Factors Influencing the Mortality of Patients with Subarachnoid Haemorrhage in the Intensive Care Unit: A Retrospective Cohort Study
Abstract
1. Background
2. Methods
2.1. Study Design
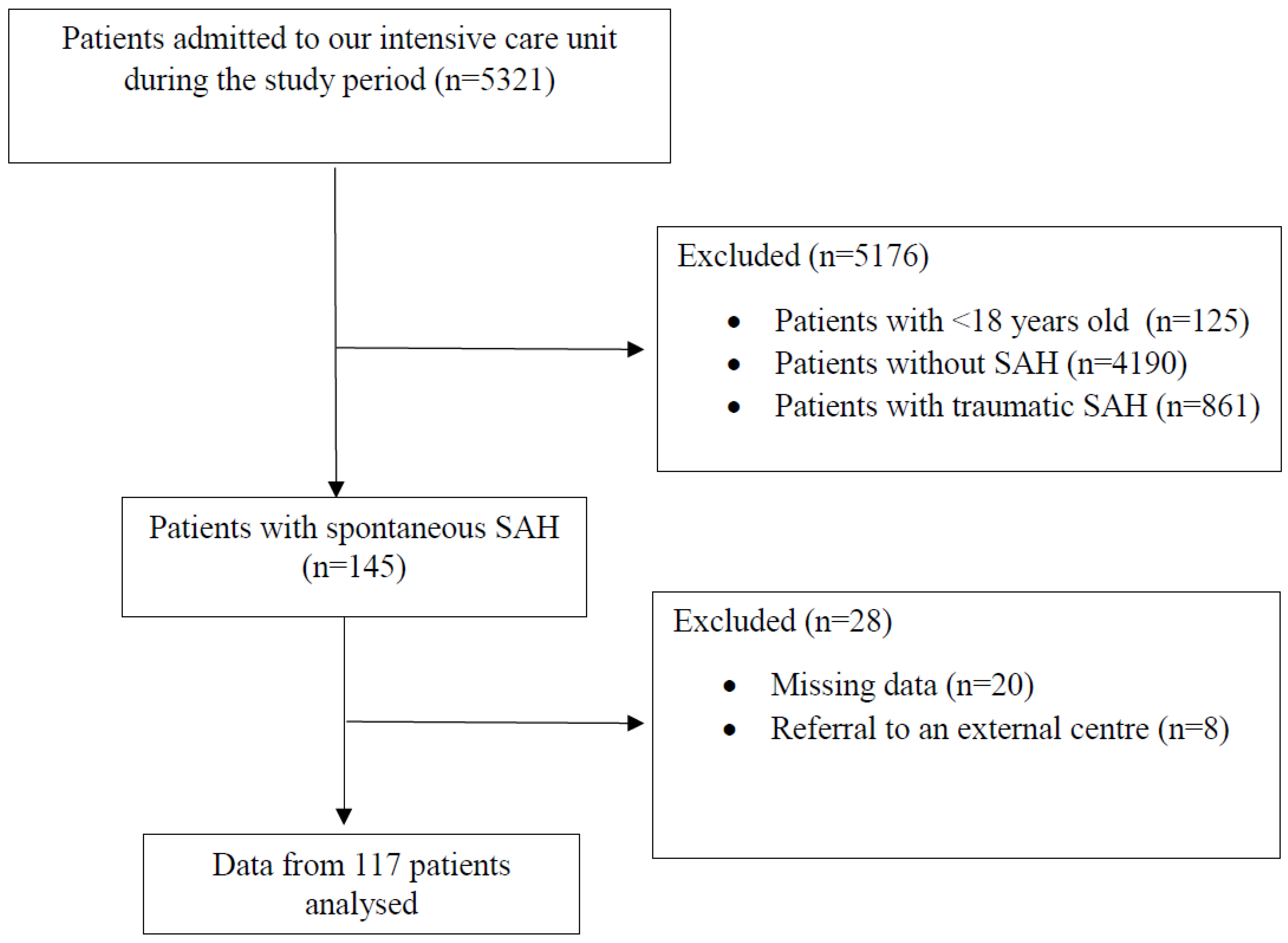
2.2. Participants
2.3. Management of Intensive Care Unit
2.4. Data Collection
2.5. Outcome
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claassen, J.; Park, S. Spontaneous subarachnoid haemorrhage. Lancet 2022, 400, 846–862. [Google Scholar] [CrossRef]
- Rincon, F.; Rossenwasser, R.H.; Dumont, A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery 2013, 73, 217–222; discussion 212–213. [Google Scholar] [CrossRef] [PubMed]
- Koshy, L.; Easwer, H.V.; Premkumar, S.; Alapatt, J.P.; Pillai, A.M.; Nair, S.; Bhattacharya, R.N.; Banerjee, M. Risk factors for aneurysmal subarachnoid hemorrhage in an Indian population. Cerebrovasc. Dis. 2010, 29, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics--2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Ranaie, G.; Erbguth, F.; Hohenhaus, M.; Wenzl, M.; Killer-Oberpfalzer, M.; Steiner, H.-H.; Janssen, H. Impact of Complications and Comorbidities on the Intensive Care Length of Stay after Aneurysmal Subarachnoid Haemorrhage. Sci. Rep. 2020, 10, 6228. [Google Scholar] [CrossRef] [PubMed]
- Koester, S.W.; Catapano, J.S.; Rhodenhiser, E.G.; Rudy, R.F.; Winkler, E.A.; Benner, D.; Cole, T.S.; Baranoski, J.F.; Srinivasan, V.M.; Graffeo, C.S.; et al. Propensity-adjusted analysis of ultra-early aneurysmal subarachnoid hemorrhage treatment and patient outcomes. Acta Neurochir. 2023, 165, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Akinci, A.T.; Akturk, Y.; Tutunculer, B.; Orakdöğen, M.; Şimşek, O. The effects of the early and ultra-early intervention on the outcome in aneurysmatic subarachnoid hemorrhage. Ulus. Travma Acil Cerrahi Derg. 2021, 27, 449–456. [Google Scholar] [CrossRef]
- Lu, J.; Wang, L.; Li, R.; Lin, F.; Chen, Y.; Yan, D.; Yang, J.; Li, R.; Li, Z.; Zhang, H.; et al. Timing of operation for poor-grade aneurysmal subarachnoid hemorrhage: Relationship with delayed cerebral ischemia and poor prognosis. CNS Neurosci. Ther. 2023, 29, 1120–1128. [Google Scholar] [CrossRef]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Chou, S.H.-Y.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hänggi, D.; Hetts, S.W.; et al. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370. [Google Scholar] [CrossRef] [PubMed]
