The Role of Blood Inflammatory Markers in Salivary Gland Carcinoma: A Scoping Review
Abstract
1. Introduction
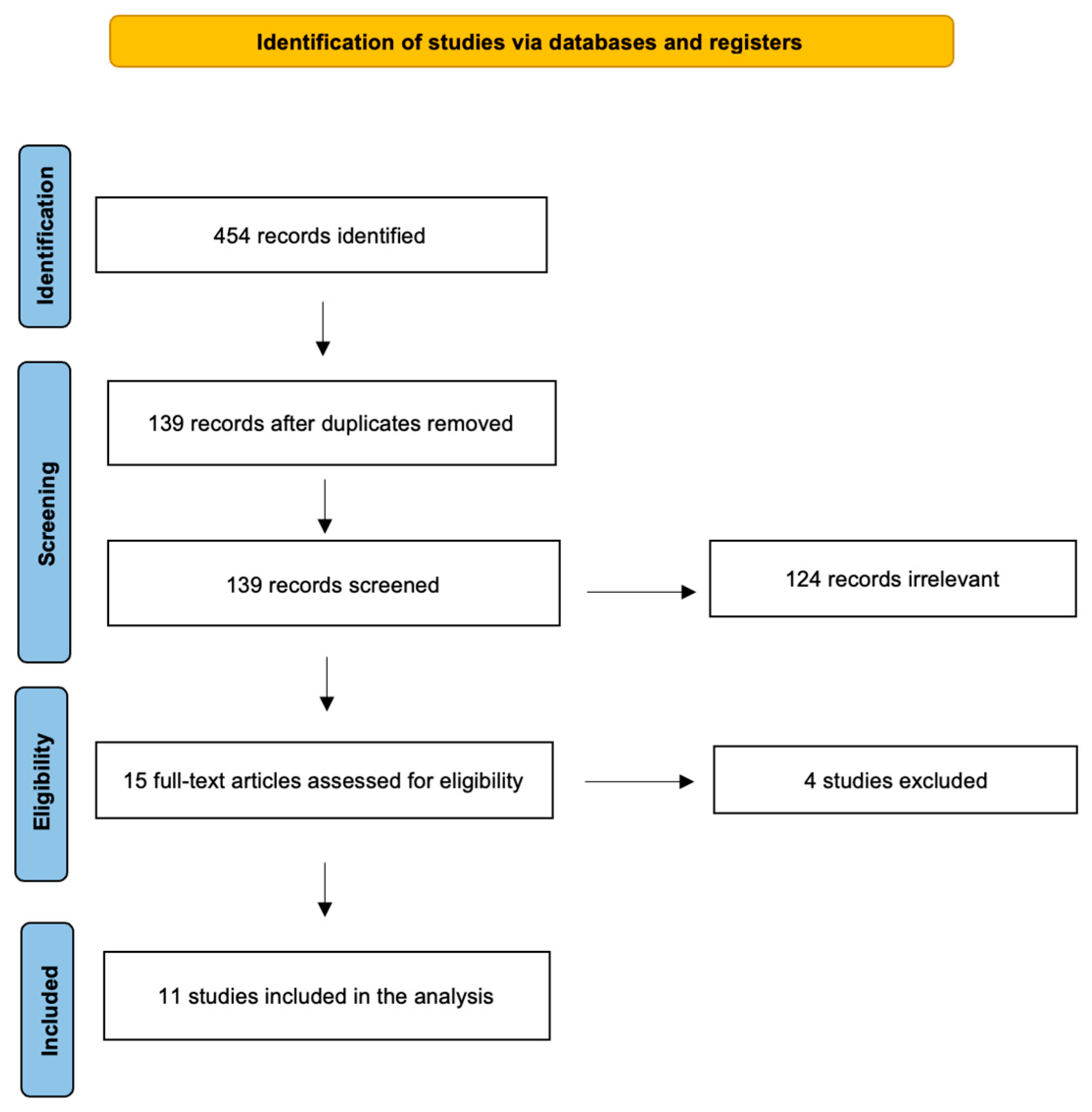
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jegadeesh, N.; Liu, Y.; Prabhu, R.S.; Magliocca, K.R.; Marcus, D.M.; Higgins, K.A.; Vainshtein, J.M.; Wadsworth, J.T.; Beitler, J.J. Outcomes and prognostic factors in modern era management of major salivary gland cancer. Oral Oncol. 2015, 51, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, K.M.; Abd-Elhay, F.A.; Kamel, M.G.; Abd El Hamid Hassan, H.H.; El Tanany, H.H.M.; Hieu, T.H.; Tieu, T.M.; Low, S.K.; Hou, V.; Dibas, M.; et al. Examined and positive lymph nodes counts and lymph nodes ratio are associated with survival in major salivary gland cancer. Head Neck 2019, 41, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, R. Malignant salivary gland tumors: A short review. Oral Maxillofac. Res. 2021, 7, 1–5. [Google Scholar] [CrossRef]
- Andreasen, S.; Stevens, E.; Bjørndal, K.; Homøe, P. Salivary gland epithelial neoplasms in pediatric population: A single-institute experience with a focus on the histologic spectrum and clinical outcome. Hum. Pathol. 2018, 73, 193–194. [Google Scholar] [CrossRef]
- Fang, Q.G.; Shi, S.; Li, Z.N.; Zhang, X.; Liu, F.Y.; Sun, C.F. Epithelial salivary gland tumors in children: A twenty-five-year experience of 122 patients. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1252–1254. [Google Scholar] [CrossRef]
- Li, L.J.; Li, Y.; Wen, Y.M.; Liu, H.; Zhao, H.W. Clinical analysis of salivary gland tumor cases in West China in past 50 years. Oral Oncol. 2008, 44, 187–192. [Google Scholar] [CrossRef]
- Kupferman, M.E.; de la Garza, G.O.; Santillan, A.A.; Williams, M.D.; Varghese, B.T.; Huh, W.; Roberts, D.; Weber, R.S. Outcomes of pediatric patients with malignancies of the major salivary glands. Ann. Surg. Oncol. 2010, 17, 3301–3307. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.S.P. WHO Classification of Head and Neck Tumours, 4th ed.; IARC Publication: Lyon, France, 2017; pp. 159–202. [Google Scholar]
- Peravali, R.K.; Bhat, H.H.; Upadya, V.H.; Agarwal, A.; Naag, S. Salivary gland tumors: A diagnostic dilemma! J. Maxillofac. Oral Surg. 2015, 14 (Suppl. S1), 438–442. [Google Scholar] [CrossRef]
- Ronchi, A.; Montella, M.; Zito Marino, F.; Panarese, I.; Pagliuca, F.; Colella, G.; Franco, R.; Cozzolino, I. Diagnostic accuracy of FNA cytology for diagnosis of salivary gland tumors in pediatric patients. Cancer Cytopathol. 2019, 127, 529–538. [Google Scholar] [CrossRef]
- Dell’Aversana Orabona, G.; Salzano, G.; Abbate, V.; Bonavolontà, P.; Committeri, U.; Seidita, F.; Petrocelli, M.; Somma, T.; Improta, G.; Vaira, L.A.; et al. Malignant tumours of the parotid gland: Management of the neck (including the clinically negative neck) and a literature review. Br. J. Oral Maxillofac. Surg. 2021, 59, 665–671. [Google Scholar] [CrossRef]
