Abstract
Background/Objectives: Isolated defects of the posterior hypopharyngeal/upper esophageal wall are rare, typically arising after cancer resection or complications following cervical spine osteosynthesis. Various local and free flaps are available for reconstruction, but we opted for a double-island anterolateral thigh (ALT) flap in this case. Methods: A scoping review was conducted (June 2024) following PRISMAScR 2018 guidelines in order to examine the coverage options available in the literature for posterior hypopharyngeal/upper esophagus wall defects while also presenting a case where such a defect was covered with a double-island anterolateral thigh (ALT) flap. Eligibility criteria: Human studies describing defect coverage of the posterior hypopharyngeal/upper esophagus wall were included. Sources of evidence: A literature search was conducted in PubMed, Cochrane Library, and Google Scholar, following PRISMAScR guidelines. Charting methods: Data on surgical techniques, outcomes, and complications were extracted and analyzed by two independent reviewers. Case report: A 57-year-old female developed a chronic posterior wall perforation following Zenker’s diverticulum treatment and C5/6 cage osteosynthesis. Reconstruction was performed using a free fasciocutaneous ALT flap with two skin paddles: one (2 × 2 cm) for the esophageal mucosa and an additional vascularized fascia layer (4 × 8 cm) to separate the cage from the hypopharyngeal defect. To enable flap monitoring in the otherwise hidden defect, a second skin island was externalized cervically. Results: Postoperative recovery was uneventful, with a continuous viable flap signal. A Gastrografin swallow test confirmed an intact esophagus without leaks or dehiscences. Oral intake resumed after 10 days. The literature review highlighted 239 cases with multiple reconstructive techniques, each with advantages and limitations. Conclusions: The double-paddle free fasciocutaneous ALT flap is a viable option for posterior hypopharyngeal/upper esophageal wall reconstruction, allowing effective postoperative monitoring. This approach offers a valuable modification for complex cases requiring enhanced structural integrity and flap assessment.
1. Introduction
The reconstruction of the posterior hypopharyngeal and upper esophageal wall is a complex surgical challenge arising after oncologic resection or complications following cervical spine procedures. Isolated defects in this region are relatively rare but can result in significant morbidity due to impaired swallowing and increased risk of fistula formation or infections, particularly when osteosynthesis material is involved [1,2].
Hypopharyngeal and upper esophageal malignancies are among the more aggressive tumors in the head and neck region, frequently diagnosed at advanced stages due to their asymptomatic progression in the early phases [3]. Squamous cell carcinoma is the predominant histologic subtype, accounting for more than 95% of cases, with risk factors including tobacco use, alcohol consumption, and human papillomavirus (HPV) infection [4]. Posterior hypopharyngeal wall tumors specifically present unique challenges in resection and reconstruction due to their proximity to critical structures and rich lymphatic drainage, which predisposes them to early metastases [5,6].
Beyond oncologic cases, defects in the posterior hypopharynx and upper esophagus can also arise from complications of Zenker’s diverticulum surgery, cervical spine instrumentation, or radiation-induced necrosis [7,8]. Postoperative dehiscence in these cases can lead to severe complications, including mediastinitis and chronic aspiration. Therefore, reconstruction strategies must aim not only to restore anatomical integrity but also to ensure functional outcomes such as swallowing and phonation while minimizing donor site morbidity [9].
Several reconstructive techniques have been described, ranging from primary closure and pedicled flaps to free tissue transfer. Local options such as the pectoralis major myocutaneous flap or supraclavicular artery island flap provide robust coverage but may be inadequate for posterior defects or cases involving hardware exposure, especially in already irradiated or operated cases [10,11]. Free flaps, particularly the radial forearm free flap (RFFF) and anterolateral thigh (ALT) flap, have been increasingly favored for their thin, pliable tissue and reliable vascularization, making them suitable for complex reconstructions [12,13]. In addition to anatomic reconstructive approaches, it is important to mention the possibility of a diversion loop, which provides an alternative pathway for food continuity. One example is the free jejunal flap, particularly in patients with impaired swallowing and aspiration [14]. However, in our case, we focused on the anatomic reconstruction of the posterior hypopharyngeal/upper esophageal wall and, therefore, chose not to further elaborate on this option.
We were faced with the challenge of reconstructing a posterior hypopharyngeal/upper esophageal wall defect ensuing after the resection of a Zenker’s diverticulum via an open approach in the presence of an intervertebral titanium cage and anterior plate at the C5/6 cervical spine level. Since the region underwent multiple orthopedic operations, a local flap was not an option anymore. Therefore, a free flap with feasible postoperative monitoring options was needed. This led to the idea of using two skin paddles: one to cover the hypopharyngeal/upper esophageal defect and the second to serve as a monitor island at the neck level.
The purpose of this study is to present a posterior hypopharyngeal/upper esophagus wall defect coverage by means of a free double-paddled anterolateral thigh (ALT) flap, showing the feasibility of this technique for delicate reconstructions requiring pliable tissues and the possibility of monitoring at a hidden localization. This method is a modification of the well-established ALT flap technique. Additionally, a scoping literature review was also conducted according to the PRISMAScR 2018-guidelines in order to examine and identify the coverage options available in the literature for posterior hypopharyngeal/upper esophageal wall defects in the context of trauma, the presence of osteosynthesis, or other etiologies, as no single standardized approach has been established.
2. Case Report
We present the case of a 57-year-old female patient with chronic dysphagia due to a Zenker’s diverticulum (Brombart grade 3), with a history of a failed attempt at a rigid and flexible transoral cricopharyngeal myotomy and subsequent surgical treatment involving a left transcervical approach, dissection, and excision of the Zenker’s diverticulum along with cricopharyngeal myotomy in March 2023. Additionally, she had a history of cervical spondylosis and foraminal stenosis at C5/6, treated with anterior cervical fusion and cage placement in 2018, followed by cage removal and revision in 2019 due to a pseudarthrosis. A new cage and anterior plate were installed at the C5/6 level. In January 2024, the patient was diagnosed with a chronic hypopharyngeal/upper esophageal wall perforation and dehiscence with exposure of the cervical spine’s anterior plate and suspected chronic instability and infection of the cervical vertebrae underlying the current cage.
