Portal Vein Thrombosis in Second Trimester of Pregnancy
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
2.1. Initial Laboratory Analysis
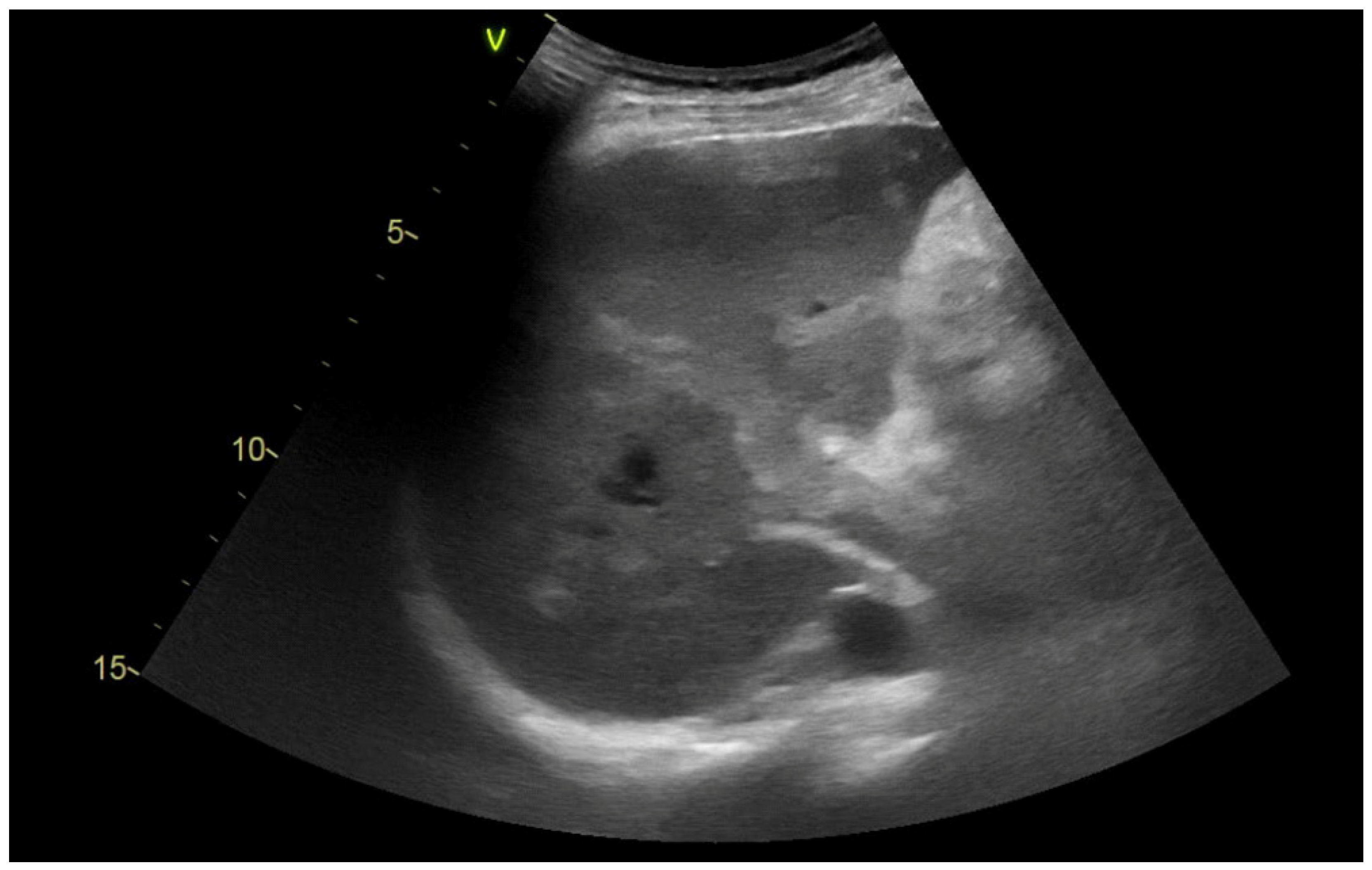
