Fibrinogen-to-Albumin Ratio as Predictor of Mortality in Acute Aortic Syndromes
Abstract
1. Introduction
1.1. Biomarkers in AAS
1.2. Fibrinogen-to-Albumin Ratio
2. Materials and Methods
2.1. Setting
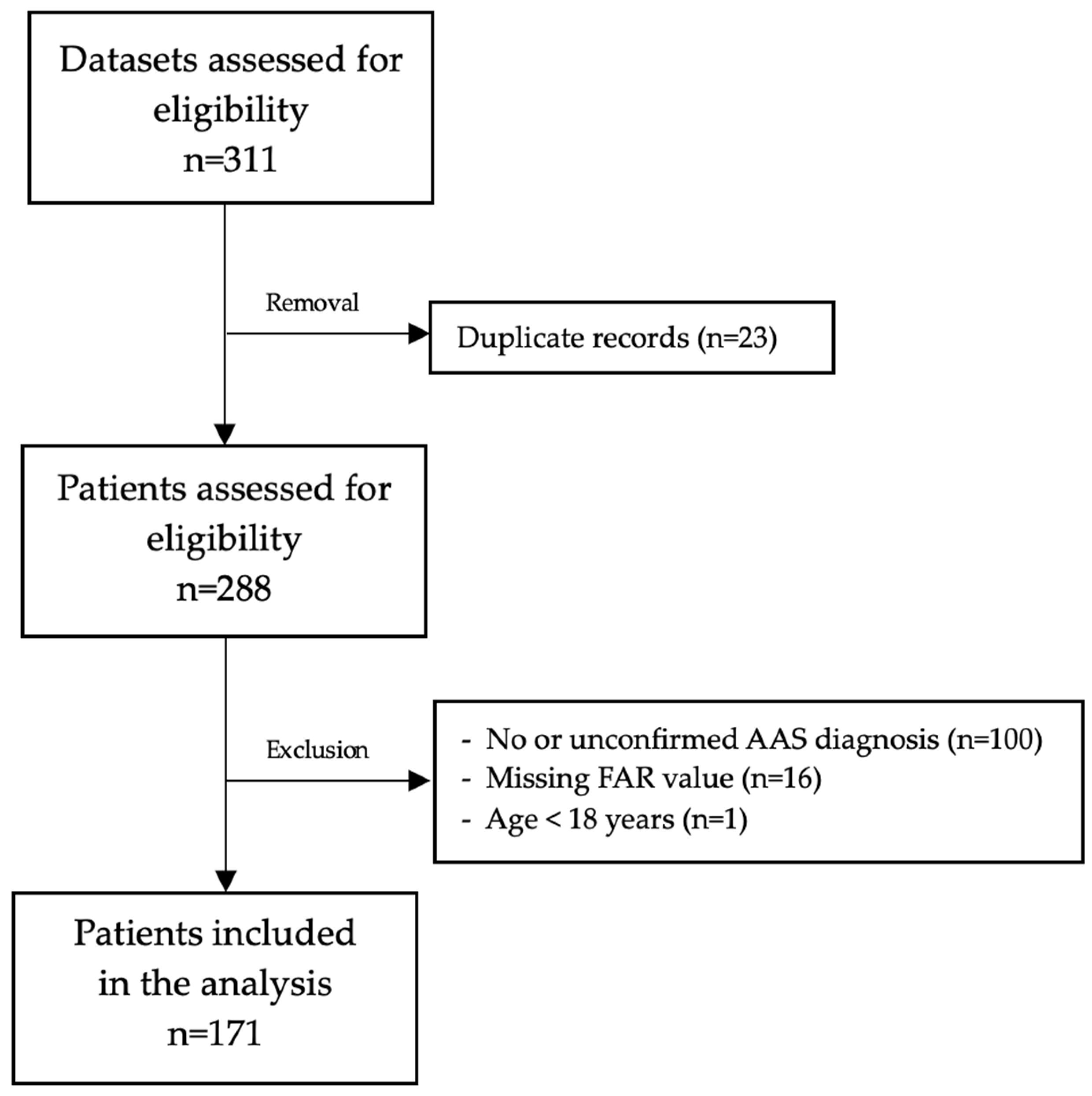
2.2. Inclusion and Exclusion
2.3. Statistical Analysis
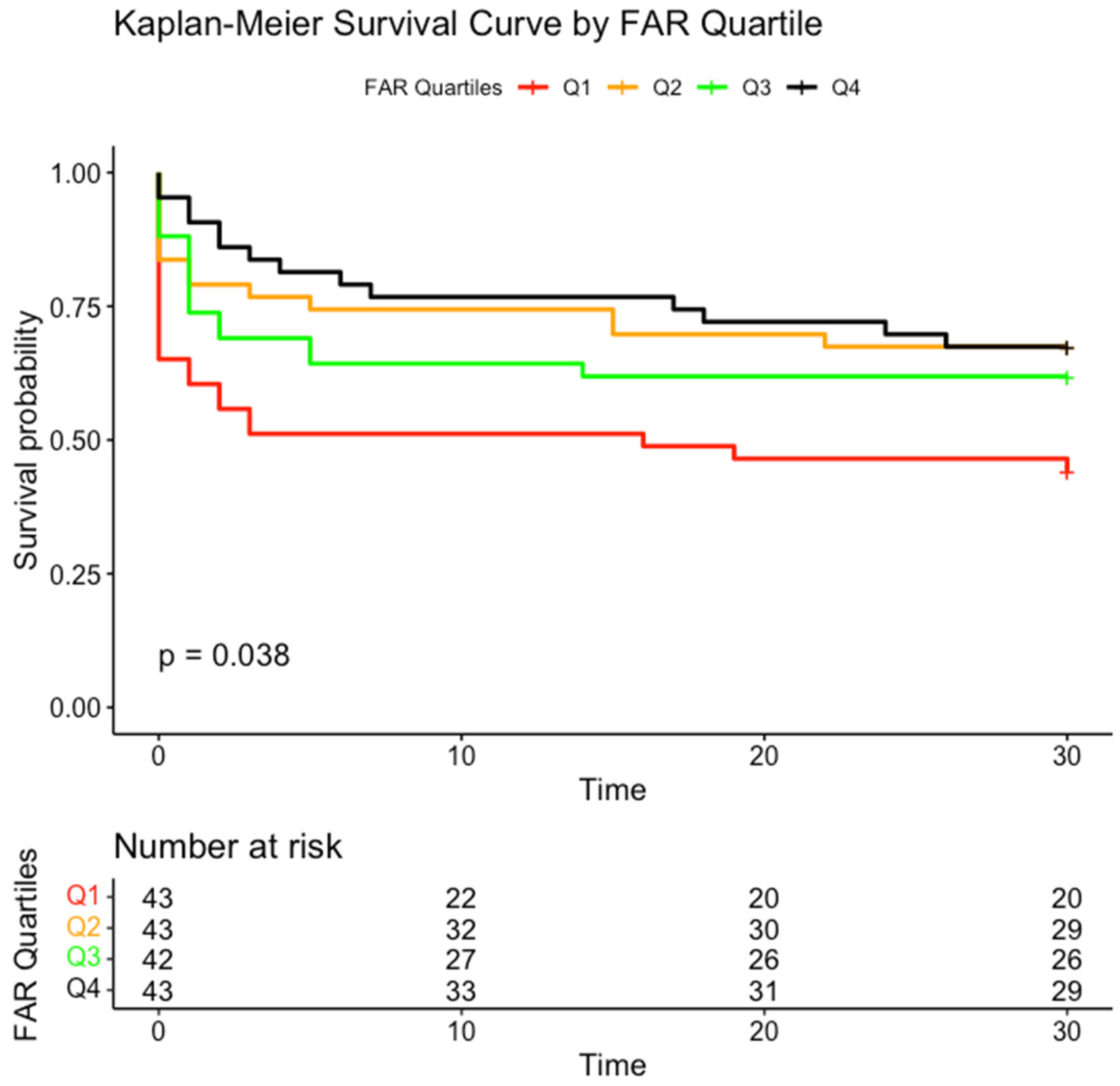
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAD | Acute aortic dissection |
| AAS | Acute aortic syndrome |
| ADD-RS | Aortic Dissecction Detection Risk Score |
| ALAT | Alanine transaminase |
| ASAT | Aspartate transaminase |
| CI | Confidence Interval |
| CPR | Cardiopulmonary resuscitation |
| CRP | C-reactive protein |
| DIC | Disseminated intravascular coagulation |
| FAR | Fibrinogen-to-albumin ratio |
| Gamma-GT | Gamma-glutamyltransferase |
| GFR | Glomerular filtration rate |
| HR | Hazard ratio |
| IMH | Intramural hematoma |
| ISTH | Internatinoal Society on Thrombosis and Haemostasis |
| IQR | Interquartile range |
| PAU | Penetrating aortic ulcer |
References
- Daily, P.O.; Trueblood, H.W.; Stinson, E.B.; Wuerflein, R.D.; Shumway, N.E. Management of Acute Aortic Dissections. Ann. Thorac. Surg. 1970, 10, 237–247. [Google Scholar] [CrossRef] [PubMed]
- De Bakey, M.E.; Henly, W.S.; Cooley, D.A.; Morris, G.C.; Crawford, E.S.; Beall, A.C. Surgical Management of Dissecting Aneurysms of the Aorta. J. Thorac. Cardiovasc. Surg. 1965, 49, 130–149. [Google Scholar] [CrossRef]
- Vilacosta, I.; San Román, J.A. Acute aortic syndrome. Heart 2001, 85, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, I.; Mórocz, J.; Szlávi, J.; Schmidt, J.; Tornóci, L.; Nagy, L.; Szép, L. Epidemiology and clinicopathology of aortic dissection. Chest 2000, 117, 1271–1278. [Google Scholar] [CrossRef]
- Bossone, E.; LaBounty, T.M.; Eagle, K.A. Acute aortic syndromes: Diagnosis and management, an update. Eur. Heart J. 2018, 39, 739–749d. [Google Scholar] [CrossRef]
- Ferrera, C.; Vilacosta, I.; Cabeza, B.; Cobiella, J.; Martínez, I.; Saiz-Pardo Sanz, M.; Bustos, A.; Serrano, F.J.; Maroto, L. Diagnosing Aortic Intramural Hematoma: Current Perspectives. Vasc. Health Risk Manag. 2020, 16, 203–213. [Google Scholar] [CrossRef]