- Bianchini, C.; Brugali, M.; Migliorelli, A.; Corazzi, V.; Cammaroto, G.; Meccariello, G.; Stomeo, F.; Ciorba, A.; Pelucchi, S. Basal cell adenoma and pleomorphic adenoma of the parotid gland: A single center experience. Minerva Surg. 2023, 78, 626–632. [Google Scholar] [CrossRef]
- Rossi, E.D.; Faquin, W.C. The Milan system for reporting salivary gland cytopathology (MSRSGC): An international effort toward improved patient care—When the roots might be inspired by Leonardo da Vinci. Cancer Cytopathol. 2018, 126, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of Salivary Gland Malignancy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef] [PubMed]
- Damar, M.; Dinç, A.E.; Erdem, D.; Aydil, U.; Kizil, Y.; Eravcı, F.C.; Bişkin, S.; Şevik Eliçora, S.; Işik, H. Pretreatment Neutrophil-Lymphocyte Ratio in Salivary Gland Tumors Is Associated with Malignancy. Otolaryngol. Head Neck Surg. 2016, 155, 988–996. [Google Scholar] [CrossRef]
- Nakayama, M.; Gosho, M.; Hirose, Y.; Nishimura, B.; Tanaka, S.; Tabuchi, K.; Okubo, H.; Wada, T.; Hara, A. Modified combination of platelet count and neutrophil “to” lymphocyte ratio as a prognostic factor in patients with advanced head and neck cancer. Head Neck 2018, 40, 1138–1146. [Google Scholar] [CrossRef]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Sethi, M.; Polesel, J.; Tomasoni, M.; Deganello, A.; Nicolai, P.; Bossi, P.; Fabbris, C.; Molteni, G.; Marchioni, D.; et al. The risk of recurrence in surgically treated head and neck squamous cell carcinomas: A conditional probability approach. Acta Oncol. 2021, 60, 942–947. [Google Scholar] [CrossRef]
- Kwon, H.C.; Kim, S.H.; Oh, S.Y.; Lee, S.; Lee, J.H.; Choi, H.J.; Park, K.J.; Roh, M.S.; Kim, S.G.; Kim, H.J.; et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012, 17, 216–222. [Google Scholar] [CrossRef]
- Hua, X.; Long, Z.Q.; Zhang, Y.L.; Wen, W.; Guo, L.; Xia, W.; Zhang, W.W.; Lin, H.X. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Breast Cancer: A Propensity Score-Matching Study. Front. Oncol. 2020, 10, 580. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; D’Alessandro, A.; Polesel, J.; Borsetto, D.; Tofanelli, M.; Deganello, A.; Tomasoni, M.; Nicolai, P.; Bossi, P.; Spinato, G.; et al. Different inflammatory blood markers correlate with specific outcomes in incident HPV-negative head and neck squamous cell carcinoma: A retrospective cohort study. BMC Cancer 2022, 22, 243. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, D.; Tada, Y.; Imanishi, Y.; Beppu, S.; Tsukahara, K.; Kano, S.; Ozawa, H.; Okami, K.; Sato, Y.; Shimizu, A.; et al. Impact of hematological inflammatory markers on clinical outcome in patients with salivary duct carcinoma: A multi-institutional study in Japan. Oncotarget 2017, 8, 1083–1091. [Google Scholar] [CrossRef]
