Endovascular Treatment of Femoro-Popliteal Disease with the Supera Stent: A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Procedure Details
2.3. Outcomes
2.4. Definitions
2.5. Clinical Follow-Up
2.6. Statistical Analysis
3. Results
Clinical Outcomes by Kaplan–Meier Estimates and Predictors of MALE
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shu, J.; Santulli, G. Update on Peripheral Artery Disease: Epidemiology and Evidence-Based Facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T. Current state of endovascular treatment of femoro- popliteal artery disease. Vasc. Med. 2007, 12, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Bishu, K.; Armstrong, E.J. Supera self-expanding stents for endovascular treatment of femoropopliteal disease: A review of the clinical evidence. Vasc. Health Risk Manag. 2015, 11, 387–395. [Google Scholar]
- Laird, J.R.; Katzen, B.T.; Scheinert, D.; Lammer, J.; Carpenter, J.; Buchbinder, M.; Dave, R.; Ansel, G.; Lansky, A.; Cristea, E.; et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: Twelve-month results from the RESILIENT randomized trial. Circ. Cardiovasc. Interv. 2010, 3, 267–276. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.W.; van den Heuvel, D.A.F.; de Vries-Werson, D.A.B.; Vos, J.A.; Fioole, B.; Vroegindeweij, D.; Elgersma, O.E.; Tutein Nolthenius, R.P.; Heyligers, J.M.M.; Bosma, G.P.T.; et al. Short-term results of the RAPID randomized trial of the legflow paclitaxel-eluting balloon with Supera stenting vs Supera stenting alone for the treatment of intermediate and long superficial femoral artery lesions. J. Endovasc. Ther. 2017, 24, 783–792. [Google Scholar] [CrossRef]
- Sabeti, S.; Mlekusch, W.; Amighi, J.; Minar, E.; Schillinger, M. Primary patency of long-segment self-expanding nitinol stents in the femoropopliteal arteries. J. Endovasc. Ther. 2005, 12, 6–12. [Google Scholar] [CrossRef]
- San Norberto, E.M.; Fuente, R.; Flota, C.M.; Taylor, J.H.; Vaquero, C. Impact of implantation defects on intermediate outcome of Supera stent for popliteal artery stenosis. Ann. Vasc. Surg. 2017, 41, 186–195. [Google Scholar] [CrossRef]