- Picetti, E.; Barbanera, A.; Bernucci, C.; Bertuccio, A.; Bilotta, F.; Boccardi, E.P.; Cafiero, T.; Caricato, A.; Castioni, C.A.; Cenzato, M.; et al. Early management of patients with aneurysmal subarachnoid hemorrhage in a hospital with neurosurgical/neuroendovascular facilities: A consensus and clinical recommendations of the Italian Society of Anesthesia and Intensive Care (SIAARTI)-Part 1. J. Anesth. Analg. Crit. Care 2022, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Ramnarayan, R.; Anto, D.; Alapatt, J. Aneurysmal Subarachnoid Hemorrhage: Geography has a Role. Asian J. Neurosurg. 2018, 13, 669–673. [Google Scholar] [CrossRef]
- Schupper, A.J.; Hardigan, T.A.; Mehta, A.; Yim, B.; Yaeger, K.A.; De Leacy, R.; Fifi, J.T.; Mocco, J.; Majidi, S. Sex and Racial Disparity in Outcome of Aneurysmal Subarachnoid Hemorrhage in the United States: A 20-Year Analysis. Stroke 2023, 54, 1347–1356. [Google Scholar] [CrossRef]
- Kimberly, H.H.; Shah, S.; Marill, K.; Noble, V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad. Emerg. Med. 2008, 15, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Germans, M.; Burkhardt, J.-K.; Neidert, M.C.; Fung, C.; Bervini, D.; Zumofen, D.; Roethlisberger, M.; Marbacher, S.; Maduri, R.; et al. Predictors of In-Hospital Death After Aneurysmal Subarachnoid Hemorrhage: Analysis of a Nationwide Database (Swiss SOS [Swiss Study on Aneurysmal Subarachnoid Hemorrhage]). Stroke 2018, 49, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Samagh, N.; Bhagat, H.; Jangra, K. Monitoring cerebral vasospasm: How much can we rely on transcranial Doppler. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.A.; Gonzalez, L.F.; Suarez, J.I. Therapies for Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2023, 39, 36–50. [Google Scholar] [CrossRef]
- Hernandez Ortiz, O.H.; Garcia Garcia, H.I.; Munoz Ramirez, F.; Flórez, J.S.C.; Gil Valencia, B.A.; Mantilla, S.E.M.; Ochoa, M.J.M.; Ochoa, J.E.S.; Jaimes, F. Development of a prediction rule for diagnosing postoperative meningitis: A cross-sectional study. J. Neurosurg. 2018, 128, 262–271. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention NHSN. Pneumonia (Ventilator-Associated [VAP] and Nonventilator-Associated Pneumonia [PNEU]). 2024. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf (accessed on 15 September 2024).
