Full-Face Allograft Retrieval in a Multiple-Organ Donation in a Maastricht III Type Donor
Abstract
1. Introduction
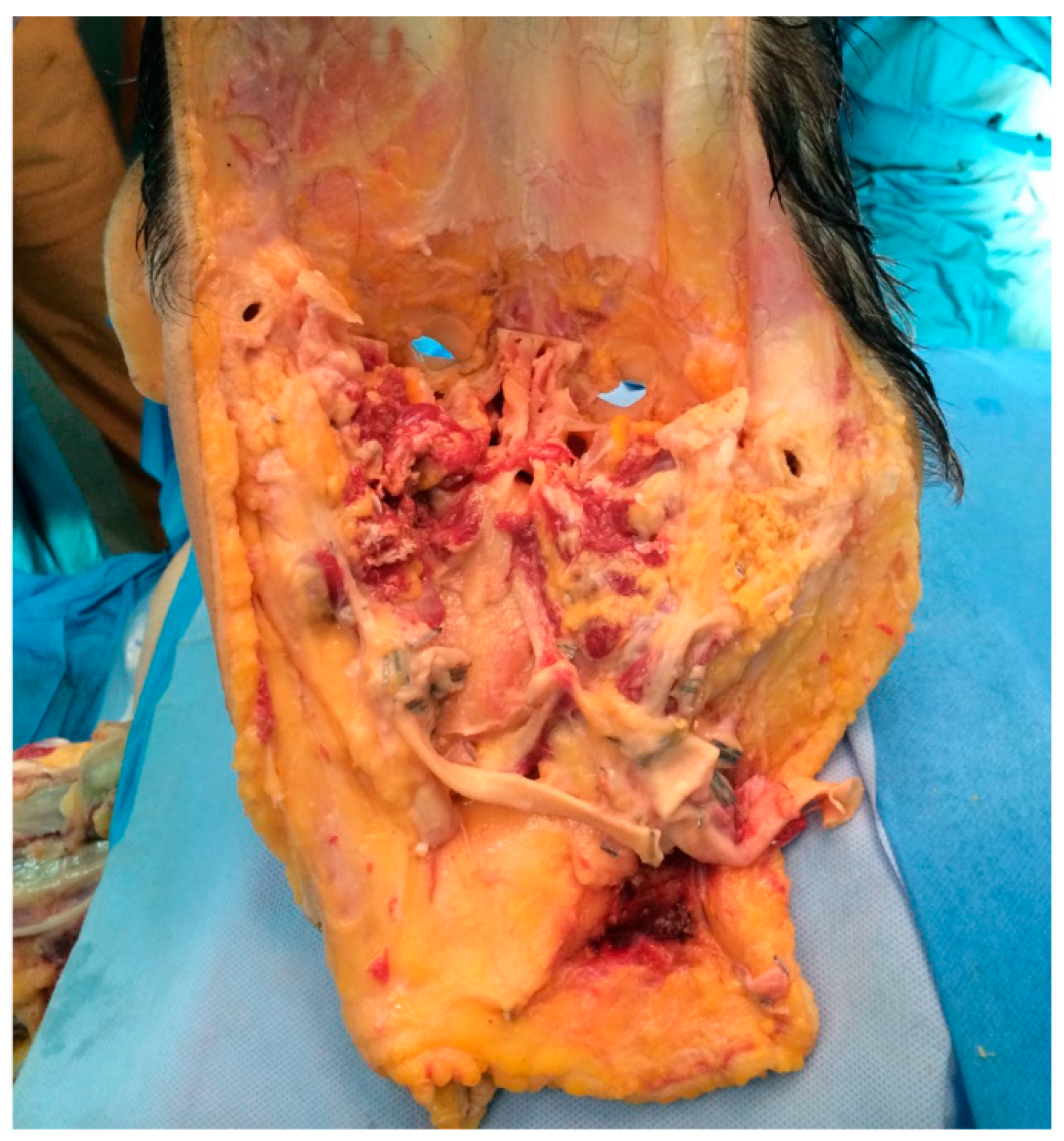
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AD | Asystole Donation |
| DCD | Donation after Cardiac Death |
| RR | Rapid Recovery |
| rATG | rabbit Antithymocyte Globulin |
| UW | University of Wisconsin |
| VCA | Vascularized Composite Allotransplantation |
| WLST | Withdrawal of Life Sustaining Therapy |
| FVCA | face Vascularized Composite Allotransplantation |
References
- Devauchelle, B.; Badet, L.; Lengelé, B.; Morelon, E.; Testelin, S.; Michallet, M.; D’Hauthuille, C.; Dubernard, J.-M. First human face allograft: Early report. Lancet 2006, 368, 203–209. [Google Scholar] [CrossRef]
- Homsy, P.; Huelsboemer, L.; Barret, J.P.; Blondeel, P.; Borsuk, D.E.; Bula, D.; Gelb, B.; Infante-Cossio, P.; Lantieri, L.; Mardini, S.; et al. An Update on the Survival of the First 50 Face Transplants Worldwide. JAMA Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Longo, B.; Pomahac, B.; Giacalone, M.; Cardillo, M.; Cervelli, V. 18 years of face transplantation: Adverse outcomes and challenges. J. Plast. Reconstr. Aesthet. Surg. 2023, 87, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Barret, J.P.; Gavaldà, J.; Bueno, J.; Nuvials, X.; Pont, T.; Masnou, N.; Colomina, M.J.; Serracanta, J.; Arno, A.; Huguet, P.; et al. Full face transplant: The first case report. Ann. Surg. 2011, 254, 252–256. [Google Scholar] [CrossRef]
- Lantieri, L. Face transplant: A paradigm change in facial reconstruction. J. Craniofac. Surg. 2012, 23, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Brazio, P.S.; Barth, R.N.; Bojovic, B.; Dorafshar, A.H.; Garcia, J.P.; Brown, E.N.; Bartlett, S.T.; Rodriguez, E.D. Algorithm for total face and multiorgan procurement from a brain-dead donor. Am. J. Transpl. 2013, 13, 2743–2749. [Google Scholar] [CrossRef]
- Bueno, J.; Barret, J.P.; Serracanta, J.; Arnó, A.; Collado, J.M.; Valles, C.; Colomina, M.J.; Diez, Y.; Pont, T.; Salamero, P.; et al. Logistics and strategy of multiorgan procurement involving total face allograft. Am. J. Transpl. 2011, 11, 1091–1097. [Google Scholar] [CrossRef]
- BOE-A-2012-15715; Real Decreto 1723/2012, de 28 de Diciembre, Por El Que SE Regulan Las Actividades de ObtencióN, UtilizacióN ClíNica Y CoordinacióN Territorial de Los ÓRganos Humanos Destinados Al Trasplante Y SE Establecen Requisitos de Calidad Y Seguridad Permalink ELI. Available online: https://www.boe.es/eli/es/rd/2012/12/28/1723 (accessed on 10 October 2024).