- Werner, M.; Paetzold, A.; Banning-Eichenseer, U.; Scheinert, S.; Piorkowski, M.; Ulrich, M.; Bausback, Y.; Bräunlich, S.; Schmidt, A.; Scheinert, D.; et al. Treatment of complex atherosclerotic femoropopliteal artery disease with a self- expanding interwoven nitinol stent: Midterm results from the Leipzig SUPERA 500 registry. EuroIntervention 2014, 10, 861–868. [Google Scholar] [CrossRef]
- Garcia, L.; Jaff, M.R.; Metzger, C.; Sedillo, G.; Pershad, A.; Zidar, F.; Patlola, R.; Wilkins, R.G.; Espinoza, A.; Iskander, A.; et al. SUPERB Trial Investigators*. Wire-interwoven nitinol stent outcome in the superficial femoral and proximal popliteal arteries: Twelve-month results of the SUPERB trial. Circ. Cardiovasc. Interv. 2015, 8, e000937. [Google Scholar] [CrossRef]
- Salamaga, S.; Stępak, H.; Żołyński, M.; Kaczmarek, J.; Błaszyk, M.; Stanišić, M.G.; Krasiński, Z. Three-Year Real-World Outcomes of Interwoven Nitinol Supera Stent Implantation in Long and Complex Femoropopliteal Lesions. J. Clin. Med. 2023, 12, 4869. [Google Scholar] [CrossRef]
- Guzzardi, G.; Spinazzola, A.; Cangiano, G.; Natrella, M.; Paladini, A.; Porta, C.; Boccalon, L.; Negroni, D.; Leati, G.; Laganà, D.; et al. Endovascular treatment of femoro-popliteal disease with the Supera stent: Results of a multicenter study. J. Public Health Res. 2021, 11, 2360. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, M.; Sabeti, S.; Loewe, C.; Dick, P.; Amighi, J.; Mlekusch, W.; Schlager, O.; Cejna, M.; Lammer, J.; Minar, E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N. Engl. J. Med. 2006, 354, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Dake, M.D.; Ansel, G.M.; Jaff, M.R.; Ohki, T.; Saxon, R.R.; Smouse, H.B.; Machan, L.S.; Snyder, S.A.; O’Leary, E.E.; Ragheb, A.O.; et al. Durable Clinical Effectiveness With Paclitaxel-Eluting Stents in the Femoropopliteal Artery: 5-Year Results of the Zilver PTX Randomized Trial. Circulation 2016, 133, 1472–1483, discussion 1483. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Jeon-Slaughter, H.; Kahlon, R.S.; Niazi, K.A.; Shammas, N.W.; Banerjee, S. Comparative Outcomes of Supera Interwoven Nitinol vs Bare Nitinol Stents for the Treatment of Femoropopliteal Disease: Insights From the XLPAD Registry. J. Endovasc. Ther. 2020, 27, 60–65. [Google Scholar] [CrossRef]