- Fang, Q.; Liu, F.; Seng, D. Oncologic outcome of parotid mucoepidermoid carcinoma in pediatric patients. Cancer Manag. Res. 2019, 11, 1081–1085. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, F.; Niu, X.; Fang, Q. Role of the pretreatment neutrophil-to-lymphocyte ratio in the survival of primary parotid cancer patients. Cancer Manag. Res. 2019, 11, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Seng, D.; Fang, Q.; Li, P.; Liu, F.; Liu, S. Prognostic Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio in Pediatric Parotid Cancer. Front. Pediatr. 2019, 7, 207. [Google Scholar] [CrossRef]
- Gao, H.; Gao, Q.; Sun, J. Significance of Pretreatment Neutrophil-to-Lymphocyte Ratio in Mucoepidermoid Carcinoma of Pediatrics: A Multicenter Study. Front. Pediatr. 2020, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Somay, E.; Yilmaz, B.; Topkan, E.; Kucuk, A.; Pehlivan, B.; Selek, U. Initial neutrophil-to-lymphocyte ratio predicts radiation-induced trismus in parotid gland cancer. Oral Dis. 2023, 29, 2772–2779. [Google Scholar] [CrossRef]
- Abbate, V.; Barone, S.; Troise, S.; Laface, C.; Bonavolontà, P.; Pacella, D.; Salzano, G.; Iaconetta, G.; Califano, L.; Dell’Aversana Orabona, G. The Combination of Inflammatory Biomarkers as Prognostic Indicator in Salivary Gland Malignancy. Cancers 2022, 14, 5934. [Google Scholar] [CrossRef]
- Committeri, U.; Barone, S.; Salzano, G.; Arena, A.; Borriello, G.; Giovacchini, F.; Fusco, R.; Vaira, L.A.; Scarpa, A.; Abbate, V.; et al. Support Tools in the Differential Diagnosis of Salivary Gland Tumors through Inflammatory Biomarkers and Radiomics Metrics: A Preliminary Study. Cancers 2023, 15, 1876. [Google Scholar] [CrossRef]
- Lee, R.H.; Truong, A.; Wu, X.; Kang, H.; Algazi, A.P.; El-Sayed, I.H.; George, J.R.; Heaton, C.M.; Ryan, W.R.; Ha, P.K.; et al. The neutrophil-to-lymphocyte ratio in salivary gland cancers treated with pembrolizumab. Head Neck 2024, 46, 129–137. [Google Scholar] [CrossRef]
- Abbate, V.; Barone, S.; Borriello, G.; Troise, S.; Bonavolontà, P.; Pacella, D.; Vaira, L.A.; Turri-Zanoni, M.; Cuéllar, C.N.; Califano, L.; et al. Diagnostic performance of inflammatory biomarkers and cytological analysis in salivary gland tumors. Head Neck 2023, 45, 3015–3023. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wu, J.; Wang, S.; Xu, S. Perioperative change in neutrophil count predicts worse survival in esophageal squamous cell carcinoma. Future Oncol. 2021, 17, 4721–4731. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Kitamiura, T.; Ashida, N.; Shimizu, K.; Takemura, K.; Yamamoto, Y.; Uno, A. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: A meta-analysis. Head Neck 2018, 40, 647–655. [Google Scholar] [CrossRef]
- Ulich, T.R.; Del Castillo, J.; Guo, K.Z. In vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCs. Blood 1989, 73, 108–110. [Google Scholar] [CrossRef]
