.; Reid, C.M.; Kirpach, B.; Shah, R.C.; Ives, D.G.; Storey, E.; et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1519–1528. [Google Scholar] [CrossRef]
- Mahady, S.E.; Margolis, K.L.; Chan, A.; Polekhina, G.; Woods, R.L.; Wolfe, R.; Nelson, M.R.; E Lockery, J.; Wood, E.M.; Reid, C.; et al. Major GI bleeding in older persons using aspirin: Incidence and risk factors in the ASPREE randomised controlled trial. Gut 2021, 70, 717–724. [Google Scholar] [CrossRef]
- Ray, I.B.; Khanra, D.; Shah, S.; Char, S.; Jia, X.; Lam, W.; Mathuria, N.; Razavi, M.; Jain, B.; Lakkireddy, D.; et al. Meta-Analysis Comparing Watchman (TM) and Amplatzer Devices for Stroke Prevention in Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 89. [Google Scholar]
- Oliva, A.; Ioppolo, A.M.; Chiarito, M.; Cremonesi, A.; Azzano, A.; Miccichè, E.; Mangiameli, A.; Ariano, F.; Ferrante, G.; Reimers, B.; et al. Left Atrial Appendage Closure Compared with Oral Anticoagulants for Patients with Atrial Fibrillation: A Systematic Review and Network Meta-Analysis. J. Am. Heart Assoc. 2024, 13, e034815. [Google Scholar] [CrossRef]
- Price, M.J.; Gibson, D.N.; Yakubov, S.J.; Schultz, J.C.; Di Biase, L.; Natale, A.; Burkhardt, J.D.; Pershad, A.; Byrne, T.J.; Gidney, B.; et al. Early safety and efficacy of percutaneous left atrial appendage suture ligation: Results from the U.S. transcatheter LAA ligation consortium. J. Am. Coll. Cardiol. 2014, 64, 565–572. [Google Scholar] [CrossRef]
- Bartus, K.; Han, F.T.; Bednarek, J.; Myc, J.; Kapelak, B.; Sadowski, J.; Lelakowski, J.; Bartus, S.; Yakubov, S.J.; Lee, R.J. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: Initial clinical experience. J. Am. Coll. Cardiol. 2013, 62, 108–118. [Google Scholar] [CrossRef]
- Pillarisetti, J.; Reddy, Y.M.; Gunda, S.; Swarup, V.; Lee, R.; Rasekh, A.; Horton, R.; Massumi, A.; Cheng, J.; Bartus, K.; et al. Endocardial (Watchman) vs epicardial (Lariat) left atrial appendage exclusion devices: Understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm 2015, 12, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Bartus, K.; Yakubov, S.J. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: Initial experience in a canine model. Circ. Cardiovasc. Interv. 2010, 3, 224–229. [Google Scholar] [CrossRef]
- Bonanad, C.; García-Blas, S.; Llergo, J.T.; Fernández-Olmo, R.; Díez-Villanueva, P.; Ariza-Solé, A.; Martínez-Sellés, M.; Raposeiras, S.; Ayesta, A.; Bertomeu-González, V.; et al. Direct Oral Anticoagulants versus Warfarin in Octogenarians with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 5268. [Google Scholar] [CrossRef] [PubMed]
- Gafoor, S.; Franke, J.; Bertog, S.; Boehm, P.; Heuer, L.; Gonzaga, M.; Bauer, J.; Braut, A.; Lam, S.; Vaskelyte, L.; et al. Left atrial appendage occlusion in octogenarians: Short-term and 1-year follow-up. Catheter. Cardiovasc. Interv. 2014, 83, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhao, Y.; Sun, J.; Wang, Q.; Li, W.; Zhang, R.; Chen, M.; Mo, B.; Feng, X.; Liu, B.; et al. Safety and efficacy of ablation for atrial fibrillation in combination with left atrial appendage occlusion in octogenarians. Clin. Cardiol. 2023, 46, 1202–1209. [Google Scholar] [CrossRef]
- Sulaiman, S.; Roy, K.; Wang, H.; De Backer, O.; Alloco, D.; Reddy, V.Y.; Holmes, D.R., Jr.; Alkhouli, M. Left Atrial Appendage Occlusion in the Elderly: Insights from PROTECT-AF, PREVAIL, and Continuous Access Registries. JACC Clin. Electrophysiol. 2023, 9, 669–676. [Google Scholar] [CrossRef]
- Han, S.; Jia, R.; Zhao, S.; Chan, J.; Bai, Y.; Cui, K. Left Atrial Appendage Closure for Atrial Fibrillation in the Elderly >75 Years Old: A Meta-Analysis of Observational Studies. Diagnostics 2022, 12, 3174. [Google Scholar] [CrossRef]