Her medical history was complex and extended, marked by numerous complications stemming from both the initial surgical interventions and subsequent attempts at management. These challenges necessitated a highly specialized multidisciplinary approach to achieve adequate tissue coverage while ensuring the highest level of safety and durability in such a delicate and anatomically concealed area. The reconstruction of a transmural defect of the posterior hypopharyngeal/upper esophageal wall requires thin, well-vascularized, and stable tissue, with donor sites offering low morbidity, ensuring safety for a high-risk patient with a history of multiple prior surgeries. Given the unique location of the defect—on the posterior wall of the hypopharynx/upper esophagus, an area that is difficult to access and visualize—the surgical team faced a critical question: could the reconstruction be successfully achieved using a free flap, specifically one that could be visually monitored for viability postoperatively?
Despite the absence of clear guidelines or case reports addressing this specific situation in the literature, we chose to proceed with an innovative surgical strategy. The solution involved the use of a free ALT flap, fashioned with two separate skin paddles. One paddle was to be employed for the reconstruction of the hypopharyngeal/upper esophageal posterior wall defect. The second paddle was supposed to be positioned externally, serving as a “monitor paddle”, allowing for real-time visual assessment of the flap’s viability during the critical postoperative period. The technical execution of this procedure required meticulous planning, given the dual objectives of reconstructing the hypopharyngeal/upper esophageal wall while simultaneously providing sufficient coverage for the osteosynthesis material at the C5/6 level.
In February 2024, an interdisciplinary surgical intervention was performed following a multidisciplinary board consensus in this complex case involving cervical implant instability, a chronic implant-associated infection of the cervical osteosynthesis material, a chronic hypopharyngeal/upper esophageal fistula, and a hypopharyngeal/upper esophageal defect requiring reconstruction. The procedure began with the Otolaryngology team performing a left cervicotomy and freshening the wound edges. Subsequently, the Orthopedics team revised the cervical spine, removing the existing plate and intervertebral cage, followed by the implantation of a new anterior cervical titanium 3D-printed highly porous cage at the C5/6 level. Intraoperative probes from the soft tissue around the spine and the bone of C5 and C6 vertebrae were collected for histologic and microbiologic analysis. The Plastic Surgery team harvested a 6 × 16 cm microvascular ALT flap from the right thigh. The two skin paddles were meticulously prepared, and vascularization of both skin paddles was checked at the donor site before clamping the pedicle (Figure 1). The first skin paddle, measuring 2 × 2 cm, was used to reconstruct the hypopharyngeal/upper esophageal mucosal defect, while a 4 × 8 cm section of vascularized fascia from the perforator was positioned between the posterior hypopharyngeal/upper esophageal wall and the cage to provide an additional protective layer (Figure 2). Despite the relatively small size of the mucosal defect, prior surgeries had left the tissue fibrotic and too rigid to be closed primarily without risking tension, dehiscence, or stenosis. This fascia placement also helped prevent future complications by acting as a buffer between the esophagus and the cervical spine hardware.
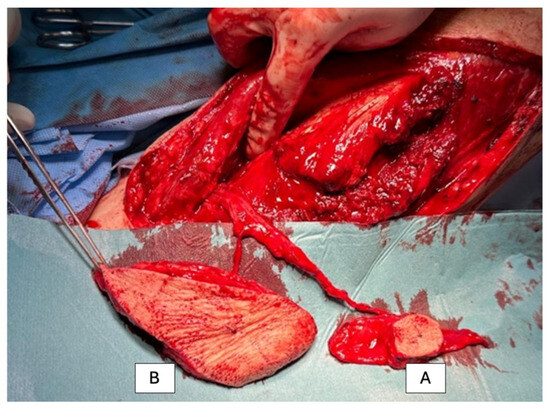
Figure 1.
Harvesting of the free ALT flap from the right thigh. Before clamping the pedicle, the flap is prepared for the subsequent reconstruction. Two skin paddles are created for coverage: Skin paddle A (2 × 2 cm skin, 4 × 8 cm fascial tissue) is used as reconstructive tissue and sutured into the posterior wall of the esophagus, with the fascial tissue providing sufficient size to cover the osteosynthesis material. Skin paddle B is designated as a monitor and will be sutured between the skin wound edges on the left neck side.
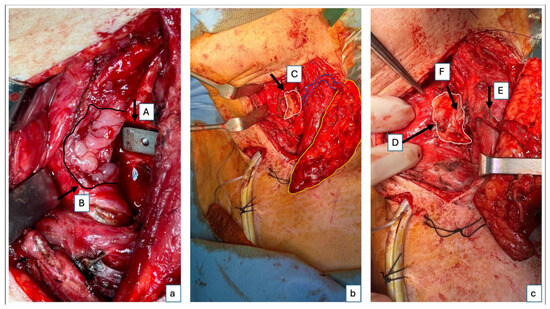
Figure 2.
Intraoperative images of flap inset. Intraoperative images after suturing of the smaller skin paddle for reconstruction of the posterior hypopharyngeal/upper esophageal wall. The second, larger skin paddle has not yet been sutured and will be positioned cervically as a monitoring paddle. (a) A close-up of the cage (A) and the posterior hypopharyngeal/upper esophageal defect marked with the black arrow and delineated by the black line (B). (b) Right lateral fixation of the flap to the defect boarder; the left lateral border of the defect is delineated with the green line, while the small skin paddle used for the defect reconstruction, the monitoring skin paddle, and the perforator going from the monitoring paddle to the skin paddle used for the posterior hypopharyngeal/upper esophageal wall reconstruction are delineated by a white, yellow, and blue line, respectively (C). (c) Complete fixation of the flap in the defect, also on the left side, with a second layer of suture for the fat tissue—the white line delineates the skin paddle used to completely close the posterior hypopharyngeal/upper esophageal wall defect (D); perforator location of the small skin island (E); additional vascularized fascia for cage coverage (F).