- Di Carlo, E.; Forni, G.; Lollini, P.; Colombo, M.P.; Modesti, A.; Musiani, P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood 2001, 97, 339–345. [Google Scholar] [CrossRef]
- Ji, H.; Houghton, A.M.; Mariani, T.J.; Perera, S.; Kim, C.B.; Padera, R.; Tonon, G.; McNamara, K.; Marconcini, L.A.; Hezel, A.; et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 2006, 25, 2105–2112. [Google Scholar] [CrossRef]
- Cassatella, M.A. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 1995, 16, 21–26. [Google Scholar] [CrossRef]
- Quigley, D.A.; Kristensen, V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol. Oncol. 2015, 9, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M. Role of Platelets and Platelet Receptors in Cancer Metastasis. J. Hematol. Oncol. 2018, 11, 125. [Google Scholar] [CrossRef]
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, thrombo-inflammation, and cancer: Collaborating with the enemy. Front. Immunol. 2019, 10, 1805. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, H.; Yan, A.; Wang, H.; Li, X.; Liu, J.; Li, W. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: A meta-analysis. BMC Cancer 2018, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Kano, S.; Homma, A.; Hatakeyama, H.; Mizumachi, T.; Sakashita, T.; Kakizaki, T.; Fukuda, S. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck 2017, 39, 247–253. [Google Scholar] [CrossRef]
| Authors (Yrs) | Country | NoP | Average Yrs (Range Yrs) | A/C | M/B | Parameters and Cut-Off | Cut-Off Calculation |
|---|---|---|---|---|---|---|---|
| Damar (2016) [15] | Turkey | 182 | 53 (16–87) | A | M: 58 B: 124 | NLR: 1.86 | ROC |
| Kawakita (2017) [23] | Japan | 140 | 64 (26–84) | A | M | NLR: 2.5 PLR: 186.2 | ROC |
| Fang (2019) [24] | China | 73 | 14.3 (8–18) | C | M | NLR: 2.48 | Mean value |
| Cheng (2019) [25] | China | 249 | 47.7 (19–73) | A | M | NLR: 2.48 | Mean value |
| Seng (2019) [26] | China | 123 | 14.3 (6–18) | C | M | NLR: 2.51 | Mean value |
| Gao (2020) [27] | China | 88 | 14.2 (6–18) | C | M | NLR: 2.32 | Mean value |
| Somay (2022) [28] | Turkey | 51 | 52 (31–75) | A | M | NLR: 2.7 | ROC |
| Abbate (2022) [29] | Italy | 74 | 56 (13–85) | A | M | NLR: 3.95 PLR: 187.6 SII: 917.585 SIRI: 2.045 | ROC |
| Committeri (2023) [30] | Italy | 117 | Warthin: 62 Pleomorphic 52.5 Malignant 53.5 | A | M: 28 B: 89 | NLR: 3.62 PLR: 133.30 SII: 594.91 SIRI: 1.61 | ROC |
| Lee (2023) [31] | USA | 20 | 60 (41–68) | A | M | NLR: 5 | Literature |
| Abbate (2023) [32] | Italy | 239 | 55 (18–87) | A | M: 99 B: 140 | NLR: 3.09 PLR: 129 SII: 788 SIRI: 0.94 | ROC |
| Authors (Yrs) | Objective | Major Results |
|---|---|---|
| Damar (2016) [15] | To evaluate the pretreatment role of NLR, percentages and leucocyte counts in benign and malignant salivary gland tumors. | Malignant tumors showed higher NLR and neutrophil percentages, and lower lymphocyte percentages. |