- Tan, M.; Takahara, M.; Soga, Y.; Mori, S.; Tsuchiya, T.; Mazaki, T.; Shintani, Y.; Noguchi, M.; Taniguchi, M.; Kobayashi, Y.; et al. Three-Year Clinical Outcomes Following Implantation of LifeStent Self-Expanding Nitinol Stents in Patients With Femoropopliteal Artery Lesions. Angiology 2022, 73, 244–251. [Google Scholar] [CrossRef]
- Scheinert, D.; Werner, M.; Scheinert, S.; Paetzold, A.; Banning-Eichenseer, U.; Piorkowski, M.; Ulrich, M.; Bausback, Y.; Bräunlich, S.; Schmidt, A. Treatment of Complex Atherosclerotic Popliteal Artery Disease with a New Self-Expanding Interwoven Nitinol Stent: 12-Month Results of the Leipzig SUPERA Popliteal Artery Stent Registry. JACC Cardiovasc. Interv. 2013, 6, 65–71. [Google Scholar] [CrossRef]
- Bhatt, H.; Kovach, R.; Janzer, S.; George, J.C. SUPERA Stent Outcomes in Above-The-Knee IntervEntions: Effects of COMPression and ELongation (SAKE-COMPEL) Sub-Study. Cardiovasc. Revasc. Med. 2018, 19, 512–515. [Google Scholar] [CrossRef]
- Haine, A.; Schmid, M.J.; Schindewolf, M.; Lenz, A.; Bernhard, S.M.; Drexel, H.; Baumgartner, I.; Dopheide, J.F. Comparison between Interwoven Nitinol and Drug Eluting Stents for Endovascular Treatment of Femoropopliteal Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 865–873. [Google Scholar] [CrossRef]
- Chan, Y.C.; Cheng, S.W.; Cheung, G.C. A Midterm Analysis of Patients Who Received Femoropopliteal Helical Interwoven Nitinol Stents. J. Vasc. Surg. 2020, 71, 2048–2055. [Google Scholar] [CrossRef]
- Nasr, B.; Gouailler, F.; Marret, O.; Guillou, M.; Chaillou, P.; Guyomarc’h, B.; Maurel, B.; Gouëffic, Y. Treatment of Long Femoropopliteal Lesions With Self-Expanding Interwoven Nitinol Stent: 24 Month Outcomes of the STELLA-SUPERA Trial. J. Endovasc. Ther. 2023, 30, 98–105. [Google Scholar] [CrossRef]
