A Decade of Ovarian Cancer in Indonesia: Epidemiology and Survival Analysis from 2010 to 2020
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Source
2.2. Study Population
2.3. Variables and Data Collection
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, P.M.; Jordan, S.J. Global epidemiology of epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Statistics Factsheets: Ovarian Cancer. National Cancer Institute. Available online: https://ocrahope.org/get-the-facts/statistics/ (accessed on 14 July 2024).
- Razi, S.; Ghoncheh, M.; Mohammadian-Hafshejani, A.; Aziznejhad, H.; Mohammadian, M.; Salehiniya, H. The incidence and mortality of ovarian cancer and their relationship with the Human Development Index in Asia. Ecancermedicalscience 2016, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Maleki, Z.; Vali, M.; Nikbakht, H.-A.; Hassanipour, S.; Kouhi, A.; Sedighi, S.; Farokhi, R.; Ghaem, H. Survival rate of ovarian cancer in Asian countries: A systematic review and meta-analysis. BMC Cancer 2023, 23, 558. [Google Scholar] [CrossRef] [PubMed]
- Noela, F.; Nuryanto, K.H. Epidemiology Data of Ovarian Cancer in Dr. Cipto Mangunkusumo Hospital, Jakarta. Indones. J. Obstet. Gynaecol. 2016, 4, 101–106. [Google Scholar] [CrossRef]
- Purbadi, S.; Tanamas, G.; Novianti, L. Advanced stage ovarian cancer survival in Jakarta. Eur. J. Gynaecol. Oncol. 2020, 41, 587–590. [Google Scholar]
- Nogueira-Rodrigues, A.; Giannecchini, G.V.; Secord, A.A. Real world challenges and disparities in the systemic treatment of ovarian cancer. Gynecol. Oncol. 2024, 185, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Javadi, S.; Ganeshan, D.M.; Qayyum, A.; Iyer, R.B.; Bhosale, P. Ovarian Cancer, the Revised FIGO Staging System, and the Role of Imaging. AJR Am. J. Roentgenol. 