| Kawakita (2017) [23] | To evaluate the role of NLR and PLR in OS and PFS in salivary duct carcinomas. | Multivariate analysis revealed a significant association between high NLR and OS; this association was not consistent with the PFS results. No notable associations were found between PLR and survival. |
| Fang (2019) [24] | To evaluate the long-term oncological outcome of pediatric patients with mucoepidermoid carcinoma of the parotid gland treated with total parotidectomy. | A high NLR was associated with recurrence in univariate analysis but not in the Cox model. High NLR was not linked to DSS in univariate analysis. |
| Cheng (2019) [25] | To assess the role of NLR in the prognosis of patients with parotid gland cancer. | NLR is an independent predictor of DSS, with high values indicating a worse prognosis. |
| Seng (2019) [26] | To assess the prognostic value of NLR in pediatric patients with parotid gland cancer. | In multivariate analysis, RFS and DSS rates were significantly reduced in patients with a high NLR. |
| Gao (2020) [27] | To evaluate the significance of pretreatment NLR in the prognosis of pediatric patients with parotid mucoepidermoid carcinoma. | Tumor histological grade and stage were significantly associated with NLR, and an NLR ≥ 2.32 was associated with a worse prognosis. |
| Somay (2022) [28] | To evaluate the significance of pretreatment NLR values for predicting radiation-induced trismus in parotid gland tumor patients treated with postoperative radiotherapy. | High pretreatment NLR levels are statistically predictive of increased rates of radiotherapy-induced trismus in patients with parotid carcinoma treated with surgery and adjuvant radiotherapy. |
| Abbate (2022) [29] | To investigate the predictive value of inflammatory biomarkers for OS in patients treated surgically for salivary gland malignancies. | SII plus SIRI can independently predict the OS of patients after surgery. The prognostic score based on these is useful in clinical decision making. |
| Committeri (2023) [30] | To increase the effectiveness of pre-surgical diagnosis and improve the differentiation between benign and malignant pathologies in salivary gland tumors using inflammatory biomarkers. | The inflammatory biomarkers NLR, PLR, SII and SIRI showed an accuracy of 0.88, 0.74, 0.76 and 0.83, respectively, in differentiating Warthin tumors from pleomorphic adenoma and malignant neoplasms. |
| Lee (2023) [31] | To investigate the association between pretreatment NLR and immune checkpoint inhibitor outcomes in patients with recurrent/metastatic salivary gland carcinoma treated with pembrolizumab. | Multivariate Cox analysis shows that pretreatment NLR remained independently associated with 6-month PFS and 2-year OS. In Kaplan–Meier analysis, patients with NLR ≥ 5 had significantly worse 6-month and 2-year PFS and OS after starting pembrolizumab therapy. |