- Palena, L.M.; Diaz-Sandoval, L.J.; Sultato, E.; Brigato, C.; Candeo, A.; Brocco, E.; Manzi, M. Feasibility and 1-Year Outcomes of Subintimal Revascularization with Supera® Stenting of Long Femoropopliteal Occlusions in Critical Limb Ischemia: The “Supersub” Study. Catheter. Cardiovasc. Interv. 2017, 89, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shi, B.; Ma, L.; Yu, C.; Zhang, X.; Li, T.; Zhang, X.; Wang, Y.; Zhuang, B. Treatment of Atherosclerotic Femoropopliteal Artery Disease with Supera Interwoven Nitinol Stent: A Real-World Study in China. Ann. Vasc. Surg. 2022, 85, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Maleckis, K.; Deegan, P.; Poulson, W.; Sievers, C.; Desyatova, A.; MacTaggart, J.; Kamenskiy, A. Comparison of femoropopliteal artery stents under axial and radial compression, axial tension, bending, and torsion deformations. J. Mech. Behav. Biomed. Mater. 2017, 75, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zlatanovic, P.; Mahmoud, A.A.; Cinara, I.; Cvetic, V.; Lukic, B.; Davidovic, L. Comparison of Long Term Outcomes After Endovascular Treatment Versus Bypass Surgery in Chronic Limb Threatening Ischaemia Patients with Long Femoropopliteal Lesions. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 258–269. [Google Scholar] [CrossRef]
- Wijnand, J.G.J.; Zarkowsky, D.; Wu, B.; van Haelst, S.T.W.; Vonken, E.P.A.; Sorrentino, T.A.; Pallister, Z.; Chung, J.; Mills, J.L.; Teraa, M.; et al. The Global Limb Anatomic Staging System (GLASS) for CLTI: Improving Inter-Observer Agreement. J. Clin. Med. 2021, 10, 3454. [Google Scholar] [CrossRef]
- Cerqueira, L.O.; Duarte, E.G.; Barros, A.L.S.; Cerqueira, J.R.; de Araújo, W.J.B. WIfI classification: The Society for Vascular Surgery lower extremity threatened limb classification system, a literature review. J. Vasc. Bras. 2020, 19, e20190070. [Google Scholar] [CrossRef]
- Brescia, A.A.; Wickers, B.M.; Correa, J.C.; Smeds, M.R.; Jacobs, D.L. Stenting of femoropopliteal lesions using interwoven nitinol stents. J. Vasc. Surg. 2015, 61, 1472–1478. [Google Scholar] [CrossRef]
- León, L.R., Jr.; Dieter, R.S.; Gadd, C.L.; Ranellone, E.; Mills, J.L., Sr.; Montero-Baker, M.F.; Gruessner, A.C.; Pacanowski, J.P., Jr. Preliminary results of the initial United States experience with the Supera woven nitinol stent in the popliteal artery. J. Vasc. Surg. 2013, 57, 1014–1022. [Google Scholar] [CrossRef]