- Morello, F.; Bima, P.; Castelli, M.; Capretti, E.; de Matos Soeiro, A.; Cipriano, A.; Costantino, G.; Vanni, S.; Leidel, B.A.; Kaufmann, B.A.; et al. Diagnosis of acute aortic syndromes with ultrasound and d-dimer: The Profundus study. Eur. J. Intern. Med. 2024, 128, 94–103. [Google Scholar] [CrossRef]
- Cesarman-Maus, G.; Hajjar, K.A. Molecular mechanisms of fibrinolysis. Br. J. Haematol. 2005, 129, 307–321. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Respir. J. 2019, 54, 543–603. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., III; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Suzuki, T.; Bossone, E.; Sawaki, D.; Jánosi, R.A.; Erbel, R.; Eagle, K.; Nagai, R. Biomarkers of aortic diseases. Am. Heart J. 2013, 165, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Distante, A.; Zizza, A.; Trimarchi, S.; Villani, M.; Salerno Uriarte, J.A.; De Luca Tupputi Schinosa, L.; Renzulli, A.; Sabino, F.; Nowak, R.; et al. Diagnosis of acute aortic dissection by D-dimer: The International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009, 119, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Třeška, V.; Topolčan, O.; Pecen, L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin. Chem. Lab. Med. 2000, 38, 1161–1164. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef]
- Chen, C.M.; Lu, C.F.; Liu, W.S.; Gong, Z.H.; Wang, X.Q.; Xu, F.; Ji, J.F.; Fang, X.X. Association between fibrinogen/albumin ratio and arterial stiffness in patients with type 2 diabetes: A cross-sectional study. Front. Pharmacol. 2022, 13, 1120043. [Google Scholar] [CrossRef]
- Sun, D.W.; An, L.; Lv, G.Y. Albumin-fibrinogen ratio and fibrinogen-prealbumin ratio as promising prognostic markers for cancers: An updated meta-analysis. World J. Surg. Oncol. 2020, 18, 9. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Xiang, Y.; Zhang, S.; Feng, W.; Yuan, Y.; Liu, Y.; Yin, S. Clinical Significance of Preoperative Fibrinogen to Albumin Ratio in Patients with Glioblastoma: A Singe Center Experience. Cancer Manag. Res. 2021, 13, 3259–3269. [Google Scholar] [CrossRef]