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.; Jong-Tjien-Fa, A.V.; Algra, A.; Rinkel, G.J. Time trends in causes of death after aneurysmal subarachnoid hemorrhage: A hospital-based study. Neurology 2016, 86, 59–63. [Google Scholar] [CrossRef]
- Molyneux, A.J.; Kerr, R.S.; Birks, J.; Ramzi, N.; Yarnold, J.; Sneade, M.; Rischmiller, J.; ISAT Collaborators. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurol. 2009, 8, 427–433. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Rinkel, G.J.; Rothwell, P.M. Time trends in outcome of subarachnoid hemorrhage: Population-based study and systematic review. Neurology 2010, 74, 1494–1501. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Zotou, A.; Koutsileou, K.; Aretha, D.; Boulovana, M.; Vrettos, T.; Sklavou, C.; Marangos, M.; Fligou, F. Fatores de risco para mortalidade após hemorragia subaracnoidea: Estudo observacional retrospectivo. Braz. J. Anesthesiol. 2019, 69, 448–454. [Google Scholar] [CrossRef]
- Küçük Pehlivanlar, M.; Öztürk, Ç.E.; Küçük, A.O.; Turunç, E.; Ülger, F. Yoğun Bakımda Spontan Subaraknoid Kanamalı Hastaların Kısa Dönem Sonuçları: Tek Merkez Tecrübeleri. Türk Yoğun Bakım Derg. 2021, 19, 174–183. [Google Scholar] [CrossRef]
- Keris, V.; Buks, M.; Macane, I.; Kalnina, Z.; Vetra, A.; Jurjane, N.; Mikelsone, A. Aneurysmal subarachnoid hemorrhage in Baltic population: Experience from Latvia (1996–2000). Eur. J. Neurol. 2002, 9, 601–607. [Google Scholar] [CrossRef]
- Vemmos, K.N.; Bots, M.L.; Tsibouris, P.K.; Zis, V.P.; Takis, C.E.; Grobbee, D.E.; Stamatelopoulos, S. Prognosis of stroke in the south of Greece: 1 year mortality, functional outcome and its determinants: The Arcadia Stroke Registry. J. Neurol. Neurosurg. Psychiatry 2000, 69, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Waweru, P.; Gatimu, S.M. Mortality and functional outcomes after a spontaneous subarachnoid haemorrhage: A retrospective multicentre cross-sectional study in Kenya. PLoS ONE 2019, 14, e0217832. [Google Scholar] [CrossRef]
- Post, R.; Germans, M.R.; Boogaarts, H.D.; Xavier, B.F.D.; Berg, R.V.D.; Coert, B.A.; Vandertop, W.P.; Verbaan, D. Short-term tranexamic acid treatment reduces in-hospital mortality in aneurysmal sub-arachnoid hemorrhage: A multicenter comparison study. PLoS ONE 2019, 14, e0211868. [Google Scholar] [CrossRef]
- Hua, X.; Gray, A.; Wolstenholme, J.; Clarke, P.; Molyneux, A.J.; Kerr, R.S.C.; Clarke, A.; Sneade, M.; Rivero-Arias, O. Survival, Dependency, and Health-Related Quality of Life in Patients With Ruptured Intracranial Aneurysm: 10-Year Follow-up of the United Kingdom Cohort of the International Subarachnoid Aneurysm Trial. Neurosurgery 2021, 88, 252–260. [Google Scholar] [CrossRef]
- Pedraza, S.; Mendez-Mendez, J. The prognostic value of computerized tomography in acute aneurysmal subarachnoid haemorrhages. Rev. Neurol. 2004, 39, 359–363. [Google Scholar] [PubMed]