2016, 206, 1351–1360. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Singh, N.; Gilks, C.B. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology 2022, 80, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Lira, R.P.C.; Antunes-Foschini, R.; Rocha, E.M. Survival analysis (Kaplan-Meier curves): A method to predict the future. Arq. Bras. Oftalmol. 2020, 83, V–VII. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Hollis, R.L.; Meynert, A.M.; Michie, C.O.; Rye, T.; Churchman, M.; Hallas-Potts, A.; Croy, I.; McCluggage, W.G.; Williams, A.R.W.; Bartos, C.; et al. Multiomic Characterization of High-Grade Serous Ovarian Carcinoma Enables High-Resolution Patient Stratification. Clin. Cancer Res. 2022, 28, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Hatano, Y.; Hatano, K.; Tamada, M.; Morishige, K.I.; Tomita, H.; Yanai, H.; Hara, A. A Comprehensive Review of Ovarian Serous Carcinoma. Adv. Anat. Pathol. 2019, 26, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Kleinmanns, K.; Bjørge, L. Enhancing precision oncology in high-grade serous carcinoma: The emerging role of antibody-based therapies. Npj Women’s Health 2024, 2, 7. [Google Scholar] [CrossRef]
- Chandler, R.L.; Damrauer, J.S.; Raab, J.R.; Schisler, J.C.; Wilkerson, M.D.; Didion, J.P.; Starmer, J.; Serber, D.; Yee, D.; Xiong, J.; et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat. Commun. 2015, 6, 6118. [Google Scholar] [CrossRef]
- Wong, O.G.W.; Li, J.; Cheung, A.N.Y. Targeting DNA Damage Response Pathway in Ovarian Clear Cell Carcinoma. Front. Oncol. 2021, 11, 666815. [Google Scholar] [CrossRef]