- Ding, Y.; Xue, L. The potential value of fibrinogen to albumin ratio (FAR) in the assessment of inflammation in spondyloarthritis. BMC Musculoskelet. Disord. 2022, 23, 864. [Google Scholar] [CrossRef]
- Dai, L.L.; Chen, C.; Wu, J.; Cheng, J.F.; He, F. The predictive value of fibrinogen-to-albumin ratio in the active, severe active, and poor prognosis of systemic lupus erythematosus: A single-center retrospective study. J. Clin. Lab. Anal. 2022, 36, e24621. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Ji, Y.; Wang, S.; Wang, T.; Wang, F.; Tang, J. Usefulness of fibrinogen-to-albumin ratio to predict no-reflow and short-term prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessel. 2019, 34, 1600–1607. [Google Scholar] [CrossRef]
- Xiao, L.; Jia, Y.; Wang, X.; Huang, H. The impact of preoperative fibrinogen-albumin ratio on mortality in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Clin. Chim. Acta 2019, 493, 8–13. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Luan, H.; Luo, C.; Kamila, K.; Zheng, T.; Tian, G. Predictive impact of fibrinogen-to-albumin ratio (FAR) for left ventricular dysfunction in acute coronary syndrome: A cross-sectional study. Eur. J. Med. Res. 2023, 28, 68. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, L.L.; Wang, J.; Ji, G. The relationship between fibrinogen and in-hospital mortality in patients with type A acute aortic dissection. Am. J. Emerg. Med. 2018, 36, 741–744. [Google Scholar] [CrossRef]
- Gao, Y.; Li, D.; Cao, Y.; Zhu, X.; Zeng, Z.; Tang, L. Prognostic value of serum albumin for patients with acute aortic dissection: A retrospective cohort study. Medicine 2019, 98, e14486. [Google Scholar] [CrossRef]
- Grafeneder, J.; Krychtiuk, K.A.; Buchtele, N.; Schoergenhofer, C.; Gelbenegger, G.; Lenz, M.; Wojta, J.; Heinz, G.; Huber, K.; Hengstenberg, C.; et al. The ISTH DIC score predicts outcome in non-septic patients admitted to a cardiovascular intensive care unit. Eur. J. Intern. Med. 2020, 79, 37–42. [Google Scholar] [CrossRef]