- Goltz, J.P.; Ritter, C.O.; Kellersmann, R.; Klein, D.; Hahn, D.; Kickuth, R. Endovascular Treatment of Popliteal Artery Segments P1 and P2 in Patients with Critical Limb Ischemia: Initial Experience Using a Helical Nitinol Stent with Increased Radial Force. J. Endovasc. Ther. 2012, 19, 450–456. [Google Scholar] [CrossRef]
| n (%) | |
|---|---|
| Age (med, range) | 68.54 ± 9.48 |
| Gender | |
| Male | 194 (66.0) |
| Female | 100 (34.0) |
| Smoking | |
| No | 95 (32.3) |
| Former | 63 (21.4) |
| Current | 136 (46.3) |
| Obesity | 63 (21.4) |
| DM | 182 (61.9) |
| Insulin treatment | 96 (32.7) |
| HTN | 251 (85.4) |
| Hypercholesterolemia | 141 (48) |
| IHD | 70 (23.8) |
| CHF | 20 (6.8) |
| Renal insufficiency | 27 (9.2) |
| Previous CV | 104 (35.4) |
| n (%) | |
|---|---|
| WiFi amputation risk | |
| Very low/low | 103 (35.0) |
| Moderate | 98 (33.3) |
| High | 93 (31.6) |
| WiFi revascularization | |
| Very low/low | 55 (18.7) |
| Moderate | 96 (32.7) |
| High | 143 (48.6) |
| Rest pain | 199 (67.7) |
| Ulcer | 99 (33.7) |
| Gangrene | 85 (28.9) |
| ABI | |
| <0.25 | 60 (20.4) |
| 0.25–0.35 | 124 (42.2) |
| 0.4–0.7 | 76 (25.9) |
| >1.2 or amputation | 34 (11.6) |
| ABI other leg | |
| <0.25 | 7 (2.4) |
| 0.25–0.35 | 37 (12.6) |
| 0.4–0.7 | 153 (52.0) |
| 0.75–1.2 | 65 (22.1) |
| >1.2 or amputation | 32 (10.9) |
| Distal run off | 263 (89.5) |
| Crural not patent | 31 (10.5) |
| Crural patent number arteries | |
| 1 | 114 (38.8) |
| 2 | 114 (38.8) |
| 3 | 35 (11.9) |
| Calcified lesion | 195 (66.3) |
| CTO lesion | 253 (86.1) |
| n (%) | |
|---|---|
| Occlusion length (cm) | |
| 0–5 | 65 (22.1) |
| 6–10 | 90 (30.6) |
| 11–15 | 85 (28.9) |
| 16–20 | 34 (11.6) |
| 20 | 20 (6.8) |
| Diseased segment | |
| a. SFA | 123 (41.8) |
| a. Poplitea | 47 (16.0) |
| Both | 124 (42.2) |
| Diseased segment length (cm) | |
| <10 | 52 (17.7) |
| 10–20 | 162 (55.1) |
| >20 | 80 (27.2) |
| GLASS femoropopliteal classification | |
| 1 | 5 (1.7) |
| 2 | 21 (7.1) |
| 3 | 125 (42.5) |
| 4 | 143 (48.6) |
| GLASS crural classification | |