- Rosen, D.S.; Macdonald, R.L. Grading of subarachnoid hemorrhage: Modification of the world World Federation of Neurosurgical Societies scale on the basis of data for a large series of patients. Neurosurgery 2004, 54, 566–575; discussion 575–566. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-K.; Chun, H.-J.; Kim, D.-W.; Im, T.-H.; Hong, H.-J.; Yi, H.-J. Acute Physiology and Chronic Health Evaluation II and Simplified Acute Physiology Score II in predicting hospital mortality of neurosurgical intensive care unit patients. J. Korean Med. Sci. 2009, 24, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Bohnstedt, B.N.; Nguyen, H.S.; Kulwin, C.G.; Shoja, M.M.; Helbig, G.M.; Leipzig, T.J.; Payner, T.D.; Cohen-Gadol, A.A. Outcomes for clip ligation and hematoma evacuation associated with 102 patients with ruptured middle cerebral artery aneurysms. World Neurosurg. 2013, 80, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ojha, M.; Finnis, M.E.; Heckelmann, M.; Raith, E.P.; Moodie, S.; Chapman, M.J.; Reddi, B.; Maiden, M.J. Outcomes following grade V subarachnoid haemorrhage: A single-centre retrospective study. Anaesth. Intensive Care 2020, 48, 289–296. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Mai, T.D.; Vu, L.D.; Dao, C.X.; Ngo, H.M.; Hoang, H.B.; Tran, T.A.; Pham, T.Q.; Pham, D.T.; Nguyen, M.H.; et al. Validation of the accuracy of the modified World Federation of Neurosurgical Societies subarachnoid hemorrhage grading scale for predicting the outcomes of patients with aneurysmal subarachnoid hemorrhage. PLoS ONE 2023, 18, e0289267. [Google Scholar] [CrossRef] [PubMed]
- Hanna, L.M.D.A.; Biguelini, S.E.; de Araujo Junior, F.A.; Matsubara, A.; Neto, P.H.d.S. Prognosis of Patients Victim of Spontaneous Subarachnoid Hemorrhage: A Comparison between Radiologic Scales. Arq. Bras. De Neurocir. Braz. Neurosurg. 2020, 39, 001–004. [Google Scholar] [CrossRef]
- Lantigua, H.; Ortega-Gutierrez, S.; Schmidt, J.M.; Lee, K.; Badjatia, N.; Agarwal, S.; Claassen, J.; Connolly, E.S.; Mayer, S.A. Subarachnoid hemorrhage: Who dies, and why? Crit. Care 2015, 19, 309. [Google Scholar] [CrossRef]
- Locksley, H.B. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J. Neurosurg. 1966, 25, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Ö.; Diren, F.; Boyalı, O.; Baysoy, B.; Kabataş, S. Factors Associated with Clinical Outcomes in Spontaneous Subarachnoid Hemorrhage. JAREM J. Acad. Res. Med. 2022, 12, 124. [Google Scholar] [CrossRef]
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Kleinpeter, G.; Lehr, S. Is hypertension a major risk factor in aneurysmal subarachnoid hemorrhage? Wien. Klin. Wochenschr. 2002, 114, 307–314. [Google Scholar]
- Rosengart, A.J.; Schultheiss, K.E.; Tolentino, J.; Macdonald, R.L. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007, 38, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Gomis, P.; Rousseaux, P.; Jolly, D.; Graftieaux, J.P. Initial prognostic factors of aneurysmal subarachnoid hemorrhage. Neurochirurgie 1994, 40, 18–30. [Google Scholar] [PubMed]