- Hoffman, B.L.; Schorge, J.O.; Halvorson, L.M.; Hamid, C.A.; Corton, M.M.; Schaffer, J.I. Ovarian Germ Cell and Sex Cord–Stromal Tumors. In Williams Gynecology, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2020. [Google Scholar]
- Pashankar, F.; Murray, M.J.; Gell, J.; MacDonald, N.; Shamash, J.; Billmire, D.F.; Klosterkemper, L.; Olson, T.; Hirsch, M.S.; Lockley, M.; et al. Consensus and controversy in the management of paediatric and adult patients with ovarian immature teratoma: The Malignant Germ Cell International Consortium perspective. eClinicalMedicine 2024, 69, 102453. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Talhouk, A.; McAlpine, J.N.; Law, M.R.; Hanley, G.E. Long-term mortality among women with epithelial ovarian cancer: A population-based study in British Columbia, Canada. BMC Cancer 2018, 18, 1039. [Google Scholar] [CrossRef]
- Reid, F.; Adams, T.; Adel, R.S.; Andrade, C.E.; Bajwa, A.; Bambury, I.G.; Benhima, N.; Bolatbekova, R.; Leon, D.C.; Charlton, P.; et al. The every woman study™ low- and middle-income countries edition protocol: A multi-country observational study to assess opportunities and challenges to improving survival and quality of life for women with ovarian cancer. PLoS ONE 2024, 19, e0298154. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.A.; Shrivastava, D. A Comprehensive Review of Screening Methods for Ovarian Masses: Towards Earlier Detection. Cureus 2023, 15, e48534. [Google Scholar] [CrossRef] [PubMed]
- Brand, N.R.; Qu, L.G.; Chao, A.; Ilbawi, A.M. Delays and Barriers to Cancer Care in Low- and Middle-Income Countries: A Systematic Review. Oncologist 2019, 24, e1371–e1380. [Google Scholar] [CrossRef] [PubMed]