| 0 | 20 (6.8) |
| 1 | 60 (20.4) |
| 2 | 92 (31.3) |
| 3 | 82 (27.9) |
| 4 | 40 (13.6) |
| Stent implantation site | |
| a. SFA | 126 (42.9) |
| a. Poplitea | 45 (15.3) |
| Both | 123 (41.8) |
| Diameter of stent (mm) | |
| 4, 4.5/5, 5.5 | 211 (71.8) |
| 6/6.5 | 83 (28.2) |
| Length of stent (cm) | |
| 6–8 | 55 (18.7) |
| 10–12 | 97 (33.0) |
| 15–18 | 107 (36.4) |
| 20 | 35 (11.9) |
| Additional stent | 61 (20.7) |
| Supera | 13 (4.4) |
| Other | 41 (13.9) |
| Both | 7 (2.4) |
| Inflow vess PTA stent | 16 (5.4) |
| Access point complication postop | 8 (2.7) |
| Technical success stenting crural | 62 (97.3) |
| Technical success stenting femoropopliteal | 294 (100.0) |
| 1 Month | 6 Months | 12 Months | 24 Months | |
|---|---|---|---|---|
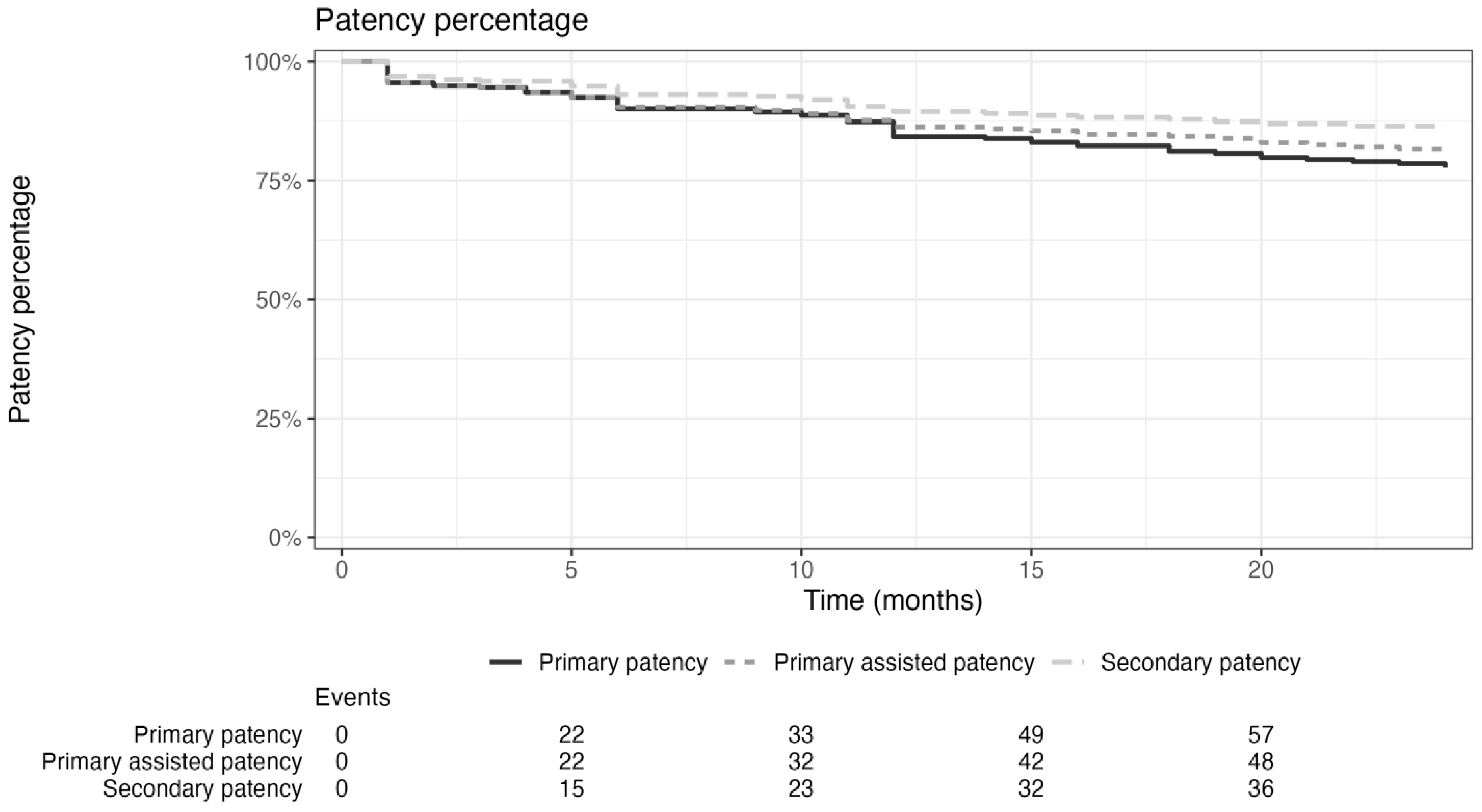
| Primary patency | 95.6% | 90.1% | 84.2% | 77.7% |
| (93.2–97.9%) | (86.7–93.4%) | (80.1–88.3%) | (72.8–82.6%) | |
| Primary assisted patency | 95.6% | 90.4% | 86.3% | 81.6% |
| (93.2–97.9%) | (87.0–93.7%) | (82.4–90.2%) | (77.1–86.1%) | |
| Secondary patency | 96.9% | 93.1% | 89.5% | 86.5% |
| (94.9–98.8%) | (90.1–96.0%) | (85.9–93.0%) | (82.4–90.6%) | |
| Bypass freedom | 99.3% | 98.6% | 98.2% | 98.2% |
| (98.3–100.0%) | (97.2–100.0%) | (96.6–99.8%) | (96.6–99.8%) | |
| Amputation free | 98.6% | 98.0% | 95.9% | 92.6% |
| (97.2–100.0%) | (96.0–100.0%) | (93.5–98.2%) | (89.5–95.7%) | |
| MALE free | 95.6% | 90.1% | 87.3% | 77.7% |
| (93.2–97.9%) | (86.8–93.4%) | (83.6–91.0%) | (72.8–82.6%) | |
| Survival | 99% | 99% | 98.3% | 98.3% |
| (97.8–100.0%) | (97.8–100.0%) | (96.7–99.9%) | (96.7–99.9%) |
| Outcome | 1 Month | 6 Months | 12 Months | 24 Months | ||||
|---|---|---|---|---|---|---|---|---|
| CLTI | IC | CLTI | IC | CLTI | IC | CLTI | IC | |
| Primary patency | 95.3 | 97.2 | 89.1 | 97.2 | 83.6 | 88.9 | 76.6 | 85.6 |
| (92.7–97.8) | (91.9–100.0) | (85.4–92.8) | (91.9–100.0) | (79.1–88.1) | (78.7–99.1) | (71.3–81.9) | (73.8–97.4) | |
| Primary ass. patency | 95.3 | 97.2 | 89.5 | 97.2 | 85.9 | 88.9 | 80.5 | 88.9 |
| (92.7–97.8) | (91.9–100.0) | (85.8–93.2) | (91.9–100.0) | (81.6–90.2) | (78.7–99.1) | (75.4–85.6) | (78.7–99.1) | |