The second skin paddle, based on a separate perforator, was externalized to the neck region for postoperative monitoring. This was a critical decision, as the location of the posterior hypopharyngeal/upper esophageal wall made visual inspection impossible without an externalized monitor island. While some may argue that flow-through couplers could be used as a monitoring tool, the literature indicates that their reliability can be inconsistent. Relying solely on them in such a high-risk area would have posed significant risks to the patient’s recovery. Furthermore, local flap options like the platysma or supraclavicular flaps were not viable due to the patient’s history of multiple surgeries.
Postoperatively, the patient’s recovery was uneventful. The flap maintained strong signals throughout, and a Gastrografin swallow test performed 9 days after surgery could exclude any leakage and wound dehiscences (Figure 3). The patient started drinking and successively eating orally 10 days after the procedure. Four months postoperatively (Figure 4), the patient demonstrated a stable course with no complications at the recipient site. The patient reported no dysphagia.
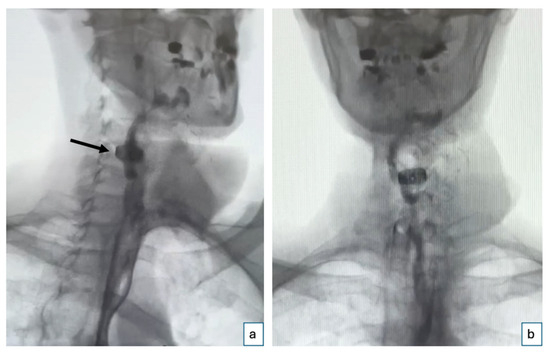
Figure 3.
Gastrografin swallow test. Postoperative Gastrografin swallow test performed on the 9th postoperative day shows an intact esophagus with no evidence of leakage or wound dehiscence: (a) oblique right view (the black arrow indicates the position of the cage) and (b) anteroposterior view.
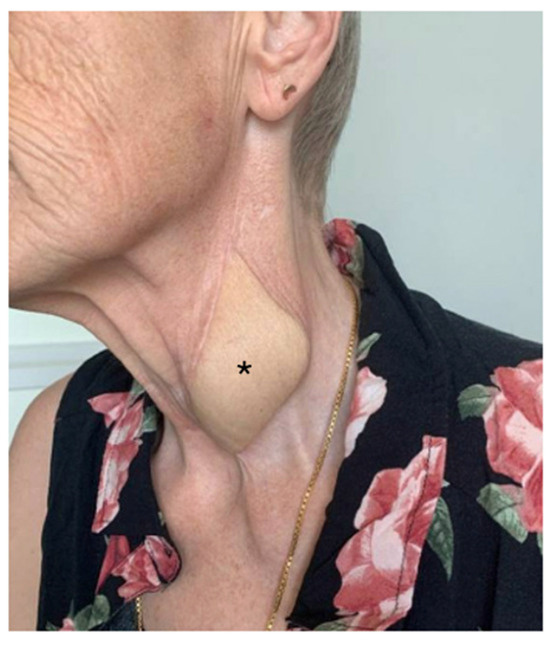
Figure 4.
Postoperative follow-up. Four months postoperatively, with unremarkable scar conditions and excess skin; * marks the monitor spindle.
3. Scoping Review of the Literature
3.1. Methods
The methodological framework for this scoping review was a literature search conducted on 20 June 2024, using PubMed, Cochrane Library, and Google Scholar, following PRISMAScR guidelines. The study selection process is summarized in a PRISMAScR flow diagram (Figure 5). The search strategy focused on identifying studies related to the reconstruction of the posterior esophageal or pharyngeal wall. It used a combination of keywords and MeSH terms, including “posterior”, “esophageal”, “pharyngeal”, and “reconstruction”, with Boolean operators to ensure comprehensive coverage of the relevant literature. To ensure reproducibility, the exact search query used in PubMed was as follows: “posterior” [All Fields] AND (“esophageal” [MeSH Terms] OR “pharyngeal” [MeSH Terms]) AND “reconstruction” [All Fields]. No filters or restrictions (e.g., publication date, study type, or full-text availability) were applied to allow for a comprehensive assessment of all the relevant literature. No restrictions were applied regarding publication years to ensure a comprehensive assessment of all the available literature. Two independent reviewers evaluated the identified articles by screening titles, abstracts, and full texts. Additional studies were included by reviewing the references of relevant articles. Any disagreements between the reviewers were resolved through discussion and consensus. The search strategy was specifically designed to include only primary research studies, excluding reviews, book chapters, and expert opinions. The following variables were extracted: (1) study design (retrospective/prospective), (2) patient population characteristics (age, comorbidities), (3) type of surgical technique used (local vs. free flaps, specific flap type), (4) reported surgical outcomes (functional swallowing, phonation, fistula formation, infection rates), and (5) complications (categorized as minor or major). Where specific outcome definitions varied across studies, data were standardized by categorizing complications into “minor” (requiring conservative management) and “major” (requiring reoperation). No assumptions were made regarding missing data, and only explicitly reported variables were included in the final analysis. Data from the included studies were extracted using a standardized data charting form, which included variables on study design, patient population, surgical techniques, outcomes, and complications. The form was developed based on key parameters identified in previous reviews and was tested for consistency before use. Data charting was performed independently by two reviewers, and discrepancies were resolved through discussion. No direct contact with study investigators was made for additional data confirmation. Articles published in English and German across any journal were considered, and data on surgical techniques, outcomes, and complications were collected. Animal studies, cadaveric studies, and other localizations of the upper digestive tract were excluded. An extensive cross-check of the references from the original publications was performed to identify additional articles. Due to the nature of this study, formal ethics application and approval by the institutional review board were not required. No prior review protocol was registered for this scoping review.