- Savona, S.J.; Daoud, E.G. Left Atrial Appendage Closure: When Does a Procedure Become Futile? JACC Clin. Electrophysiol. 2022, 8, 1103–1105. [Google Scholar] [CrossRef]
- Mesnier, J.; Cruz-González, I.; Arzamendi, D.; Freixa, X.; Nombela-Franco, L.; Peral, V.; Caneiro-Queija, B.; Mangieri, A.; Trejo-Velasco, B.; Asmarats, L.; et al. Incidence and Predictors of Early Death in Patients Undergoing Percutaneous Left Atrial Appendage Closure. JACC Clin. Electrophysiol. 2022, 8, 1093–1102. [Google Scholar] [CrossRef]
- Vij, V.; Piayda, K.; Nelles, D.; Gloekler, S.; Galea, R.; Fürholz, M.; Meier, B.; Valgimigli, M.; O’hara, G.; Arzamendi, D.; et al. Clinical and Echocardiographic Risk Factors for Device-Related Thrombus After Left Atrial Appendage Closure: An Analysis from the Multicenter EUROC-DRT Registry. Clin. Res. Cardiol. 2022, 111, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Simard, T.; Jung, R.G.; Lehenbauer, K.; Piayda, K.; Pracoń, R.; Jackson, G.G.; Flores-Umanzor, E.; Faroux, L.; Korsholm, K.; Chun, J.K.; et al. Predictors of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. J. Am. Coll. Cardiol. 2021, 78, 297–313. [Google Scholar] [CrossRef]
- Alkhouli, M.; Busu, T.; Shah, K.; Osman, M.; Alqahtani, F.; Raybuck, B. Incidence and Clinical Impact of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion: A Meta-Analysis. JACC Clin. Electrophysiol. 2018, 4, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Cinaud, A.; Brigadeau, F.; Lepillier, A.; Pierre, B.; Abbey, S.; Fatemi, M.; Franceschi, F.; Guedeney, P.; Jacon, P.; et al. Device-Related Thrombosis After Percutaneous Left Atrial Appendage Occlusion for Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 71, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Simard, T.S.; Hibbert, B.; Alkhouli, M.A.; Abraham, N.A.; Holmes, D.H. Device-related thrombus following left atrial appendage occlusion. EuroIntervention 2022, 18, 224–232. [Google Scholar] [CrossRef]
- Dukkipati, S.R.; Kar, S.; Holmes, D.R.; Doshi, S.K.; Swarup, V.; Gibson, D.N.; Maini, B.; Gordon, N.T.; Main, M.L.; Reddy, V.Y. Device-Related Thrombus After Left Atrial Appendage Closure: Incidence, Predictors, and Outcomes. Circulation 2018, 138, 874–885. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Afzal, M.R.; Lee, R.J.; Nagaraj, H.; Tschopp, D.; Gidney, B.; Ellis, C.; Altman, E.; Lee, B.; Kar, S.; et al. Short and Long-Term Outcomes of Percutaneous Left Atrial Appendage Suture Ligation: Results from a US Multicenter Evaluation. Heart Rhythm 2016, 13, 1030–1036. [Google Scholar] [CrossRef]
- Dukkipati, S.R.; Holmes, D.R., Jr.; Doshi, S.K.; Kar, S.; Singh, S.M.; Gibson, D.; Price, M.J.; Natale, A.; Mansour, M.; Sievert, H.; et al. Impact of Peridevice Leak on 5-Year Outcomes After Left Atrial Appendage Closure. J. Am. Coll. Cardiol. 2022, 80, 469–483. [Google Scholar] [CrossRef]
- Alkhouli, M.; Du, C.; Killu, A.; Simard, T.; Noseworthy, P.A.; Friedman, P.A.; Curtis, J.P.; Freeman, J.V.; Holmes, D.R. Clinical Impact of Residual Leaks Following Left Atrial Appendage Occlusion: Insights from the NCDR LAAO Registry. JACC Clin. Electrophysiol. 2022, 8, 766–778. [Google Scholar] [CrossRef]
- Pillai, A.M.; Kanmanthareddy, A.; Earnest, M.; Reddy, M.; Ferrell, R.; Nath, J.; Pillarisetti, J.; Vallakati, A.; Lakkireddy, D. Initial experience with post Lariat left atrial appendage leak closure with Amplatzer septal occluder device and repeat Lariat application. Heart Rhythm 2014, 11, 1877–1883. [Google Scholar] [CrossRef]