| Secondary patency | 96.5 | 100.0 | 92.1 | 94.3 | 88.8 | 94.3 | 85.3 | 94.3 |
| (94.3–98.6) | / | (88.8–95.4) | (86.6–100.0) | (84.9–92.7) | (86.6–100.0) | (80.8–89.8) | (86.0–100.0) | |
| By pass freedom | 99.2 | 100.0 | 98.4 | 100.0 | 98.0 | 100.0 | 98.0 | 100.0 |
| (98.2–100.0) | / | (96.8–99.9) | / | (96.2–99.8) | / | (96.2–99.8) | / | |
| Amputation | 98.4 | 100.0 | 96.5 | 100.0 | 95.3 | 100.0 | 92.0 | 96.7 |
| (96.8–99.9) | / | (94.3–98.6) | / | (92.7–97.8) | / | (88.5–95.5) | (90.2–100.0) | |
| MALE | 95.3 | 97.2 | 91.8 | 97.2 | 83.6 | 88.9 | 76.6 | 85.6 |
| (92.7–97.8) | (91.9–100.0) | (88.5–95.1) | (91.9–100.0) | (79.1–88.1) | (78.7–99.1) | (71.3–81.9) | (73.8–97.4) | |
| Death | 98.8 | 100.0 | 98.8 | 100.0 | 98.1 | 100.0 | 98.1 | 100.0 |
| (97.4–100.0) | / | (97.4–100.0) | / | (96.3–99.9) | / | (96.3–99.9) | / | |
| Outcome | 1 Month | 6 Months | 12 Months | 24 Months | ||||
|---|---|---|---|---|---|---|---|---|
| GLASS 1–3 | GLASS 4 | GLASS 1–3 | GLASS 4 | GLASS 1–3 | GLASS 4 | GLASS 1–3 | GLASS 4 | |
| Primary patency | 96.7% | 94.4% | 92.0% | 88.1% | 89.3% | 78.9% | 85.3% | 69.7% |
| (93.8–99.6%) | (90.7–98.1%) | (87.7–96.3%) | (82.8–93.4%) | (84.4–94.2%) | (72.2–85.6%) | (79.4–91.2%) | (61.8–77.5%) | |
| Primary ass. patency | 96.7% | 94.4% | 92.7% | 88.1% | 90.6% | 81.7% | 89.0% | 73.7% |
| (93.8–99.6%) | (90.7–98.1%) | (88.6–96.8%) | (82.8–93.4%) | (85.9–95.3%) | (75.4–88.0%) | (83.9–94.1%) | (66.0–81.3%) | |
| Secondary patency | 98.7% | 95.1% | 95.2% | 90.8% | 93.8% | 85.0% | 92.1% | 80.5% |
| (96.9–100.4%) | (91.6–98.6%) | (91.7–98.7%) | (86.1–95.5%) | (89.8–97.7%) | (79.1–90.9%) | (87.6–96.6%) | (73.6–87.3%) | |
| By pass freedom | 99.3% | 95.6% | 96.0% | 94.3% | 95.3% | 90.7% | 95.3% | 90.7% |
| (97.9–100.6%) | (92.7–98.5%) | (92.9–99.1%) | (90.6–98.0%) | (92.0–98.6%) | (85.8–95.6%) | (92.0–98.6%) | (85.8–95.6%) | |
| Amputation | 100.0% | 97.2% | 98.7% | 95.1% | 98.0% | 93.7% | 94.8% | 90.4% |
| (/) | (94.4–99.9%) | (96.9–100%) | (91.6–98.6%) | (95.6–100%) | (89.8–97.6%) | (91.1–98.5%) | (85.3–95.5%) | |
| MALE | 96.7% | 94.4% | 92.0% | 88.1% | 89.3% | 78.9% | 85.3% | 69.7% |