Figure 5.
A PRISMAScR diagram of the scoping literature review.
3.2. Results
The scoping review revealed a variety of reconstructive options for posterior hypopharyngeal/upper esophageal wall defects, ranging from pedicled to free tissue transfers, each with their own advantages and limitations. In our scoping review, 31 of the initial 411 publications treating the reconstruction of posterior hypopharyngeal/upper esophageal defects were included in the final analysis with a total number of 239 patients in 31 publications (225 hypopharyngeal/upper esophageal cancer cases, 11 complications related to osteosynthesis of the spine, 2 spine tumor cases, 1 case after corrosive injury (Figure 6)). The study characteristics, including study design, patient population, surgical techniques, and outcomes, are detailed in Table 1. Posterior hypopharyngeal/upper esophageal wall reconstruction has been performed using various types of flaps (Figure 7 and Figure 8): 105 free flaps and 118 local/pedicled flaps with a mean follow-up time of 26 months (1.5–72 months). Furthermore, two autologous hypopharyngeal/upper esophageal patch techniques and two studies with multiple procedures or other materials (two) were included. Heatmaps of study designs by categories and types of reconstruction by defect etiology are provided in Figure 9. Extracted data were summarized using descriptive statistics, including frequencies for categorical variables (e.g., surgical techniques and complications). Data were organized into figures/tables and visualized through heatmaps to illustrate patterns in reconstructive approaches and complication rates. Complications and revisions related to the flaps are summarized in Table 2. In the thirteen patients treated with acellular dermal matrix, one pharyngeal fistula occurred, with no revisions reported. In total, the complication rate for the local, pedicled, and free flaps was 0% (0/3), 32.2% (38/118), and 19% (20/105), respectively. However, despite the complications described, not every paper reported the revisions performed and what exactly was conducted every time, accounting for the limitations of the retrospective studies.
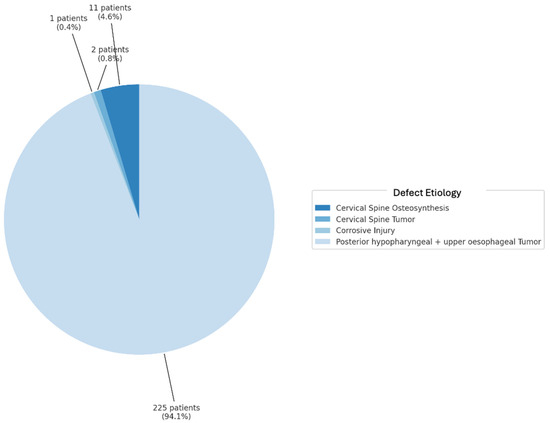
Figure 6.
Defect etiology in percentages.

Table 1.
Overview of studies on posterior hypopharyngeal/upper esophageal wall reconstruction.
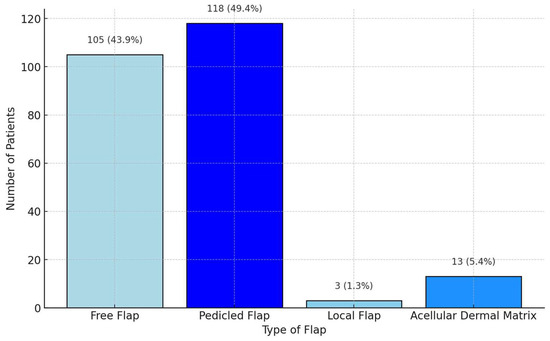
Figure 7.
Grouped distribution of reconstruction methods.
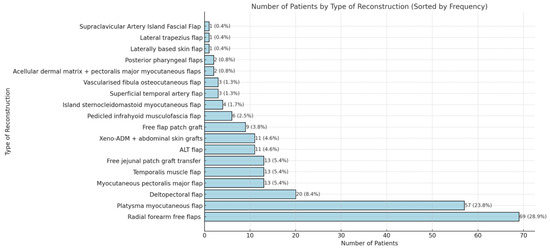
Figure 8.
Detailed distribution of reconstruction methods. Xeno-ADM: Xeno-Acellular Dermal Matrix; ALT: adipofascial anterolateral thigh.
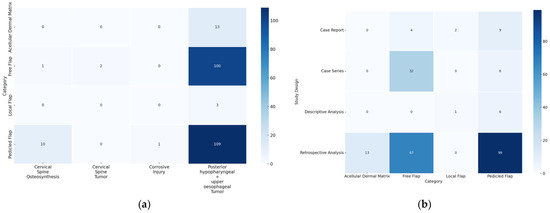
Figure 9.
(a) Heatmap of categories by defect etiology. The y-axis displays the different reconstructive categories (local, pedicled, free flap, and acellular dermal matrix), while the x-axis represents the defect etiology. The color coding indicates the frequency: the darker the color, the more frequently the corresponding combination of category and defect etiology occurs. (b) Heatmap of study designs by categories. The y-axis displays the study designs, while the x-axis represents the different reconstructive categories (local, pedicled, free flap, and acellular dermal matrix). The color coding indicates the frequency: the darker the color, the more frequently the corresponding combination of study design and category occurs.

Table 2.
Complications and revisions for local, pedicled, and free flaps (n = 226 *).
4. Discussion
This study revealed different etiologies for posterior wall defects of the hypopharyngeal/upper esophageal region (posterior hypopharyngeal/upper esophageal carcinoma, spine surgery, chordoma, corrosive trauma) as well as different reconstructive options. In contrast to the small number of cases described in the literature, a wide range of techniques are described. With twelve case reports, five case series, twelve retrospective studies, and two descriptive studies, there are various models that shed light on this topic. The use of a free double-paddle flap for a posterior hypopharyngeal/upper esophageal wall defect has been described only once in the literature [2]. The follow-up of our patient revealed minimal morbidity at the donor site, a patient-centered solution, and a satisfactory postoperative course, with no dysphagia reported 4 months postoperatively.