- Suzuki, K.; Wada, H.; Imai, H.; Iba, T.; Thachil, J.; Toh, C.H. A re-evaluation of the D-dimer cut-off value for making a diagnosis according to the ISTH overt-DIC diagnostic criteria: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1442–1444. [Google Scholar] [CrossRef]
- Lee, D.; Kim, Y.W.; Kim, T.Y.; Lee, S.; Do, H.H.; Seo, J.S.; Lee, J.H. Age-Adjusted D-Dimer in Ruling Out Acute Aortic Syndrome. Emerg. Med. Int. 2022, 2022, 6864756. [Google Scholar] [CrossRef]
- Otani, T.; Abe, T.; Ichiba, T.; Kashiwa, K.; Naito, H. D-dimer measurement is useful irrespective of time from the onset of acute aortic syndrome symptoms. Am. J. Emerg. Med. 2023, 71, 7–13. [Google Scholar] [CrossRef]
- Kotani, Y.; Toyofuku, M.; Tamura, T.; Shimada, K.; Matsuura, Y.; Tawa, H.; Uchikawa, M.; Higashi, S.; Fujimoto, J.; Yagita, K.; et al. Validation of the diagnostic utility of D-dimer measurement in patients with acute aortic syndrome. Eur. Heart J. Acute Cardiovasc. Care 2017, 6, 223–231. [Google Scholar] [CrossRef]
- Hazui, H.; Nishimoto, M.; Hoshiga, M.; Negoro, N.; Muraoka, H.; Murai, M.; Ohishi, Y.; Fukumoto, H.; Morita, H. Young Adult Patients With Short Dissection Length and Thrombosed False Lumen Without Ulcer-Like Projections are Liable to Have False-Negative Results of D-Dimer Testing for Acute Aortic Dissection Based on a Study of 113 Cases. Circ. J. 2006, 70, 1598–1601. [Google Scholar] [CrossRef]
- Ohlmann, P.; Faure, A.; Morel, O.; Petit, H.; Kabbaj, H.; Meyer, N.; Cheneau, E.; Jesel, L.; Epailly, E.; Desprez, D.; et al. Diagnostic and prognostic value of circulating D-Dimers in patients with acute aortic dissection. Crit. Care Med. 2006, 34, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Arima, D.; Suematsu, Y.; Yamada, R.; Matsumoto, R.; Kurahashi, K.; Nishi, S.; Yoshimoto, A. Relationship of acute type A aortic dissection and disseminated intravascular coagulation. J. Vasc. Surg. 2022, 75, 1553–1560.e1551. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.L.; Li, L.; Jiang, W.J.; Gong, M.; Li, H.Y.; Liu, Y.Y.; Wang, X.L.; Zhang, H.J. Low preoperative serum fibrinogen level is associated with postoperative acute kidney injury in patients with in acute aortic dissection. J. Cardiothorac. Surg. 2023, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xue, Y.; Liu, J.; Zhang, H.; Jiang, W. Is fibrinogen plasma level a risk factor for the first 24-hour death of medically treated acute type A aortic dissection patients? Ann. Transl. Med. 2020, 8, 1015. [Google Scholar] [CrossRef]
- Rogers, A.M.; Hermann, L.K.; Booher, A.M.; Nienaber, C.A.; Williams, D.M.; Kazerooni, E.A.; Froehlich, J.B.; O’Gara, P.T.; Montgomery, D.G.; Cooper, J.V.; et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: Results from the international registry of acute aortic dissection. Circulation 2011, 123, 2213–2218. [Google Scholar] [CrossRef]
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; Eusanio, M.D.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018, 137, 1846–1860. [Google Scholar] [CrossRef]
| Parameter | Total (n = 171) | Survived (n = 103) | Died Within 30 Days (n = 68) |
|---|---|---|---|
| Age [years], mean (SD) | 67 (13.7) | 65 (13) | 67 (15) |
| Female, n (%) | 56 (33) | 30 (29.1) | 26 (38.2) |
| Cardiopulmonary resuscitation (yes), n (%) | 35 (20.4) | 5 (4.9) | 30 (44.1) |
| Catecholamines (yes), n (%) | 100 (58.1) | 45 (43.7) | 53 (79.4) |
| Bleeding on admission (yes), n (%) | 146 (84.9) | 82 (79.6) | 64 (94.1) |
| White blood cell count [G/L, mean (SD) | 12.4 (5.4) | 12.4 (5.1) | 12.5 (5.9) |
| C-reactive protein [mg/dL], median (IQR) | 0.79 (0.23–3.06) | 0.89 (0.31–3.74) | 0.66 (0.21–1.76) |
| Platelet count [G/L], mean (SD) | 202 (82) | 224 (82) | 169 (73) |
| D-Dimer 1 [µg/mL], median (IQR) | 3.92 (2.25–16.04) | 8.1 (18.4) | 63.8 (100.2) |
| Creatinine [mg/dL], median (IQR) | 1.2 (0.9–1.6) | 1 (0.9–1.4) | 1.31 (1.07–2.01) |
| Bilirubin [mg/dL], median (IQR) | 0.44 (0.3–0.7) | 0.49 (0.3–0.7) | 0.4 (0.21–0.71) |
| Cholinesterase [kU/L], median (IQR) | 5.83 (4.4–7) | 6.05 (4.75–7.48) | 5.6 (4–6.8) |
| ASAT [U/L], median (IQR) | 26.5 (19–51) | 24 (17–35) | 38 (23–76) |
| ALAT [U/L], median (IQR) | 23 (14–42) | 21 (14–35) | 27.5 (16–60) |
| Gamma-GT [U/L], median (IQR) | 29 (18–57) | 26 (17–47) | 31 (20–64) |