- Duran, L.; Balci, K.; Kati, C.; Akdemir, H.U.; Kocabicak, E.; Doğruel, C. Has admission blood pressure any prognostic value in patients with subarachnoid hemorrhage: An emergency department experience. J. Clin. Hypertens. 2013, 15, 737–741. [Google Scholar] [CrossRef]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, N.K.; Linn, F.H.H.; van der Plas, J.A.; Algra, A.; Rinkel, G.J.E. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef]
- Harrison, C.H.; Taquet, M.; Harrison, P.J.; Watkinson, P.J.; Rowland, M.J. Sex and age effects on risk of non-traumatic subarachnoid hemorrhage: Retrospective cohort study of 124,234 cases using electronic health records. J. Stroke Cerebrovasc. Dis. 2023, 32, 107196. [Google Scholar] [CrossRef]
- Orakdogen, M.; Emon, S.T.; Somay, H.; Engin, T.; Ates, O. Prognostic Factors in Patients who Underwent Aneurysmal Clipping due to Spontaneous Subarachnoid Hemorrhage. Turk. Neurosurg. 2016, 26, 840–848. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chotai, S.; Ahn, S.-Y.; Moon, H.-J.; Kim, J.-H.; Chung, H.-S.; Chung, Y.-G.; Kwon, T.-H. Prediction of outcomes in young adults with aneurysmal subarachnoid hemorrhage. Neurol. Med. Chir. 2013, 53, 157–162. [Google Scholar] [CrossRef]
- Lindekleiv, H.; Sandvei, M.S.; Njølstad, I.; Løchen, M.-L.; Romundstad, P.; Vatten, L.; Ingebrigtsen, T.; Vik, A.; Mathiesen, E. Sex differences in risk factors for aneurysmal subarachnoid hemorrhage: A cohort study. Neurology 2011, 76, 637–643. [Google Scholar] [CrossRef]
- Koffijberg, H.; Buskens, E.; Granath, F.; Adami, J.; Ekbom, A.; Rinkel, G.J.E.; Blomqvist, P. Subarachnoid haemorrhage in Sweden 1987–2002: Regional incidence and case fatality rates. J. Neurol. Neurosurg. Psychiatry 2008, 79, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Fernandez, A.; Schmidt, J.M.; Claassen, J.; Wartenberg, K.E.; Badjatia, N.; Parra, A.; Connolly, E.S.; Mayer, S.A. Impact of nosocomial infectious complications after subarachnoid hemorrhage. Neurosurgery 2008, 62, 80–87; discussion 87. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cui, A.; Yu, K.; Huang, C.; Zhu, M.; Chen, M. Risk Factors Associated with Meningitis after Neurosurgery: A Retrospective Cohort Study in a Chinese Hospital. World Neurosurg. 2018, 111, e546–e563. [Google Scholar] [CrossRef] [PubMed]
- Flinspach, A.N.; Konczalla, J.; Seifert, V.; Zacharowski, K.; Herrmann, E.; Balaban, Ü.; Adam, E.H. Detecting Sepsis in Patients with Severe Subarachnoid Hemorrhage during Critical Care. J. Clin. Med. 2022, 11, 4229. [Google Scholar] [CrossRef] [PubMed]
| Overall | Survival (n = 69, 59%) | Mortality (n = 48, 41%) | p | ||
|---|---|---|---|---|---|
| Age, years | 54.9 ± 15.1 | 52.8 ± 1.4 | 57.9 ± 15.8 | 0.072 | |
| Age group | Age <50 years | 49 (41.9%) | 32 (46.4%) | 17 (35.4%) | 0.237 |
| Age ≥50 years | 68 (58.1%) | 37 (53.6%) | 31 (64.6%) | ||
| Gender | Male | 65 (55.6%) | 34 (49.3%) | 31 (64.6%) | 0.101 |
| Female | 52 (44.4%) | 35 (50.7%) | 17 (35.4%) | ||
| Habits | Smoking | 28 (23.9%) | 20 (29%) | 8 (16.7%) | 0.125 |
| Alcohol abuse | 6 (5.1%) | 2 (2.9%) | 4 (8.3%) | 0.190 | |