The reconstruction of the hypopharyngeal and hypopharyngeal/upper esophageal regions after oncologic resection presents significant challenges due to the need to restore critical functions such as swallowing, phonation, and airway integrity. This review explores a variety of reconstructive techniques, including local flaps, pedicled flaps, free flaps, and synthetic materials, each with distinct advantages and potential complications.
4.1. Local Flaps
Historically, in 1974, Seda described a surgical approach for carcinomas of the posterior hypopharyngeal/upper esophageal and hypopharyngeal wall, where reconstruction was achieved with a laterally based skin flap in two stages. His technique preserved a functioning larynx and reestablished the continuity of the upper alimentary tract. This historical approach marked the beginning of using vascularized flaps for reconstructing the posterior hypopharyngeal/upper esophageal wall [3]. The posterior pharyngeal flap has proven to be a reliable and effective method for reconstructing cervical hypopharyngeal/upper esophageal defects, enabling the restoration of hypopharyngeal/upper esophageal continuity in a single-stage operation, with minimal postoperative complications, such as fistula formation [4,5]. The local flap option is reserved for smaller defects and is not suitable for covering osteosynthesis material, as required in our patient’s case.
4.2. Pedicled Flaps
Myocutaneous flaps, such as the pectoralis major flap, have been successfully used for posterior tracheal wall reconstruction, offering stability and reliable coverage in complex cases with extensive resections [6,27]. This flap remains a well-established option for large, circumferential defects, particularly in patients who are poor candidates for microvascular surgery. It provides reliable bulk and coverage for large defects; however, its bulk can sometimes impair functional outcomes, particularly in terms of swallowing and speech recovery [7,25].
For cases requiring thinner, more pliable tissue, the supraclavicular artery island flap has gained prominence. Studies have shown that this flap offers reliable, thin tissue with minimal donor site morbidity, making it ideal for reconstructing moderate-sized defects with functional demands [10,29]. The platysma flap, while less commonly used, offers a simpler alternative for smaller defects, with good functional outcomes and minimal morbidity. It provides a one-stage solution for reconstructing smaller posterior hypopharyngeal/upper esophageal wall defects, offering minimal donor site morbidity and acceptable functional outcomes in swallowing and respiration [7,20,24,28]. A notable limitation of supraclavicular and platysma flaps is their dependence on a neck that has not been subjected to multiple prior surgeries.
The lateral trapezius flap presents another option for reconstructing extensive posterior hypopharyngeal/upper esophageal wall defects, with successful outcomes in maintaining airway patency and swallowing function in complex cases [15]. The temporalis muscle flap, with its robust blood supply and versatility, has also shown promise in reconstructing posterior hypopharyngeal/upper esophageal wall defects. It provides flexible coverage and reduces donor site morbidity compared to other regional flaps. While less commonly used, it has demonstrated reliable outcomes for restoring swallowing and airway patency without compromising speech [17]. Despite these advancements, complications such as fistula formation and flap necrosis remain significant challenges across all reconstructive methods [29]. While both of these options seem viable, head and neck reconstruction is nowadays more focused on the “reconstructive elevator”, with free tissue transfer being the first choice in such cases, in order to disturb the surrounding tissues as little as possible. Moreover, the lateral trapezius flap could be associated with accessory nerve problems, while donor site morbidity after temporalis muscle harvest entails contour deformity and an extensive undermined area in order to place the flap in the desired defect.
The deltopectoral flap continues to be a reliable and versatile option for head and neck reconstruction, particularly for posterior hypopharyngeal/upper esophageal wall defects, due to its technical simplicity and consistent survival rates [6]. However, the pedicle of the flap remains visible, therefore deforming the sternoclavicular area. Newer methods, such as the island sternocleidomastoid myocutaneous flap, have also shown promising results in managing posterior hypopharyngeal/upper esophageal wall defects after cervical spine surgeries, but they are indicated in cases without previous neck surgeries [25,34].
4.3. Free Flaps
Free flaps are a cornerstone in reconstructive surgery, particularly for addressing complex defects with extensive tissue loss and functional demands. Due to the necessity of two skin paddles, Fritz et al. reported their experiences, like our case, with a free double-paddle posterior tibial artery flap. The indication was a leakage of the hypopharynx after bougienage, which occurred through stenosis due to radiotherapy after tumor resection. Compared to our case, where no external skin was required, soft tissue with a skin paddle was also required in the neck area, as previous operations and radiotherapy had led to severe fibrosis of the neck. A purposeful result was achieved, and the authors appreciated the possibility of monitoring the otherwise hidden flap reconstruction [2]. This work differs from our technique in that the monitoring skin paddle was intentionally used to provide additional soft tissue coverage for the neck, whereas in our case, this was not necessary, and we plan to remove the skin paddle 1–2 years postoperatively. Moreover, we consider the thickness of a lower-leg free flap a disadvantage for posterior hypopharyngeal/upper esophageal wall reconstruction, where thin tissue is necessary.
The radial forearm free flap (RFFF) remains a gold standard for larger and more complex reconstructions due to its pliability, long vascular pedicle, adaptability to different anatomical regions, and suitability for irradiated areas [20]. It is particularly useful for posterior hypopharyngeal/upper esophageal wall defects and has been associated with faster recovery of swallowing and decannulation [9,18,20,22]. Reconstruction with RFFF in posterior hypopharyngeal/upper esophageal wall resections demonstrated functional voice preservation and acceptable morbidity in healthy patients, though patients with significant comorbidities exhibited increased complications [21]. However, the donor site morbidity of the RFFF is often criticized. Basaran et al. published original work in 2020 about the reconstruction of posterior esophagus defects using radial forearm flaps. Compared to our result (10 days after surgery), the patients achieved oral intake in a median time of 74 days after surgery. The authors concluded that tumor resection does not compromise the swallowing function permanently if hypopharyngeal/upper esophageal wall reconstruction is performed with a free flap [16]. RFFF reconstruction was also shown to achieve acceptable functional results in patients undergoing larynx-preserving posterior pharyngectomy, with six out of seven patients resuming oral intake, although long-term PEG dependence remained a risk for patients with swallowing difficulties [19]. The U-shaped RFFF has been identified as a reliable technique also for reconstructing the hypopharynx, offering functional benefits such as a well-maintained swallowing process in most patients while minimizing complications associated with jejunal grafts [11].