- Alkhouli, M.; De Backer, O.; Ellis, C.R.; Nielsen-Kudsk, J.E.; Sievert, H.; Natale, A.; Lakkireddy, D.; Holmes, D.R. Peridevice Leak After Left Atrial Appendage Occlusion: Incidence, Mechanisms, Clinical Impact, and Management. JACC Cardiovasc. Interv. 2023, 16, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Ellis, C.R.; Nielsen-Kudsk, J.E.; Thaler, D.; Gupta, N.; Koulogiannis, K.; Anderson, J.A.; Gage, R.; Lakkireddy, D. Peridevice Leak After Transcatheter Left Atrial Appendage Occlusion: An Analysis of the Amulet IDE Trial. JACC Cardiovasc. Interv. 2022, 15, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Teiger, E.; Eschalier, R.; Amabile, N.; Rioufol, G.; Ducrocq, G.; Garot, P.; Lepillier, A.; Bille, J.; Elbaz, M.; Defaye, P.; et al. Left atrial appendage closure in very elderly patients in the French National Registry. Heart 2024, 110, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, K.; Khalil, M.; Eid, M.M.; Hassib, M.; Ibrahim, F.; Khalife, W.; Chatila, K.; Pandey, R.; Abdou, C.; Bandyopadhyay, D.; et al. Outcomes and Readmissions After Left Atrial Appendage Occlusion in Octogenarians: A Contemporary Analysis. J. Am. Med. Dir. Assoc. 2024, 25, 356.e1–356.e6. [Google Scholar] [CrossRef]
- Pelliccia, F.; Gragnano, F.; Pasceri, V.; Cesaro, A.; Zimarino, M.; Calabrò, P. Risk Scores of Bleeding Complications in Patients on Dual Antiplatelet Therapy: How to Optimize Identification of Patients at Risk of Bleeding after Percutaneous Coronary Intervention. J. Clin. Med. 2022, 11, 3574. [Google Scholar] [CrossRef]
| Clinical Contraindications | Anatomical Contraindications |
|---|---|
| History of cardiac surgery | Superiorly or backwards orientated LAA with the anterior lobe behind the pulmonary trunk |
| Renal failure with dialysis | Left rotated heart |
| Pectus excavates | LAA width > 50 mm |
| History of thoracic radiation | Multiple lobes with different orientations and wider distance than 50 mm |
| NYHA IV classification | Adipositas BMI > 50 |
| Planned cardiac surgery with surgical LAA resection | Thrombus in LAA |
| Adhesions (uremic pericarditis) |
| Characteristic | n = Number (%) |
|---|---|
| Gender (male) | 24 (53.3%) |
| Mean age (y) | 82.6 ± 2 |
| CHADSVASC score | 4.7 ± 1 |
| HASBLED score | 3.6 ± 0.5 |
| Ejection fraction (%) | 60 ± 10.5 |
| Coronary heart disease | 24 (53.3%) |
| Prior pulmonary vein isolation | 4 (8.8%) |
| Prior stroke | 7 (15.5%) |
| Characteristic | n = Number (%) |
|---|---|
| Duration in min | 87 ± 27 |
| Radiation time in min | 16.9 ± 14 |
| Successful ligation | 95.5% |
| Major complications | 1 |
| Minor complications | 2 |
| 6-Weeks FUP | 12-Week FUP | 12-Month FUP | Mean 38-Month FUP | |
|---|---|---|---|---|
| Gap | 4 | 1 | 0 * | 0 * |
| Thrombus | 3 | 1 | 0 * | 0 * |
| Embolic event | 0 | 0 | 0 | 1 |
| Death | 2 | 2 | 0 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nentwich, K.; Kazaishvilli, N.; Sauer, E.; Berkovitz, A.; Mueller, J.; Barth, S.; Deneke, T. Epicardial Ligation of the Left Atrial Appendage in Octogenarians: Safety and Long-Term Efficacy. J. Clin. Med. 2025, 14, 1787. https://doi.org/10.3390/jcm14061787
Nentwich K, Kazaishvilli N, Sauer E, Berkovitz A, Mueller J, Barth S, Deneke T. Epicardial Ligation of the Left Atrial Appendage in Octogenarians: Safety and Long-Term Efficacy. Journal of Clinical Medicine. 2025; 14(6):1787. https://doi.org/10.3390/jcm14061787
Chicago/Turabian StyleNentwich, Karin, Nuki Kazaishvilli, Elena Sauer, Artur Berkovitz, Julian Mueller, Sebastian Barth, and Thomas Deneke. 2025. "Epicardial Ligation of the Left Atrial Appendage in Octogenarians: Safety and Long-Term Efficacy" Journal of Clinical Medicine 14, no. 6: 1787. https://doi.org/10.3390/jcm14061787
APA StyleNentwich, K., Kazaishvilli, N., Sauer, E., Berkovitz, A., Mueller, J., Barth, S., & Deneke, T. (2025). Epicardial Ligation of the Left Atrial Appendage in Octogenarians: Safety and Long-Term Efficacy. Journal of Clinical Medicine, 14(6), 1787. https://doi.org/10.3390/jcm14061787