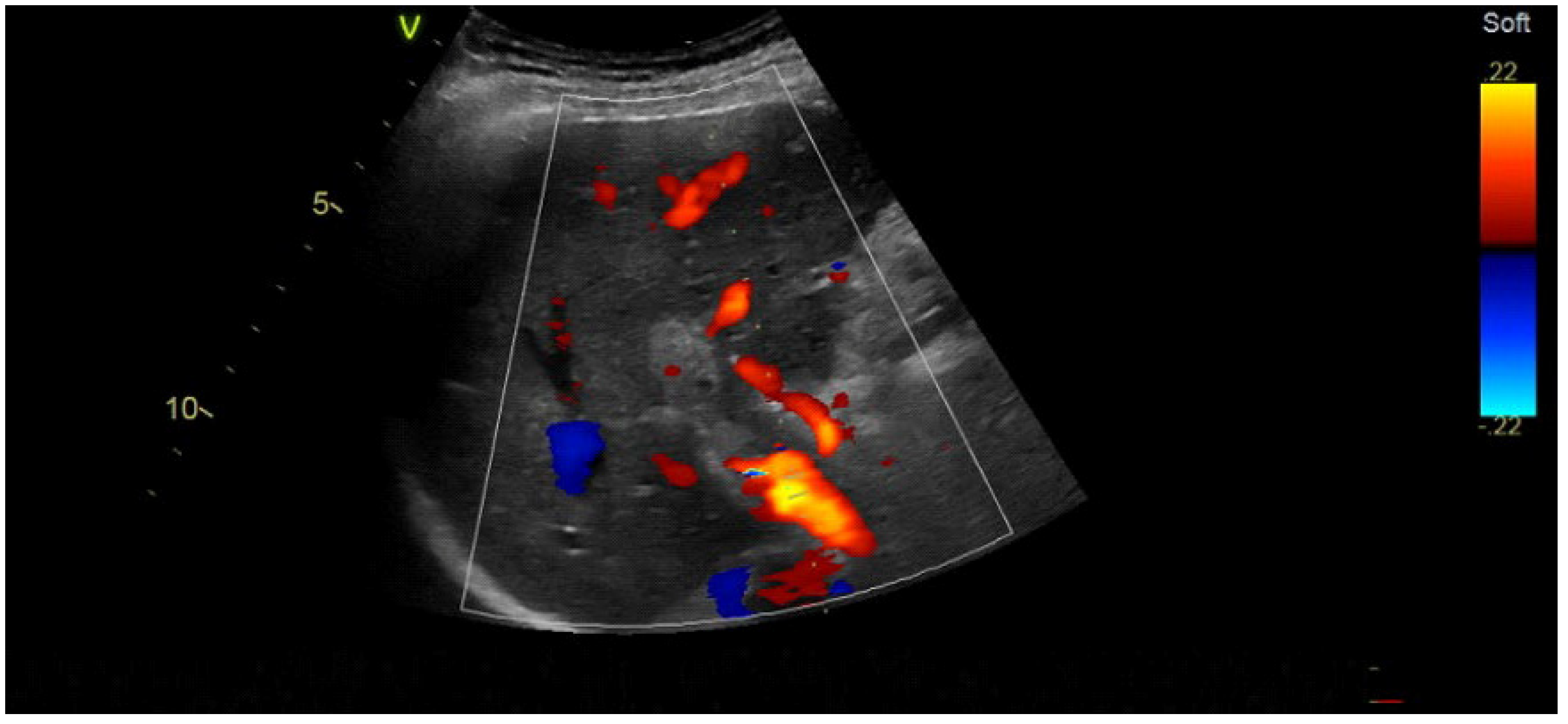
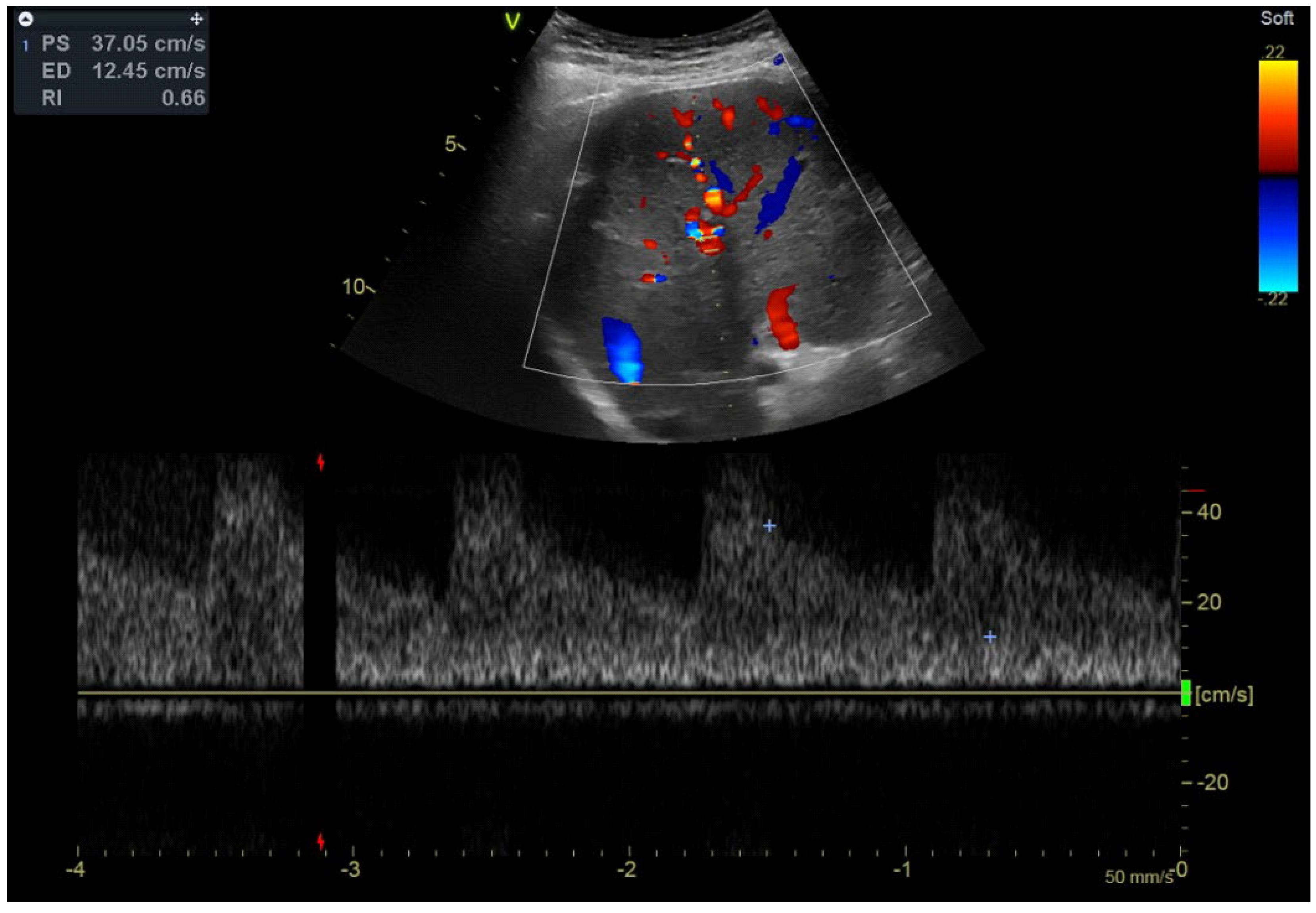
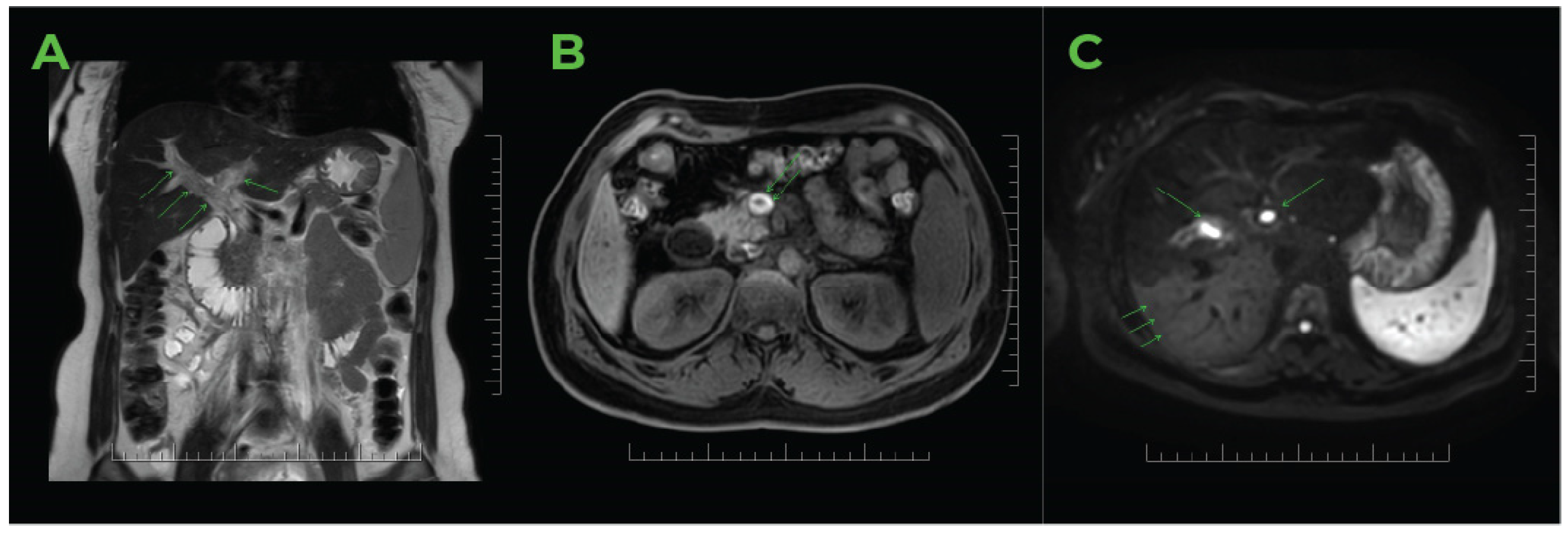
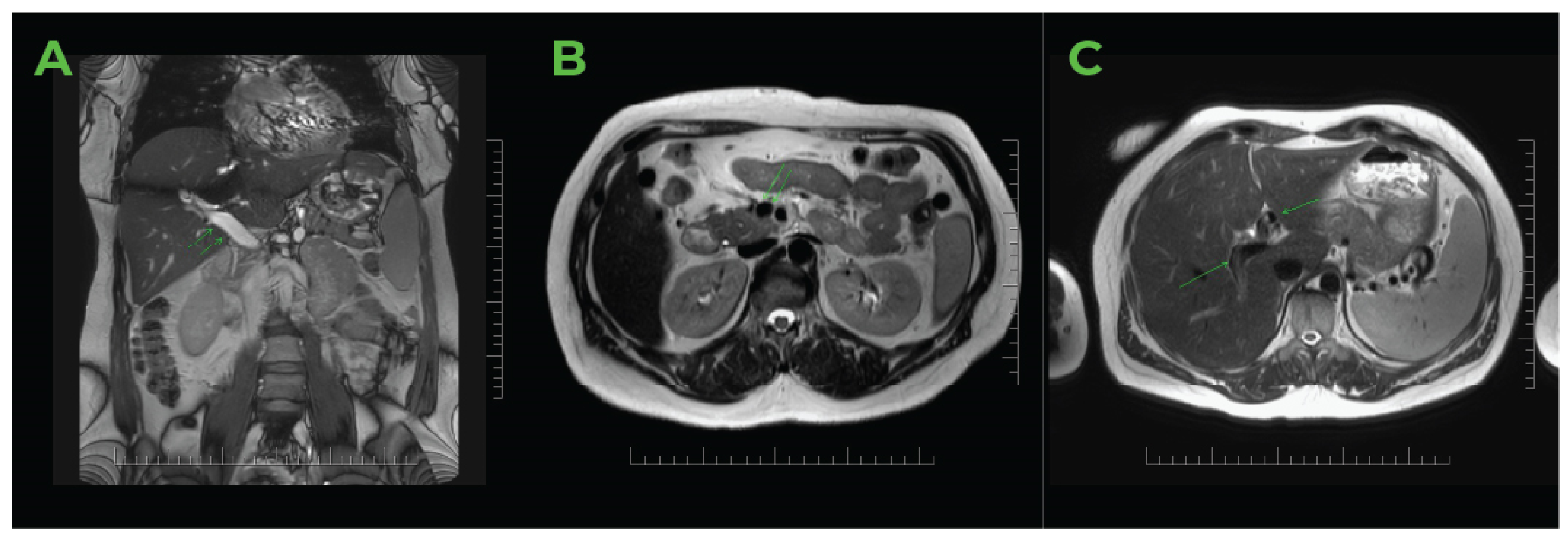
2.2. Imaging Methods
2.3. Therapy
2.4. Details of Further Therapeutic Considerations Due to Family History
2.5. During and Following Hospitalisation in an Internal Medicine Clinic
- (a)
- Thrombocyte aggregation induced by ADP: Aggreg.Tr-ADP-10 µM: 88%, Aggreg.Tr-ADP-2.34 µM: 86%, Aggreg.Tr-ADP-1.17 µM: 79%, Aggreg.Tr-ADP-0.58 µM: 24%.