- Siemionow, M.; Ozturk, C. Donor operation for face transplantation. J. Reconstr. Microsurg. 2012, 28, 35–42. [Google Scholar] [CrossRef]
- Quilichini, J.; Hivelin, M.; Benjoar, M.D.; Bosc, R.; Meningaud, J.P.; Lantieri, L. Restoration of the donor after face graft procurement for allotransplantation: Report on the technique and outcomes of seven cases. Plast. Reconstr. Surg. 2012, 129, 1105–1111. [Google Scholar] [CrossRef]
- Lantieri, L.; Hivelin, M.; Audard, V.; Benjoar, M.D.; Meningaud, J.P.; Bellivier, F.; Ortonne, N.; Lefaucheur, J.P.; Gilton, A.; Suberbielle, C.; et al. Feasibility, reproducibility, risks and benefits of face transplantation: A prospective study of outcomes. Am. J. Transpl. 2011, 11, 367–378. [Google Scholar] [CrossRef]
- Lewis, H.C.; Cendales, L.C. Vascularized composite allotransplantation in the United States: A retrospective analysis of the Organ Procurement and Transplantation Network data after 5 years of the Final Rule. Am. J. Transpl. 2021, 21, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Cavadas, P.C.; Ibáñez, J.; Thione, A. Surgical aspects of a lower face, mandible, and tongue allotransplantation. J. Reconstr. Microsurg. 2012, 28, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Meningaud, J.P.; Hivelin, M.; Benjoar, M.D.; Toure, G.; Hermeziu, O.; Lantieri, L. The procurement of allotransplants for ballistic trauma: A preclinical study and a report of two clinical cases. Plast. Reconstr. Surg. 2011, 127, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Mathes, D.W.; Zenn, M.R.; Neligan, P.C. The application of indocyanine green fluorescence angiography in plastic surgery. J. Reconstr. Microsurg. 2011, 27, 355–364. [Google Scholar] [CrossRef]
- Dalal, A. Face transplantation: Anesthetic challenges. World J. Transpl. 2016, 6, 646–649. [Google Scholar] [CrossRef]
- Bueno, E.M.; Diaz-Siso, J.R.; Pomahac, B. A multidisciplinary protocol for face transplantation at Brigham and Women’s Hospital. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1572–1579. [Google Scholar] [CrossRef]
- Gordon, C.R.; Siemionow, M.; Papay, F.; Pryor, L.; Gatherwright, J.; Kodish, E.; Paradis, C.; Coffman, K.; Mathes, D.; Schneeberger, S.; et al. The world’s experience with facial transplantation: What have we learned thus far? Ann. Plast. Surg. 2009, 63, 572–578. [Google Scholar] [CrossRef]