| (93.8–99.6%) | (90.7–98.1%) | (87.7–96.3%) | (82.8–98.4%) | (84.4–94.2%) | (72.2–85.6%) | (79.4–91.2%) | (61.8–77.5%) | |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| p | HR | 95% CI | p | HR | 95% CI | |
| WiFi revascularization | 0.044 | 1.439 | 1.010–2.049 | |||
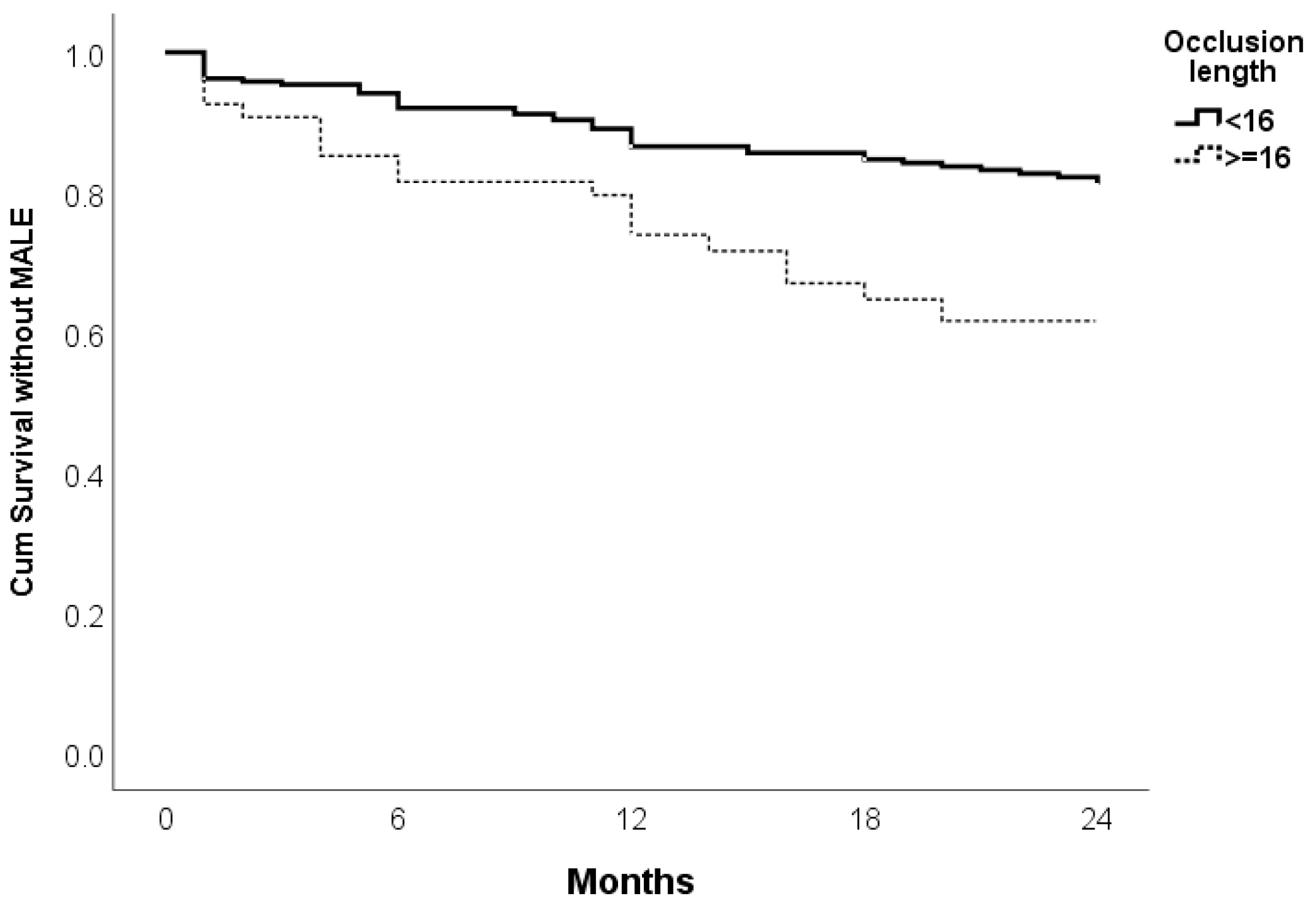
| Occlusion length | 0.002 | 2.309 | 1.343–3.970 | 0.020 | 1.933 | 1.108–3.374 |
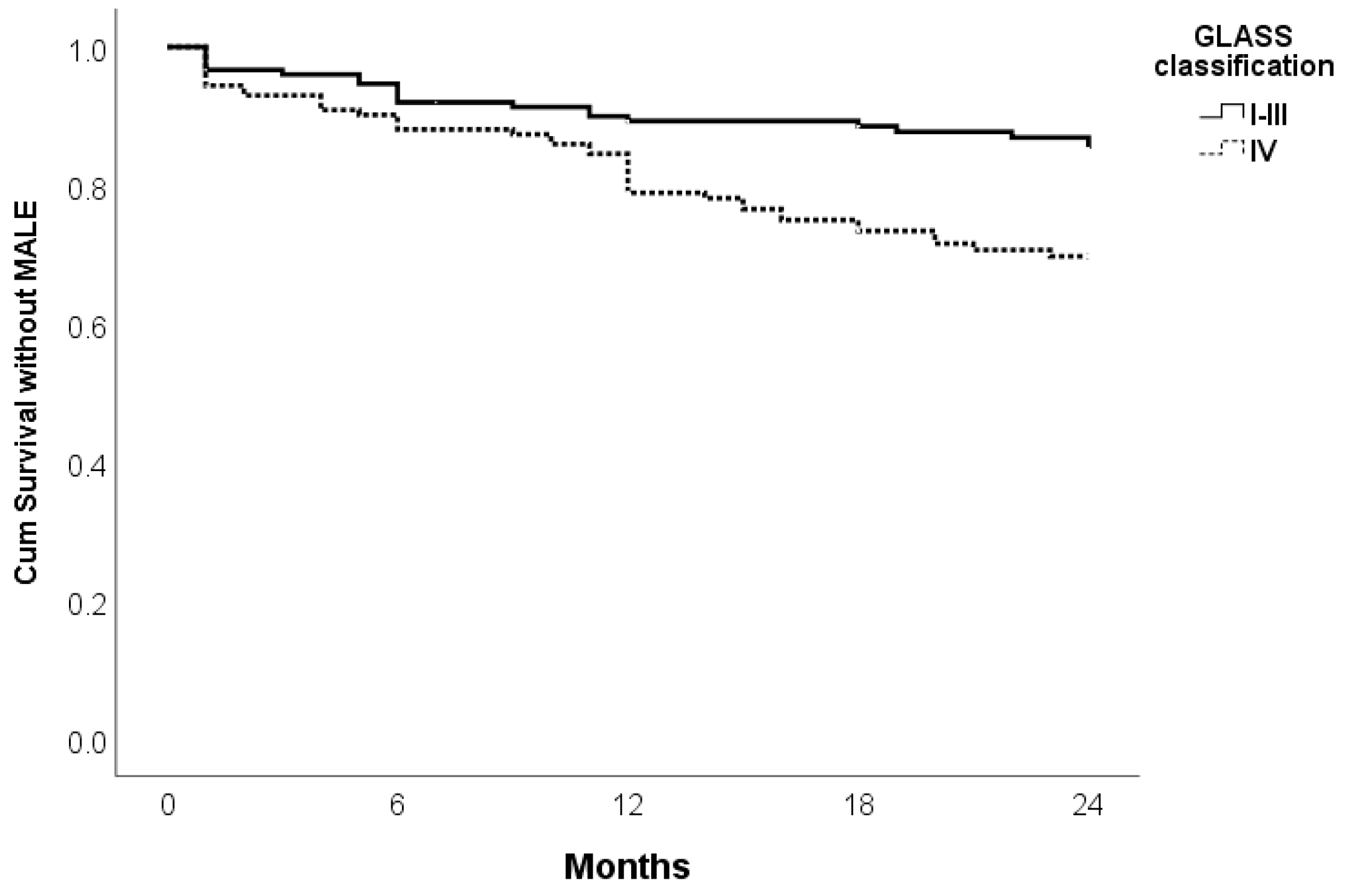
| GLASS I–III vs. IV | 0.003 | 2.223 | 1.313–3.763 | 0.015 | 1.963 | 1.143–3.373 |
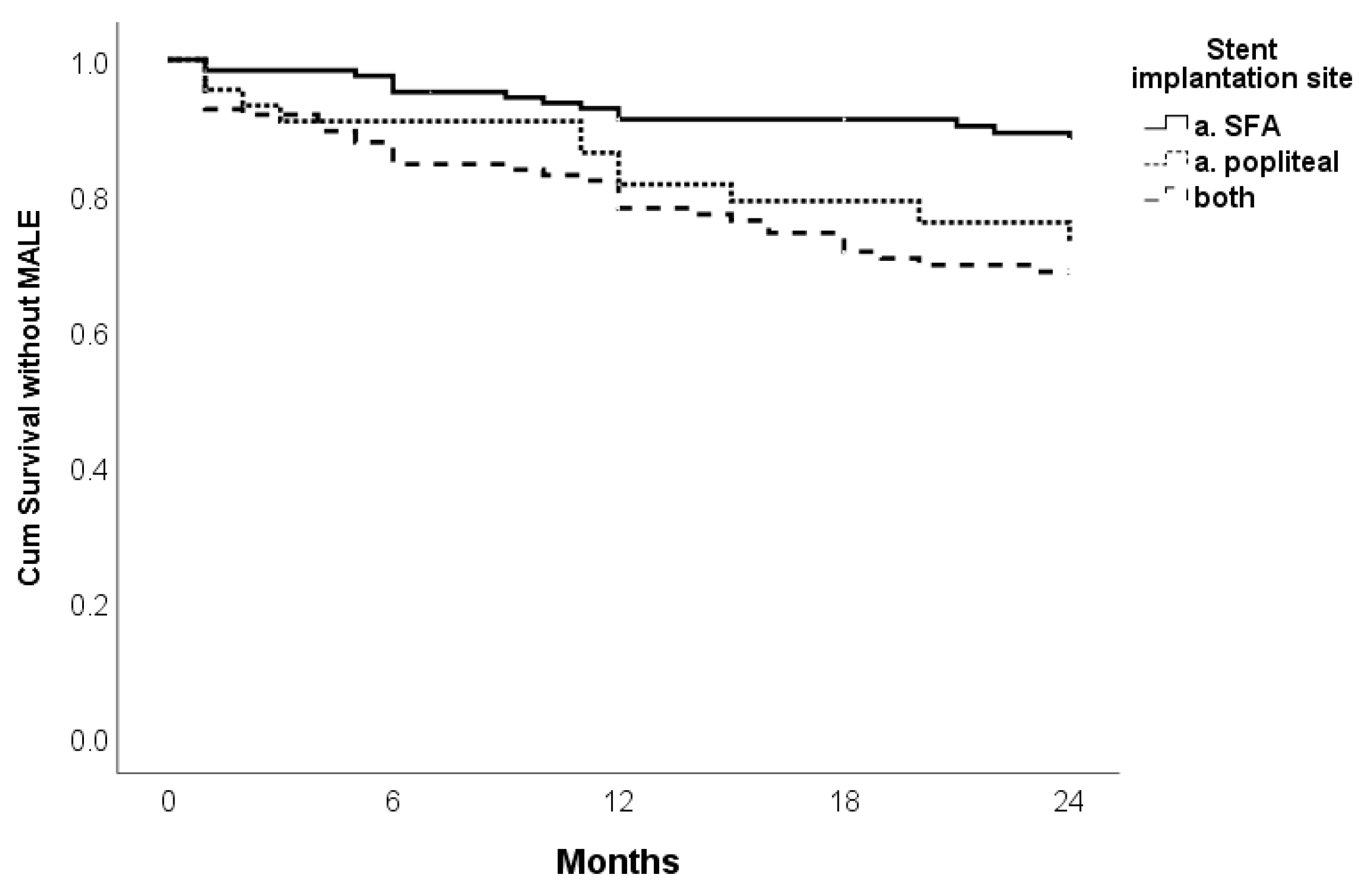
| Stent site | ||||||
| SFA, reference | / | / | / | |||
| a. Poplitea | 0.027 | 2.437 | 1.106–5.370 | |||
| a. SFA+ a. Poplitea | 0.001 | 3.000 | 1.622–5.550 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukic, B.; Miletic, M.; Milosevic, S.; Dragas, M.; Saponjski, J.; Koncar, I.; Zlatanovic, P.; Lukic, F.; Mirkovic, A.; Lazic, D.; et al. Endovascular Treatment of Femoro-Popliteal Disease with the Supera Stent: A Single Center Experience. J. Clin. Med. 2025, 14, 1704. https://doi.org/10.3390/jcm14051704
Lukic B, Miletic M, Milosevic S, Dragas M, Saponjski J, Koncar I, Zlatanovic P, Lukic F, Mirkovic A, Lazic D, et al. Endovascular Treatment of Femoro-Popliteal Disease with the Supera Stent: A Single Center Experience. Journal of Clinical Medicine. 2025; 14(5):1704. https://doi.org/10.3390/jcm14051704
Chicago/Turabian StyleLukic, Borivoje, Marko Miletic, Stefan Milosevic, Marko Dragas, Jovica Saponjski, Igor Koncar, Petar Zlatanovic, Filip Lukic, Aleksandar Mirkovic, Dimitrije Lazic, and et al. 2025. "Endovascular Treatment of Femoro-Popliteal Disease with the Supera Stent: A Single Center Experience" Journal of Clinical Medicine 14, no. 5: 1704. https://doi.org/10.3390/jcm14051704
APA StyleLukic, B., Miletic, M., Milosevic, S., Dragas, M., Saponjski, J., Koncar, I., Zlatanovic, P., Lukic, F., Mirkovic, A., Lazic, D., Markovic, K., Milic, N., & Cvetic, V. (2025). Endovascular Treatment of Femoro-Popliteal Disease with the Supera Stent: A Single Center Experience. Journal of Clinical Medicine, 14(5), 1704. https://doi.org/10.3390/jcm14051704