The adipofascial anterolateral thigh (ALT) flap is another option that offers thinner, more pliable tissue than the pectoralis major muscle flap (PMMF) and avoids bulk, which can compromise functional recovery [12,26]. It is particularly beneficial for smaller, complex defects requiring both functional and aesthetic considerations [26]. The ALT flap is highlighted as an effective option for complex reconstructions, providing adequate tissue volume and a robust vascular supply, particularly beneficial in challenging anatomical regions [30]. However, the skin can be reliably preserved in order to provide an even more stable inner lining of the esophagus. Fasciocutaneous free flaps, such as the ALT flap or RFFF, have been effectively utilized for hypopharyngeal/upper esophageal wall reconstruction, with favorable oncologic outcomes and an 84% success rate in restoring oral nutritional intake [8]. One-stage reconstruction utilizing a free vascularized fibula steocutaneous flap has also been described for simultaneous restoration of the posterior hypopharyngeal/upper esophageal wall and cervical spine, offering a comprehensive solution for complex defects [23]. However, the fibula flap was not considered necessary in our case as no bone reconstruction was required, and the patient’s slim physique made the ALT flap an excellent option due to its versatility and adequacy for soft tissue reconstruction.
Free jejunal patch graft transfer after partial hypopharyngectomy has demonstrated satisfactory swallowing function with low complication rates, making it a viable reconstruction option for posterior hypopharyngeal/upper esophageal wall defects. Notably, 95% of patients resumed oral intake, with outcomes varying slightly based on defect extent [13]. Similarly, the free jejunal graft is an established technique for circumferential hypopharyngeal/upper esophageal defects, providing early restoration of oral intake and low rates of fistula formation [22,33]. However, the intraabdominal access can increase morbidity postoperatively.
4.4. Acellular Dermal Matrix
The use of a xenogeneic acellular dermal matrix (xeno-ADM) in combination with traditional flaps, such as the PMMF, offers an alternative technique that reduces donor site morbidity and promotes faster recovery of swallowing function [32,33]. ADM is suitable when well-vascularized tissue is available as a foundation; however, in our case, the presence of an osteosynthesis plate rendered this option inapplicable.
4.5. Venous Flow Coupler in Head and Neck
We are aware that medical technological innovations are available for monitoring buried flaps and that these techniques are also utilized in the head and neck region [35]. The study by Troob et al. highlights the reliability and utility of the venous flow coupler in monitoring free flap reconstructions in the head and neck region. With a sensitivity of 90% and specificity of 94.9%, the device proved highly effective, particularly with its negative predictive value of 98.9%, ensuring the reliable identification of viable flaps. Among 217 cases, 20 required revisions for flap compromise, with 16 successfully salvaged. Notably, the coupler detected five compromised flaps before arterial signal changes occurred, enabling timely interventions and improved salvage rates. Although the false-positive rate was 5.1%, it increased to 24.1% when two couplers were used simultaneously, likely due to complex flow dynamics. The coupler’s ability to continuously monitor venous flow makes it particularly valuable for detecting venous thrombosis early, even in the absence of arterial signal loss. Despite occasional challenges, such as false signals due to backflow, the venous flow coupler remains a reliable tool for enhancing the early detection of complications and improving flap salvage outcomes [35]. Nevertheless, a residual risk of limited reliability remains. In this patient with multiple previous operations, we specifically aimed to minimize the risk of false-negative monitoring and the associated need for unnecessary revision surgery.
Various techniques for posterior hypopharyngeal/upper esophageal wall reconstruction, including free flaps like radial forearm, fibula, and ALT flaps, have been described, each with specific advantages and disadvantages. An individualized multimodal approach tailored to each patient is crucial, and the choice depends on defect size, comorbidities, and prior radiotherapy [31]. Our described technique, while innovative and effective, requires specialized microvascular expertise, limiting its use in centers without advanced reconstructive capabilities. Long-term functional outcomes, such as swallowing and phonation, warrant further investigation. Future studies should evaluate flap durability, especially in high-risk patients [34].
4.6. Limitations and Future Directions
The main criticism of this technique is the actual reliability of the monitoring skin island, since it can also provide false-negative or false-positive signals, potentially misleading the assessment of the reconstructive component.
Further, this study is limited by the rarity of isolated posterior hypopharyngeal/upper esophageal wall defects, restricting generalizability. Long-term outcomes, including stricture formation and functional recovery, require further study. Additionally, the scoping review process was limited by the variability in study designs, differences in reporting standards, and potential publication bias, which may have influenced the availability of comparable data. The exclusion of non-English and non-German studies may have further impacted the comprehensiveness of the findings. Future research should focus on improving other advanced monitoring methods since multicenter validation and statistically relevant comparative studies are rather impossible due to the rarity of and the variability in these types of defects.
5. Conclusions
Several methods have been proposed for reconstructing the posterior hypopharyngeal/upper esophageal wall, though no single technique has demonstrated clear superiority. Our case report suggests that posterior hypopharyngeal/upper esophageal wall reconstruction with a double-island free fasciocutaneous ALT flap is feasible and allows monitoring in the postoperative course in an otherwise not visible area, when one of the islands is buried (for reconstructive purposes) and the other one is externalized (for monitoring purposes). Our approach offers a potential solution for cases involving complex hypopharyngeal/upper esophageal defects and osteosynthesis material coverage, particularly when traditional methods are unsuitable. Future studies should evaluate long-term functional outcomes, complication rates, and comparative efficacy with other reconstructive techniques in larger patient cohorts.