| Triglyceride [mg/dL], median (IQR) | 104.5 (76–146) | 100 (72–153) | 109 (77–142) |
| GFR [ml/min/1,73 m2], median (IQR) | 40.02 (27.6–50.04) | 40.71 (30–53.87) | 39 (23.5–46.21) |
| Parameter | Survived | Died | p-Value |
|---|---|---|---|
| Fibrinogen [g/L] | 3.75/(1.55) | 2.76 (1.60) | <0.001 |
| Albumin [g/L] | 36.48 (6.49) | 32.48 (8.97) | 0.001 |
| FAR | 10.74 (5.44) | 8.89 (4.97) | 0.025 |
| Parameter | HR (95% CI) | p-Value |
|---|---|---|
| FAR | 0.92 (0.87–0.98) | 0.010 |
| Sex [male/female] | 1.85 (1.12–3.07) | 0.017 |
| Age [years] | 1.02 (1.00–1.04) | 0.116 |
| CPR [yes/no] | 6.00 (3.50–10.29) | <0.001 |
| Catecholamine administration [yes/no] | 2.02 (1.09–3.73) | 0.026 |
| Bleeding on admission [yes/no] | 2.47 (0.87–7.00) | 0.089 |
| Type of AAS | 0.80 (0.58–1.09) | 0.159 |
| Points | n | % |
|---|---|---|
| 0 | 7 | 18.9 |
| 1 | 12 | 32.4 |
| 2 | 8 | 21.6 |
| 3 | 7 | 18.9 |
| 4 | 2 | 5.4 |
| 6 | 1 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipa, A.J.; Andreikovits, P.; Stoeckl, M.; Domanovits, H.; Schoergenhofer, C.; Schwameis, M.; Grafeneder, J. Fibrinogen-to-Albumin Ratio as Predictor of Mortality in Acute Aortic Syndromes. J. Clin. Med. 2025, 14, 1669. https://doi.org/10.3390/jcm14051669
Lipa AJ, Andreikovits P, Stoeckl M, Domanovits H, Schoergenhofer C, Schwameis M, Grafeneder J. Fibrinogen-to-Albumin Ratio as Predictor of Mortality in Acute Aortic Syndromes. Journal of Clinical Medicine. 2025; 14(5):1669. https://doi.org/10.3390/jcm14051669
Chicago/Turabian StyleLipa, Alexandra Julia, Patrick Andreikovits, Marco Stoeckl, Hans Domanovits, Christian Schoergenhofer, Michael Schwameis, and Juergen Grafeneder. 2025. "Fibrinogen-to-Albumin Ratio as Predictor of Mortality in Acute Aortic Syndromes" Journal of Clinical Medicine 14, no. 5: 1669. https://doi.org/10.3390/jcm14051669
APA StyleLipa, A. J., Andreikovits, P., Stoeckl, M., Domanovits, H., Schoergenhofer, C., Schwameis, M., & Grafeneder, J. (2025). Fibrinogen-to-Albumin Ratio as Predictor of Mortality in Acute Aortic Syndromes. Journal of Clinical Medicine, 14(5), 1669. https://doi.org/10.3390/jcm14051669