- (b)
- Thrombocyte aggregation induced by epinephrine: Aggreg. Tr-EPI-300 µM: 90%, Aggreg.Tr-EPI-11.0 µM: 88%, Aggreg.Tr-EPI-1.1 µM: 77%, Aggreg.Tr-EPI-0.55 µM: 67%. Regulatory assessment for antiphospholipid syndrome: PTT-LT control: 33.6 s, PTT-LT patient: 36.1 s, PTT-LT ratio: 1.07. PTT-LA control: 32.7 s, PTT-LA patient: 36.0 s, PTT-LA ratio: 1.10. Kaolin clotting time: 0.91, dRVVT patient screening: 33.1 s, dRVVT screen control: 34.4 s, dRVVT screen ratio: 0.96 TTI 1:50 ratio: 1.35 s, TTI 1:500 ratio: 1.53 Anti-ß2 GPI-IgG: 1.3 GU/mL, Anti-ß2 GPI-IgM: 1.3 MU/mL.
2.6. Neonatal Outcome and Further Condition of the Offspring
2.7. Further Outpatients’ Care
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turatti, G.; Fedeli, U.; Valerio, L.; Klok, F.A.; Cohen, A.T.; Hunt, B.J.; Simioni, P.; Middeldorp, S.; Ageno, W.; Kucher, N.; et al. Splanchnic vein thrombosis-related mortality in the Veneto region (Italy), 2008–2019: Retrospective analysis of epidemiological data. Thromb. Res. 2022, 209, 41–46. [Google Scholar] [CrossRef]
- Valeriani, E.; Riva, N.; Di Nisio, M.; Ageno, W. Splanchnic vein thrombosis: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 449–461. [Google Scholar] [CrossRef]
- Gherman, R.B.; Goodwin, T.M.; Leung, B.; Byrne, J.D.; Hethumumi, R.; Montoro, M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet. Gynecol. 1999, 94, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.F.; Skjeldestad, F.E.; Sandset, P.M. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium: A register-based case-control study. Am. J. Obstet. Gynecol. 2008, 198, 233.e1–233.e7. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.S.; Steffensen, F.H.; Sørensen, H.T.; Nielsen, G.L.; Olsen, J. The cumulative incidence of venous thromboembolism during pregnancy and puerperium: An 11-year Danish population-based study of 63,300 pregnancies. Acta Obstet. Gynecol. Scand. 1998, 77, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.; Dahlbäck, B.; Marsál, K. Thrombotic risk during pregnancy: A population study. Obstet. Gynecol. 1999, 94, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Lawrenson, R.A.; Nightingale, A.L.; Farmer, R.D. Venous thromboembolism in pregnancy and the puerperium: Incidence and additional risk factors from a London perinatal database. BJOG Int. J. Obstet. Gynaecol. 2001, 108, 56–60. [Google Scholar] [CrossRef]
- Liu, S.; Rouleau, J.; Joseph, K.S.; Sauve, R.; Liston, R.M.; Young, D.; Kramer, M.S. Epidemiology of pregnancy-associated venous thromboembolism: A population-based study in Canada. J. Obstet. Gynaecol. Can. 2009, 31, 611–620. [Google Scholar] [CrossRef]
- James, A.H.; Jamison, M.G.; Brancazio, L.R.; Myers, E.R. Venous thromboembolism during pregnancy and the postpartum period: Incidence, risk factors, and mortality. Am. J. Obstet. Gynecol. 2006, 194, 1311–1315. [Google Scholar] [CrossRef]