Author Contributions
Conceptualization, I.L.; methodology, L.G.D. and I.L.; validation, L.G.D. and I.L.; formal analysis, I.L.; investigation, L.G.D. and I.L.; data curation, L.G.D.; writing—original draft preparation, L.G.D.; writing—review and editing, V.A.P., M.A.C., M.C.D., R.G. and I.L.; visualization, L.G.D.; supervision, I.L. and R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. In our institution, ethical approval is not required for case reports.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Masarapu, V.; Xia, E.; Son, H. Esophageal Emergencies: Another Important Cause of Acute Chest Pain. Insights Imaging 2020, 11, 109. [Google Scholar] [CrossRef]
- Fritz, C.; Meroni, M.; Fritsche, E.; Rajan, G.; Scaglioni, M.F. Free double-paddle posterior tibial artery perforator flap for hypopharynx reconstruction: A case report and literature review. Microsurgery 2021, 41, 660–665. [Google Scholar] [CrossRef]
- Rubinstein, M.; Creter, D.; Rotem, Y. Reconstruction of Upper Cervical Esophagus. Arch. Otolaryngol.-Head Neck Surg. 1975, 101, 695–697. [Google Scholar] [CrossRef]
- Li, L.; Zou, L.; Fang, J. A Case Report of Application of Posterior Pharyngeal Flap in Resection and Reconstruction of Posterior Pharyngeal Wall Carcinomas Located at the Level of the Cricoid Cartilage. Medicine 2019, 98, e14412. [Google Scholar] [CrossRef]
- Seda, H.J.; Snow, J.B. Surgery for Carcinoma of the Posterior Pharyngeal Wall. Arch. Otolaryngol.-Head Neck Surg. 1974, 99, 297–299. [Google Scholar] [CrossRef]
- Chan, R.C.L.; Chan, J.Y.W. Deltopectoral Flap in the Era of Microsurgery. Surg. Res. Pract. 2014, 2014, 420892. [Google Scholar] [CrossRef][Green Version]
- Alvarez, J.C.; Suarez, C.; Sota, E.; Rodrigo, J.P.; Fernandez, J.A.; Llorente, J.L.; Diaz, C. Tumours of the Posterior Pharyngeal Wall: The Use of the Platysma Flap. Clin. Otolaryngol. Allied Sci. 1997, 22, 246–250. [Google Scholar] [CrossRef]
- Joo, Y.-H.; Cho, K.-J.; Park, J.-O.; Nam, I.-C.; Kim, M.-S. Role of Larynx-Preserving Partial Hypopharyngectomy with and without Postoperative Radiotherapy for Squamous Cell Carcinoma of the Hypopharynx. Oral Oncol. 2012, 48, 168–172. [Google Scholar] [CrossRef]
- Steinhart, H.; Constantinidis, J.; Iro, H. Kehlkopferhaltende Chirurgie bei Karzinomen der Rachenhinterwand durch Rekonstruktion mit freien Lappen. HNO 1998, 46, 135–139. [Google Scholar] [CrossRef]
- Gibson, A.W.; Gobillot, T.A.; Bass, D.I.; Zakarevicius, Z.; Rizvi, Z.H.; Ravanpay, A.C. Case of Esophageal Perforation and Repair with a Supraclavicular Artery Island Fascial Flap 15 Years After Anterior Spine Surgery. World Neurosurg. 2020, 143, 102–107. [Google Scholar] [CrossRef]
- Bootz, F.; Weber, A.; Oeken, J.; Keiner, S. Rekonstruktion des Hypopharynx nach Pharyngolaryngektomie mit U-förmigem Unterarmlappen. Laryngo-Rhino-Otol. 2002, 81, 17–21. [Google Scholar] [CrossRef]
- Coelho, R.; Ekberg, T.; Svensson, M.; Mani, M.; Rodriguez-Lorenzo, A. Reconstruction of Late Esophagus Perforation after Anterior Cervical Spine Fusion with an Adipofascial Anterolateral Thigh Free Flap: A Case Report. Microsurgery 2017, 37, 684–688. [Google Scholar] [CrossRef]
- Miyamoto, S.; Sakuraba, M.; Asano, T.; Hayashi, R.; Ebihara, M.; Miyazaki, M.; Daiko, H.; Shinozaki, T.; Kimata, Y. Free Jejunal Patch Graft for Reconstruction After Partial Hypopharyngectomy with Laryngeal Preservation. Arch. Otolaryngol. Neck Surg. 2011, 137, 181. [Google Scholar] [CrossRef]
- Losco, L.; Aksoyler, D.; Chen, S.; Bolletta, A.; Velazquez-Mujica, J.; Di Taranto, G.; Lo Torto, F.; Marcasciano, M.; Cigna, E.; Chen, H. Pharyngoesophageal Reconstruction with Free Jejunum or Radial Forearm Flap as Diversionary Conduit: Functional Outcomes of Patients with Persistent Dysphagia and Aspiration. Microsurgery 2020, 40, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Singh, A.; Abrari, A.; Singh, N. Monophasic Synovial Sarcoma of Posterior Pharyngeal Wall: A Rare Case Report with Unique Reconstruction Using Lateral Trapezius Flap. Eur. Arch. Otorhinolaryngol. 2017, 274, 2059–2064. [Google Scholar] [CrossRef]
- Başaran, B.; Ünsaler, S. Carcinoma of the Posterior Wall of the Hypopharynx: Surgical Treatment with Larynx Preservation. Braz. J. Otorhinolaryngol. 2022, 88, 174–180. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Utley, D.S.; Goode, R.L. The Temporalis Muscle Flap for Reconstruction After Head and Neck Oncologic Surgery. Laryngoscope 2001, 111, 1719–1725. [Google Scholar] [CrossRef]