- Olawade, D.B.; Marinze, S.; Qureshi, N.; Weerasinghe, K.; Teke, J. Transforming organ donation and transplantation: Strategies for increasing donor participation and system efficiency. Eur. J. Intern Med. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Manninen, A.A.; Törnwall, J.; Horelli, J.C.; Heliövaara, A.K.; Mesimäki, K.V.; Lindford, A.J.; Wilkman, T.S.; Lassus, P. Virtual 3D planning and prediction accuracy in two bimaxillary face transplantations in Helsinki. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 605–612. [Google Scholar] [CrossRef]
- Cho, K.H.; Papay, F.A.; Yanof, J.; West, K.; Gharb, B.B.; Rampazzo, A.; Gastman, B.; Schwarz, G.S. Mixed Reality and 3D Printed Models for Planning and Execution of Face Transplantation. Ann. Surg. 2021, 274, e1238–e1246. [Google Scholar] [CrossRef]
- Abidin, Z.U.; Naqvi, R.A.; Haider, A.; Kim, H.S.; Jeong, D.; Lee, S.W. Recent deep learning-based brain tumor segmentation models using multi-modality magnetic resonance imaging: A prospective survey. Front. Bioeng. Biotechnol. 2024, 22, 1392807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aqvi, R.A.; Haider, A.; Kim, H.S.; Jeong, D.; Lee, S.W. Transformative Noise Reduction: Leveraging a Transformer-Based Deep Network for Medical Image Denoising. Mathematics 2024, 12, 2313. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barret, J.P.; Dopazo, C.; Sandiumenge, A.; Bilbao, I.; Charco, R. Full-Face Allograft Retrieval in a Multiple-Organ Donation in a Maastricht III Type Donor. J. Clin. Med. 2025, 14, 1682. https://doi.org/10.3390/jcm14051682
Barret JP, Dopazo C, Sandiumenge A, Bilbao I, Charco R. Full-Face Allograft Retrieval in a Multiple-Organ Donation in a Maastricht III Type Donor. Journal of Clinical Medicine. 2025; 14(5):1682. https://doi.org/10.3390/jcm14051682
Chicago/Turabian StyleBarret, Juan P., Cristina Dopazo, Alberto Sandiumenge, Itxarone Bilbao, and Ramón Charco. 2025. "Full-Face Allograft Retrieval in a Multiple-Organ Donation in a Maastricht III Type Donor" Journal of Clinical Medicine 14, no. 5: 1682. https://doi.org/10.3390/jcm14051682
APA StyleBarret, J. P., Dopazo, C., Sandiumenge, A., Bilbao, I., & Charco, R. (2025). Full-Face Allograft Retrieval in a Multiple-Organ Donation in a Maastricht III Type Donor. Journal of Clinical Medicine, 14(5), 1682. https://doi.org/10.3390/jcm14051682