- Chornokur, G.; Amankwah, E.K.; Schildkraut, J.M.; Phelan, C.M. Global ovarian cancer health disparities. Gynecol. Oncol. 2013, 129, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.; Walter, F.M.; Rubin, G.; Neal, R.D. Improving early diagnosis of symptomatic cancer. Nat. Rev. Clin. Oncol. 2016, 13, 740–749. [Google Scholar] [CrossRef] [PubMed]
| Variables | Descriptive Statistics |
|---|---|
| Age, years [mean (SD)] | 52.41 (12.56) |
| Parity, children [mean (SD)] | 1.64 (1.05) |
| Overall Survival, months [mean (SD)] | 5.07 (6.50) |
| Province, N (%) | |
| DKI Jakarta | 483 (45.35%) |
| Jawa Barat | 383 (35.96%) |
| Banten | 79 (7.42%) |
| Other | 120 (11.27%) |
| Ethnic, N (%) | |
| Jawa | 695 (61.88%) |
| Sunda | 158 (14.84%) |
| Betawi | 100 (9.39%) |
| Other | 148 (13.90%) |
| Occupation, N (%) | |
| Unemployed | 807 (75.77%) |
| Employed | 258 (24.23%) |
| FIGO stage, N (%) | |
| Stage IA | 49 (4.60%) |
| Stage IB | 13 (1.22%) |
| Stage IC1 | 46 (4.32%) |
| Stage IC2 | 2 (0.19%) |
| Stage IC3 | 8 (0.75%) |
| Stage IIA | 8 (0.75%) |
| Stage IIB | 17 (1.60%) |
| Stage IIIA | 18 (1.69%) |
| Stage IIIA1 | 5 (0.47%) |
| Stage IIIA2 | 3 (0.28%) |
| Stage IIIB | 18 (1.69%) |
| Stage IIIC | 112 (10.52%) |
| Stage IVA | 38 (3.57%) |
| Stage IVB | 15 (1.41%) |
| Unknown | 713 (66.95%) |
| Tumor Types, N (%) | |
| clear cell carcinoma | 87 (8.17%) |
| dysgerminoma | 21 (1.97%) |
| endometrioid carcinoma | 66 (6.20%) |
| adult granulosa cell tumor | 32 (3.00%) |
| immature teratoma | 14 (1.31%) |
| mucinous carcinoma | 71 (6.67%) |
| serous carcinoma | 727 (68.26%) |
| undifferentiated and dedifferentiated carcinomas | 30 (2.82%) |