- García-Pagán, J.C.; Hernández-Guerra, M.; Bosch, J. Extrahepatic portal vein thrombosis. Semin. Liver Dis. 2008, 28, 282–292. [Google Scholar] [CrossRef]
- Denninger, M.-H.; Chaït, Y.; Casadevall, N.; Hillaire, S.; Guillin, M.-C.; Bezeaud, A.; Erlinger, S.; Briere, J.; Valla, D. Cause of portal or hepatic venous thrombosis in adults: The role of multiple concurrent factors. Hepatology 2000, 31, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Jouini, M.S.; Fakunle, Y.; Azazy, A.A. Portal vein thrombosis in pregnancy: Case report and review of the literature. Ann. Saudi Med. 2002, 22, 227–229. [Google Scholar] [CrossRef]
- Tabaraii, R.; Farahmand, H.; Ahmadi, J.; Rezvan, S.; Arjmandnia, M.H.; Yosefi, M.; Naye, M.R.; Barati, A.; Razavinia, F.S. Deep vein thrombosis in pregnancy: A review article. J. Vessel. Circ. 2020, 1, 27–34. [Google Scholar] [CrossRef]
- Valla, D. Portal vein thrombosis. In Vascular Liver Disease; Springer: Berlin/Heidelberg, Germany, 2011; pp. 183–196. [Google Scholar]
- Hackmon-Ram, R.; Holcberg, G.; Bashiri, A.; Sapir, O.; Tov, G.Y.; Yermiahu, T.; Mazor, M. Thalassemia intermedia and cavernous transformation of portal vein thrombosis in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 107, 101–104. [Google Scholar] [CrossRef]
- Bayraktar, Y.; Tuncer, Z.; Kabukçu, A.; Uzunalimoğlu, B.; Ayhan, A. Pregnancy complicated by congenital hepatic fibrosis with cavernous transformation of the portal vein: A case report. Am. J. Obstet. Gynecol. 1997, 177, 459–461. [Google Scholar] [CrossRef]
- Wax, J.R.; Pinette, M.G.; Cartin, A.; Winn, S.; Blackstone, J. Cavernous transformation of the portal vein complicates pregnancy. Obstet. Gynecol. 2006, 108, 782–784. [Google Scholar] [CrossRef]
- Ducarme, G.; Plessier, A.; Thuillier, C.; Ceccaldi, P.; Valla, D.; Luton, D. Pregnancy and delivery in patients with portal vein cavernoma. Gynecol. Obstet. Investig. 2009, 68, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Dasari, P.; Balusamy, S.L. Portal vein thrombosis during pregnancy. BMJ Case Rep. 2013, 2013, bcr2012008325. [Google Scholar] [CrossRef]
- Correa, T.L.; Sastre, J.A. Caesarean section in a patient with chronic portal vein thrombosis and thrombocytopenia: Case report. Colomb. J. Anesthesiol. 2017, 45, 251–255. [Google Scholar] [CrossRef]
- Agarwal, M.; Singh, S.; Sinha, U.; Bhushan, D. Extrahepatic portal vein thrombosis in a pregnant patient with COVID-19: A rare thrombotic event survivor. BMJ Case Rep. 2021, 14, e243697. [Google Scholar] [CrossRef]
- Bartlett, K. Successful term pregnancy in a patient with acute portal vein thrombosis following in vitro fertilisation. Univ. West. Ont. Med. J. 2018, 86, 3–5. [Google Scholar]
- Bojalian, M.; Akingba, A.; Andersen, J.; Swerdlow, P.; Bove, P.; Brown, O.; Rubin, J. Sticky platelet syndrome: An unusual presentation of arterial ischemia. Ann. Vasc. Surg. 2010, 24, 691.e1–691.e6. [Google Scholar] [CrossRef] [PubMed]