- Iyer, S.; Thankappan, K.; Duarah, S.; Trivedi, N.; Panikar, D.; Kuriakose, M. Vascularised Fibula Osteocutaneous Flap for Cervical Spinal and Posterior Pharyngeal Wall Reconstruction. Indian J. Plast. Surg. 2009, 42, 259. [Google Scholar] [CrossRef]
- Jol, J.-K.A.D.; Quak, J.J.; De Bree, R.; Leemans, C.R. Larynx Preservation Surgery for Advanced Posterior Pharyngeal Wall Carcinoma with Free Flap Reconstruction: A Critical Appraisal. Oral Oncol. 2003, 39, 552–558. [Google Scholar] [CrossRef]
- Julieron, M.; Kolb, F.; Schwaab, G.; Marandas, P.; Billard, V.; Lusinchi, A.; Le Ridant, A.-M.; Luboinski, B. Surgical Management of Posterior Pharyngeal Wall Carcinomas: Functional and Oncologic Results. Head Neck 2001, 23, 80–86. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Kraus, D.H.; Cordeiro, P.G.; Hidalgo, D.A.; Shah, J.P. Posterior Pharyngeal Carcinoma Resection with Larynx Preservation and Radial Forearm Free Flap Reconstruction: A Preliminary Report. Head Neck 1996, 18, 501–505. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Harii, K.; Ueda, K.; Ebihara, S.; Asai, M.; Hirano, K.; Yoshizumi, T.; Sugasawa, T. Preservation of the Larynx after Resection of a Carcinoma of the Posterior Wall of the Hypopharynx: Versatility of a Free Flap Patch Graft. Head Neck 1997, 19, 137–142. [Google Scholar] [CrossRef]
- Ng, R.L.; Beahm, E.; Clayman, G.L.; Hassenbusch, S.J.; Miller, M.J. Simultaneous reconstruction of the posterior pharyngeal wall and cervical spine with a free vascularized fibula osteocutaneous flap. Plast Reconstr Surg. 2002, 109, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Suárez Nieto, C.; Lorenzo Gallego, L.; Galán Cortés, J.C. Reconstruction of the posterior wall of the pharynx using a myocuta-neous platysma flap. Br. J. Plast. Surg. 1983, 36, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Pfitzmann, R. Plastic Reconstruction of an Extended Corrosive Injury of the Posterior Tracheal Wall with an Autologous Esophageal Patch. Eur. J. Cardiothorac. Surg. 2003, 24, 463–465. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, A.; Rydevik Mani, M.; Thor, A.; Gudjonsson, O.; Marklund, N.; Olerud, C.; Ekberg, T. Fibula Osteo-Adipofascial Flap for Reconstruction of a Cervical Spine and Posterior Pharyngeal Wall Defect: Fibula Osteo-Adipofascial Flap in Cervical Spine Reconstruction. Microsurgery 2014, 34, 314–318. [Google Scholar] [CrossRef]
- Salmerón-González, E.; García-Vilariño, E.; Antolin-Santamaria, P.A.; Sanchez-Garcia, A.; Valverde-Navarro, A.A. Reconstruction of a Posterior Tracheal Wall Defect With a Myocutaneous Pectoralis Major Flap After Salvage Cervical Exenteration for a Squamous Carcinoma of the Upper Third of the Esophagus. Plast. Surg. Nurs. 2018, 38, 162–165. [Google Scholar] [CrossRef]
- Seidl, R.O.; Niedeggen, A.; Todt, I.; Westhofen, M.; Ernst, A. Infrahyoid Muscle Flap for Pharyngeal Fistulae after Cervical Spine Surgery: A Novel Approach—Report of Six Cases. Eur. Spine J. 2007, 16, 501–505. [Google Scholar] [CrossRef][Green Version]
- Shen, L.; Fan, G.-K.; Zhu, Y.; Xu, F.; Zhan, W. Superficial Temporal Artery Flap: A New Option for Posterior Hypopharyngeal Wall Reconstruction. Eur. Arch. Otorhinolaryngol. 2011, 268, 1017–1021. [Google Scholar] [CrossRef]
- Mathy, J.A.; Proctor, M.R.; Rogers, G.F. Cranial Expansion in an Infant with Malignant Osteopetrosis. Plast. Reconstr. Surg. 2010, 126, 134e–135e. [Google Scholar] [CrossRef]
- Spiro, R.H.; Kelly, J.; Vega, A.L.; Harrison, L.B.; Strong, E.W. Squamous carcinoma of the posterior pharyngeal wall. Am. J. Surg. 1990, 160, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Feng, S.; Yang, H.; Guo, X. Comparison of Xenogeneic Acellular Dermal Matrix and Skin Grafts in Reconstruction of Postoperative Defects of Hypopharyngeal Cancer: A Retrospective Cohort Study. Medicine 2020, 99, e19361. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.-P.; Zhang, W.-C.; Li, W.; Anniko, M. Reconstruction of Defects Following Surgery for Hypopharyngeal Carcinoma Using Artificial Biological Material. Acta Otolaryngol. 2010, 130, 1293–1299. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, R.; Wang, G.; Chen, Y.; Ding, P.; Yang, X.; Zhao, Z.; Zhang, Y. Island Sternocleidomastoid Myocutaneous Flap for Posterior Pharyngeal Wall Defect Repair after Anterior Cervical Spine Surgery. Int. Wound J. 2022, 19, 169–177. [Google Scholar] [CrossRef]
- Troob, S.H.; Self, Q.; Gerecci, D.; Hodgson, M.; González-Castro, J.; Petrisor, D.; Wax, M.K. Venous Flow Coupler in Head and Neck Free Flap Reconstruction. Otolaryngol. Neck Surg. 2021, 164, 574–579. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).