| yolk sac tumor | 17 (1.60%) |
| Category, N (%) | |
| clear cell tumors | 87 (8.17%) |
| endometrioid tumors | 66 (6.20%) |
| germ cell tumors | 52 (4.88%) |
| mucinous tumors | 71 (6.67%) |
| pure sex cord tumors | 30 (2.82%) |
| serous tumors | 32 (3.00%) |
| other carcinomas | 727 (68.26%) |
| Degree Differentiation, N (%) | |
| Grade 1 | 82 (7.70%) |
| Grade 2 | 47 (4.41%) |
| Grade 3 | 90 (8.45%) |
| Unknown | 846 (79.44%) |
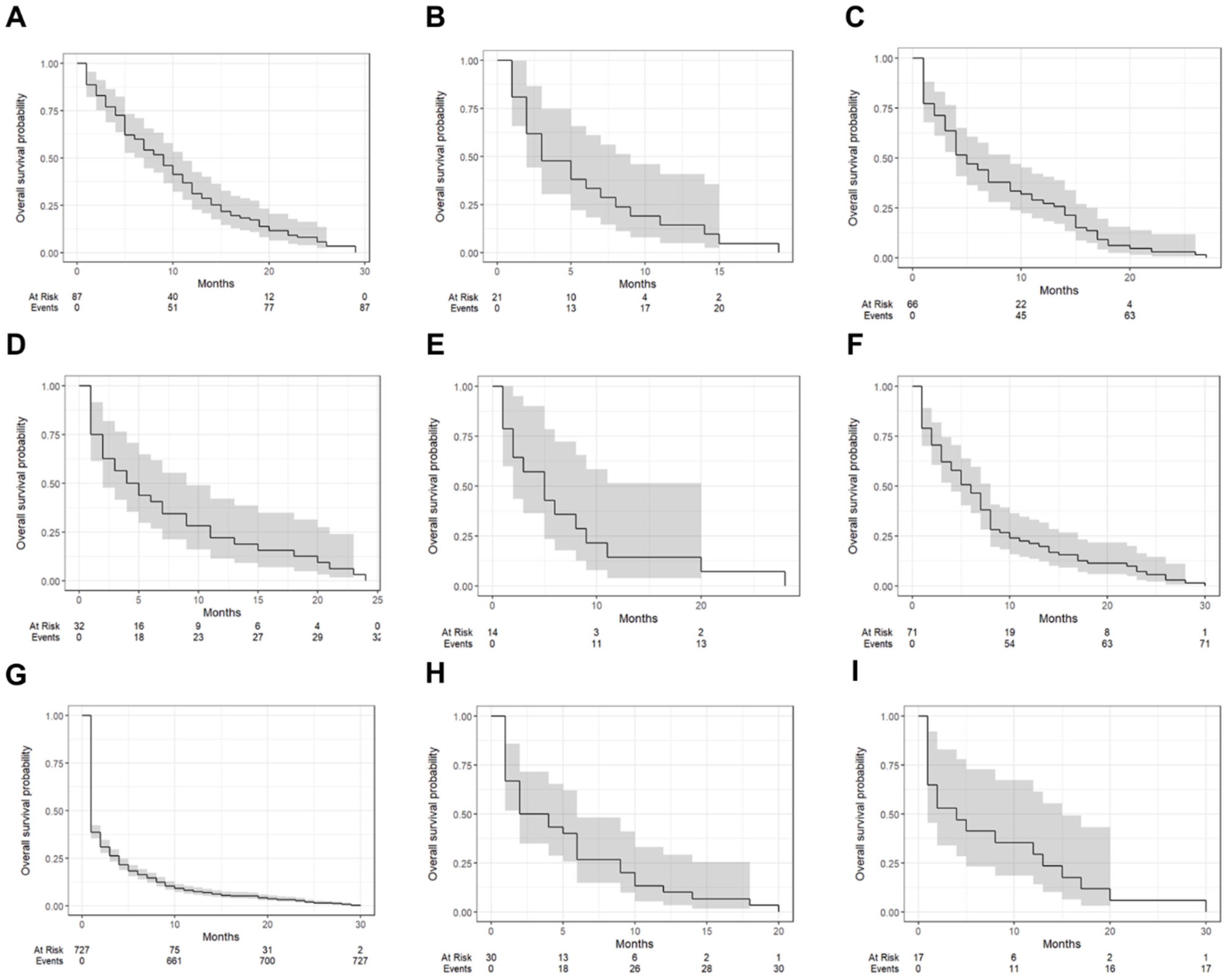
| Tumor Types | One-Year Survival (95% CI) | Median Survival Time (Months [IQR]) |
|---|---|---|
| clear cell carcinoma | 31.0% (22.7–42.5%) | 9 (7–11) |
| dysgerminoma | 14.3% (5.0–40.7%) | 3 (2–9) |
| endometrioid carcinoma | 27.3% (18.4–40.4%) | 5 (4–9) |
| adult granulosa cell tumor | 21.9% (11.4–42.1%) | 4.5 (2–9) |
| immature teratoma | 14.3% (39.6–51.5%) | 5 (2–20) |
| mucinous carcinoma | 21.1% (13.5–33.1%) | 6 (4–8) |
| serous carcinoma | 7.4% (5.6–9.6%) | 1 (1–2) |
| undifferentiated and dedifferentiated carcinomas | 10.0% (3.4–29.3%) | 3 (2–6) |
| yolk sac tumor | 29.4% (14.2–61.4%) | 4 (1–15) |
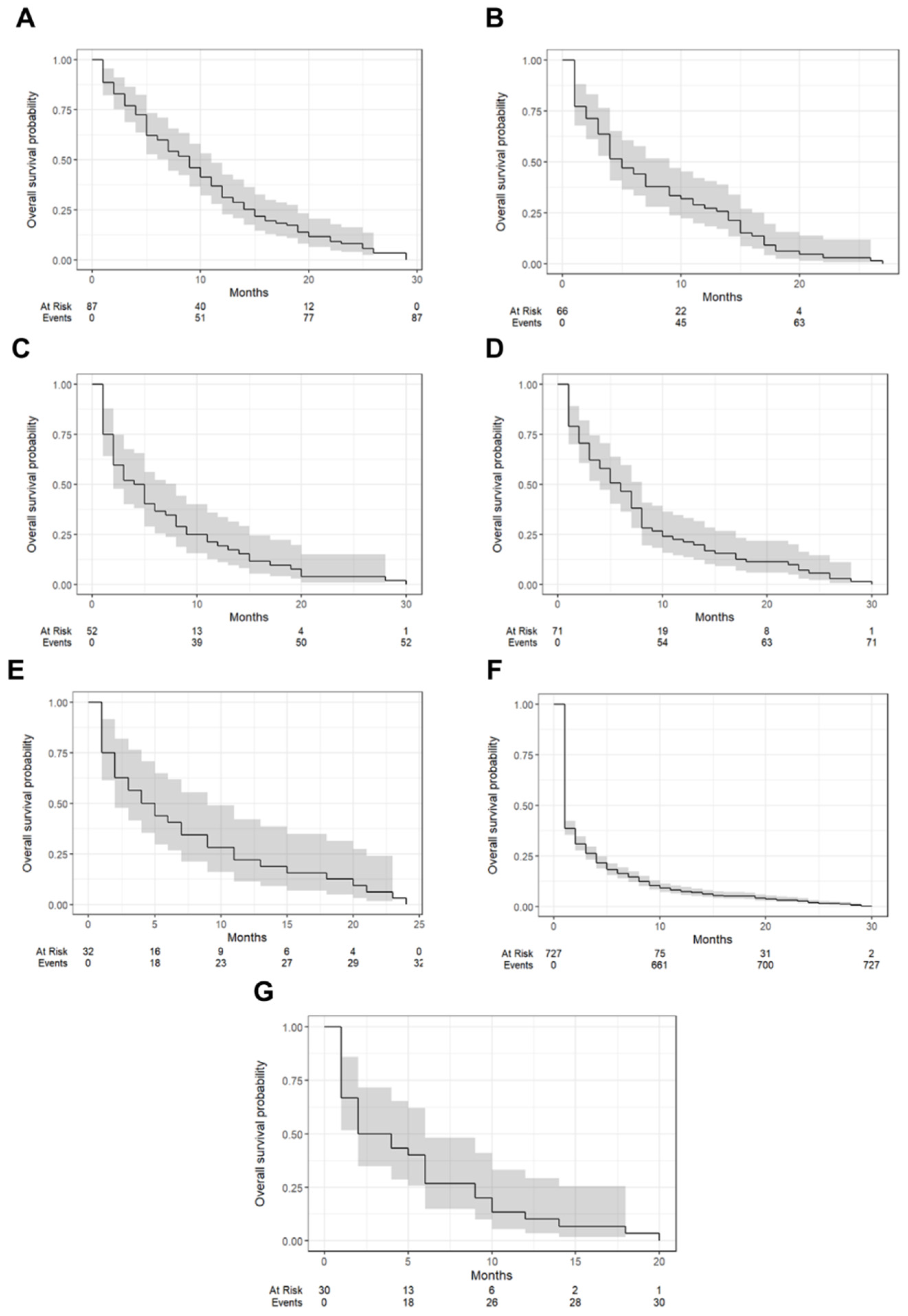
| Tumor Types | One-Year Survival (95% CI) | Median Survival Time (Months [IQR]) |
|---|---|---|
| clear cell tumors | 31.0% (22.7–42.5%) | 9 (7–11) |
| endometrioid tumors | 27.3% (18.4–40.4%) | 5 (4–9) |
| germ cell tumors | 19.2% (1.10–33.6%) | 4.5 (2–8) |
| mucinous tumors | 21.1% (13.5–33.1%) | 6 (4–8) |
| pure sex cord tumors | 21.9% (11.4–42.1%) | 4.5 (2–9) |
| serous tumors | 7.4% (5.8–9.6%) | 1 (1–2) |
| other carcinomas | 10.0% (3.4–29.3%) | 3 (2–6) |
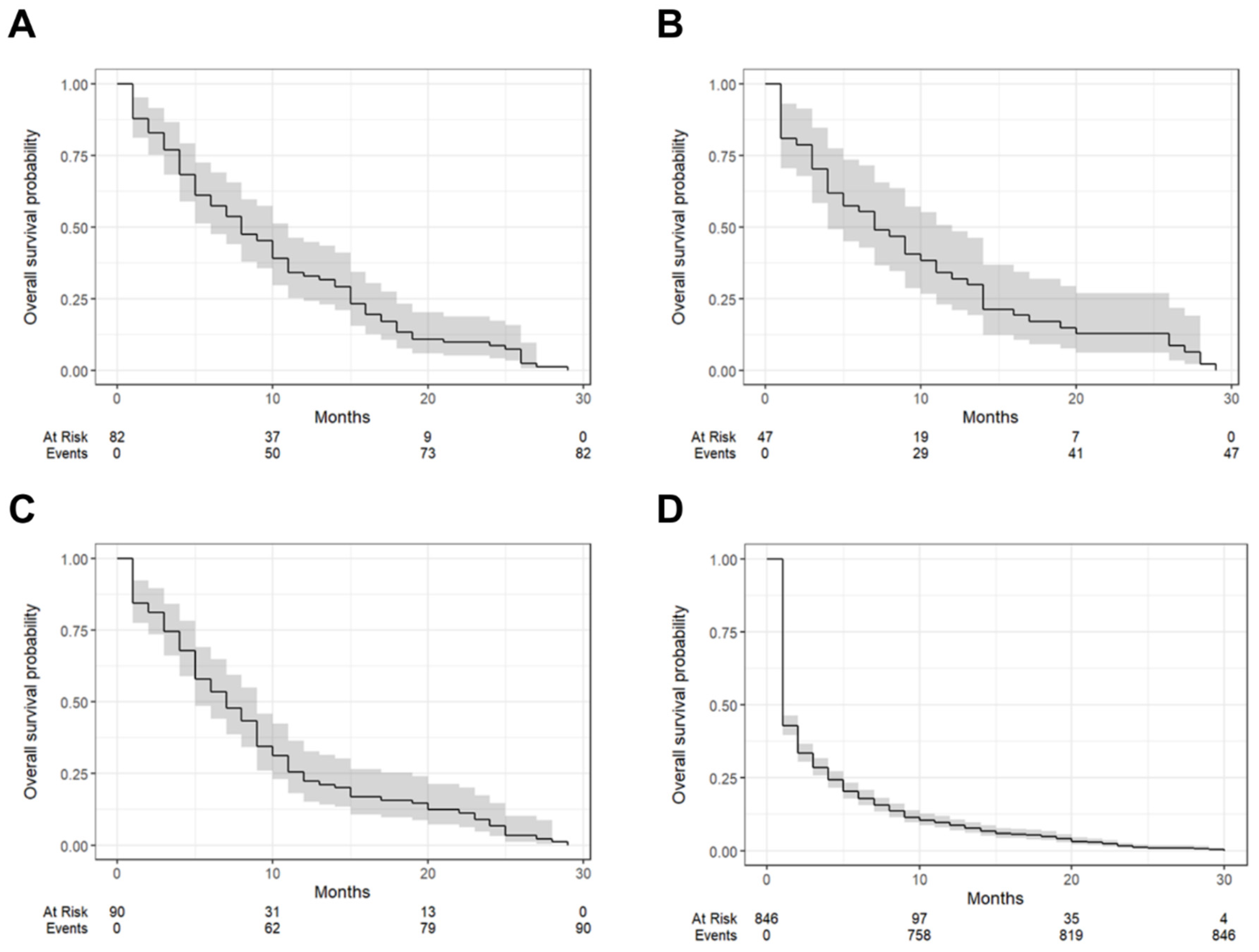