- García-Navarrete, Y.I.; Vallejo-Villalobos, M.F.; Olivares-Gazca, J.M.; Cantero-Fortiz, Y.; León-Peña, A.A.; Olivares-Gazca, J.C.; Murrieta-Álvarez, I.; Ruiz-Delgado, G.J.; Ruiz-Argüelles, G.J. Primary thrombophilia xv: Antithrombotic treatment of sticky platelet syndrome worldwide. Ann. Blood 2019, 4, 15. [Google Scholar] [CrossRef]
- Minutti-Zanella, C.; Villarreal-Martínez, L.; Ruíz-Argüelles, G.J. Primary thrombophilia xvii: A narrative review of sticky platelet syndrome in Mexico. J. Clin. Med. 2022, 11, 4100. [Google Scholar] [CrossRef]
- Chawla, Y.; Duseja, A.; Dhiman, R.K. Review article: The modern management of portal vein thrombosis. Aliment. Pharmacol. Ther. 2009, 30, 881–894. [Google Scholar] [CrossRef]
- Eastwood, K.; Mohan, A.R. Imaging in pregnancy. Obstet. Gynaecol. 2019, 21, 255–262. [Google Scholar] [CrossRef]
- Masselli, G.; Subcommittee, E.F.P.I.; Derchi, L.; McHugo, J.; Rockall, A.; Vock, P.; Weston, M.; Spencer, J. Acute abdominal and pelvic pain in pregnancy: ESUR recommendations. Eur. Radiol. 2013, 23, 3485–3500. [Google Scholar] [CrossRef]
- Moreno, C.C.; Mittal, P.; Miller, F.H. Non-fetal imaging during pregnancy. Radiol. Clin. N. Am. 2020, 58, 363–380. [Google Scholar] [CrossRef]
- Costache, R.S.; Dragomirică, A.S.; Dumitraș, E.A.; Mariana, J.; Căruntu, A.; Popescu, A.; Costache, D.O. Portal vein thrombosis: A concise review. Exp. Ther. Med. 2021, 22, 759. [Google Scholar] [CrossRef]
- Franchis, R.D. Evolving consensus in portal hypertension report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J. Hepatol. 2005, 43, 167–176. [Google Scholar] [CrossRef]
- DeLeve, L.D.; Valla, D.; García-Tsao, G. Vascular disorders of the liver. Hepatology 2009, 49, 1729–1764. [Google Scholar] [CrossRef] [PubMed]
- Nisio, M.D.; Valeriani, E.; Riva, N.; Schulman, S.; Beyer-Westendorf, J.; Ageno, W. Anticoagulant therapy for splanchnic vein thrombosis. J. Thromb. Haemost. 2020, 18, 1562–1568. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregušová, A.; Slováčiková, M.; Gazdíková, K.; Dobrotová, M.; Jezberová, M.; Žigrai, M. Portal Vein Thrombosis in Second Trimester of Pregnancy. J. Clin. Med. 2025, 14, 1713. https://doi.org/10.3390/jcm14051713
Gregušová A, Slováčiková M, Gazdíková K, Dobrotová M, Jezberová M, Žigrai M. Portal Vein Thrombosis in Second Trimester of Pregnancy. Journal of Clinical Medicine. 2025; 14(5):1713. https://doi.org/10.3390/jcm14051713
Chicago/Turabian StyleGregušová, Adriana, Martina Slováčiková, Katarína Gazdíková, Miroslava Dobrotová, Michaela Jezberová, and Miroslav Žigrai. 2025. "Portal Vein Thrombosis in Second Trimester of Pregnancy" Journal of Clinical Medicine 14, no. 5: 1713. https://doi.org/10.3390/jcm14051713
APA StyleGregušová, A., Slováčiková, M., Gazdíková, K., Dobrotová, M., Jezberová, M., & Žigrai, M. (2025). Portal Vein Thrombosis in Second Trimester of Pregnancy. Journal of Clinical Medicine, 14(5), 1713. https://doi.org/10.3390/jcm14051713