| Abbate (2023) [32] | To evaluate the diagnostic efficacy of inflammatory biomarkers compared to FNAC alone in salivary gland tumors. | Combining SIRI with cytological analysis significantly increases the sensitivity to 82.8%, allowing it to be used routinely to increase the accuracy of preoperative diagnosis. |
| Authors (Yrs) | Pros | Cons |
|---|---|---|
| Damar (2016) [15] | Large sample size. First study to analyze the role of inflammatory markers in salivary gland tumors. Cut-off selection method. | Retrospective study. Use of NLR only. Disparity between malignant and benign tumors, with a reduced malignant sample. |
| Kawakita (2017) [23] | Large sample size. Multi-parameter analysis. Cut-off selection method. | Retrospective study. |
| Fang (2019) [24] | Large sample size. | Retrospective study. Use of NLR only. Cut-off selection method. |
| Cheng (2019) [25] | Large sample size. | Retrospective study. Use of NLR only. Cut-off selection method. |
| Seng (2019) [26] | Large sample size. | Retrospective study. Use of NLR only. Cut-off selection method. |
| Gao (2020) [27] | Large sample size. | Retrospective study. Use of NLR only. Cut-off selection method. |
| Somay (2022) [28] | Analyzing a specific subcategory. Cut-off selection method. | Retrospective study. Use of NLR only. |
| Abbate (2022) [29] | Multi-parameter analysis. Cut-off selection method. | Retrospective study. |
| Committeri (2023) [30] | Multi-parameter analysis. Cut-off selection method. | Retrospective study. Disparity between malignant and benign tumors, with a reduced malignant sample. |
| Lee (2023) [31] | Analyzing a specific subcategory. | Retrospective study. Use of NLR only. Cut-off selection method. |
| Abbate (2023) [32] | Multi-parameter analysis. Cut-off selection method. Large sample size. | Retrospective study. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorelli, A.; Manuelli, M.; Ciorba, A.; Stomeo, F.; Pelucchi, S.; Bianchini, C. The Role of Blood Inflammatory Markers in Salivary Gland Carcinoma: A Scoping Review. J. Clin. Med. 2025, 14, 1762. https://doi.org/10.3390/jcm14051762
Migliorelli A, Manuelli M, Ciorba A, Stomeo F, Pelucchi S, Bianchini C. The Role of Blood Inflammatory Markers in Salivary Gland Carcinoma: A Scoping Review. Journal of Clinical Medicine. 2025; 14(5):1762. https://doi.org/10.3390/jcm14051762
Chicago/Turabian StyleMigliorelli, Andrea, Marianna Manuelli, Andrea Ciorba, Francesco Stomeo, Stefano Pelucchi, and Chiara Bianchini. 2025. "The Role of Blood Inflammatory Markers in Salivary Gland Carcinoma: A Scoping Review" Journal of Clinical Medicine 14, no. 5: 1762. https://doi.org/10.3390/jcm14051762
APA StyleMigliorelli, A., Manuelli, M., Ciorba, A., Stomeo, F., Pelucchi, S., & Bianchini, C. (2025). The Role of Blood Inflammatory Markers in Salivary Gland Carcinoma: A Scoping Review. Journal of Clinical Medicine, 14(5), 1762. https://doi.org/10.3390/jcm14051762