| Tumor Types | One-Year Survival (95% CI) | Median Survival Time (Months [IQR]) |
|---|---|---|
| Grade 1 | 32.9% (5.2–44.8%) | 8 (6–11) |
| Grade 2 | 31.9% (21.0–48.5%) | 7 (4–12) |
| Grade 3 | 22.2% (15.1–32.7%) | 7 (5–9) |
| Unknown | 8.51% (6.82–10.6%) | 1 (1–2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rustamadji, P.; Wiyarta, E.; Nuryanto, K.H.; Anggraeni, T.D.; Kusuma, F.; Purwoto, G.; Winarto, H.; Heliyanti, T.; Tjahjadi, H.; Hayati, A.; et al. A Decade of Ovarian Cancer in Indonesia: Epidemiology and Survival Analysis from 2010 to 2020. J. Clin. Med. 2025, 14, 1692. https://doi.org/10.3390/jcm14051692
Rustamadji P, Wiyarta E, Nuryanto KH, Anggraeni TD, Kusuma F, Purwoto G, Winarto H, Heliyanti T, Tjahjadi H, Hayati A, et al. A Decade of Ovarian Cancer in Indonesia: Epidemiology and Survival Analysis from 2010 to 2020. Journal of Clinical Medicine. 2025; 14(5):1692. https://doi.org/10.3390/jcm14051692
Chicago/Turabian StyleRustamadji, Primariadewi, Elvan Wiyarta, Kartiwa Hadi Nuryanto, Tricia Dewi Anggraeni, Fitriyadi Kusuma, Gatot Purwoto, Hariyono Winarto, Tantri Heliyanti, Hartono Tjahjadi, Amal Hayati, and et al. 2025. "A Decade of Ovarian Cancer in Indonesia: Epidemiology and Survival Analysis from 2010 to 2020" Journal of Clinical Medicine 14, no. 5: 1692. https://doi.org/10.3390/jcm14051692
APA StyleRustamadji, P., Wiyarta, E., Nuryanto, K. H., Anggraeni, T. D., Kusuma, F., Purwoto, G., Winarto, H., Heliyanti, T., Tjahjadi, H., Hayati, A., Sartika, R. A. D., Prasetyo, S., & Andrijono, A. (2025). A Decade of Ovarian Cancer in Indonesia: Epidemiology and Survival Analysis from 2010 to 2020. Journal of Clinical Medicine, 14(5), 1692. https://doi.org/10.3390/jcm14051692