| Substance use | 4 (3.4%) | 3 (4.3%) | 1 (2.1%) | 0.507 | |
| Comorbidities | CCI | 1 (0–9) | 1 (0–7) | 2 (0–9) | 0.023 |
| Hypertension | 75 (64.1%) | 41 (59.4%) | 34 (70.8%) | 0.206 | |
| Diabetes mellitus | 22 (18.8%) | 13 (18.8%) | 9 (18.8%) | 0.990 | |
| Systemic rheumatological disease | 2 (1.7%) | 1 (1.4%) | 1 (2.1%) | 0.795 | |
| Passed SAH | 9 (7.7%) | 5 (7.2%) | 4 (8.3%) | 0.828 | |
| Autosomal dominant PKD | 6 (5.1%) | 4 (5.8%) | 2 (4.2%) | 0.694 | |
| Clinical and vital signs | Headache | 89 (76.1%) | 61 (88.4%) | 28 (58.2%) | <0.001 |
| Neck stiffness | 10 (8.5%) | 4 (5.8%) | 6 (12.5%) | 0.202 | |
| Neck pain | 4 (3.4%) | 1 (1.4%) | 3 (6.3%) | 0.160 | |
| Loss of consciousness | 38 (32.5%) | 10 (14.5%) | 28 (58.3%) | <0.001 | |
| Nausea and vomiting | 28 (23.9%) | 17 (24.6%) | 11 (22.9%) | 0.830 | |
| Seizure | 14 (12%) | 8 (11.6%) | 6 (12.5%) | 0.882 | |
| Focal neurological deficit | 7 (6%) | 4 (5.8%) | 3 (6.3%) | 0.919 | |
| SBP on admissionmmHg | 140 (130–165) | 140 (130–160) | 140 (130–170) | 0.824 | |
| ICU admission time, hours | 7 (4–11) | 8 (4–14) | 7 (3.5–9.5) | 0.108 |
| Overall (n = 117) | Survival (n = 69, %59) | Mortality (n = 48, %41) | p | ||
|---|---|---|---|---|---|
| Angiography | Positive | 72 (61.5%) | 48 (69.5%) | 24 (50%) | 0.032 |
| Negative | 45 (38.4%) | 21 (30.4%) | 24 (50%) | ||
| Aetiology | Aneurysm | 65 (55.6%) | 42 (60.8%) | 23 (47.9%) | 0.110 |
| AVM | 7 (6%) | 6 (8.7%) | 1 (2.1%) | ||
| Other | 45 (38.5%) | 21 (30.4%) | 24 (50%) | ||
| Modified Fisher Scale | Stage 1 | 28 (23.9%) | 24 (34.8%) | 4 (8.3%) b | <0.001 |
| Stage 2 | 19 (16.2%) | 15 (21.7%) | 4 (8.3%) b | ||
| Stage 3 | 13 (11.1%) | 10 (14.5%) a | 3 (6.3%) b | ||
| Stage 4 | 57 (48.4%) | 20 (29%) a | 37 (77.1%) b | ||
| Aneurysm side | Right | 32 (27.4%) | 21 (30.4%) | 11 (22.9%) | 0.280 |
| Left | 24 (20.5%) | 17 (24.6%) | 7 (14.6%) | ||
| Both sides | 9 (7.7%) | 4 (5.8%) | 5 (10.4%) | ||
| Aneurysm location | Saptanmadı | 52 (44.4%) | 27 (39.1%) | 25 (52.1%) | 0.353 |
| ICA | 10 (8.5%) | 7 (10.1%) | 3 (6.3%) | ||
| ACA | 29 (24.8%) | 19 (27.5%) | 10 (20.8%) | ||
| MCA | 16 (13.7%) | 12 (17.4%) | 4 (8.3%) | ||
| BA | 6 (5.1%) | 2 (2.9%) | 4 (8.3%) | ||
| PCoA | 4 (3.4%) | 2 (2.9%) | 2 (4.2%) | ||
| Aneurysm size | Not detected | 52 (44.4%) | 27 (39.1%) | 25 (52.1%) | 0.434 |
| <5 mm | 25 (21.4%) | 18 (26.1%) | 7 (14.6%) | ||
| 5–9,9 mm | 26 (22.2%) | 17 (24.6%) | 9 (18.8%) | ||
| 10–24,9 mm | 11 (9.4%) | 6 (8.7%) | 5 (10.4%) | ||
| ≥25 mm | 3 (2.6%) | 1 (1.4%) | 2 (4.2%) |
| Scores | Overall (n = 117) | Survival (n = 69, %59) | Mortality (n = 48, %41) | p | |
|---|---|---|---|---|---|
| GCS | 11 (6–14) | 14 (12–15) | 5 (8–3) | <0.001 | |
| GCS (group) | 3–8 | 47 (40.2%) | 10 (14.5%) | 37 (77.1%) | <0.001 |
| 9–12 | 18 (15.4%) | 12 (17.4%) | 6 (12.5%) | ||
| 13–15 | 52 (44.4%) | 47 (68.1%) | 5 (10.4%) | ||
| APACHE II | 14 (2–35) | 11 (2–29) | 22 (6–35) | <0.001 | |
| WFNS | 3 (2–5) | 2 (1–4) | 5 (4–5) | <0.001 | |
| WFNS (distribution) | Stage 1 | 23 (19.7%) | 22 (31.9%) a | 1 (2.1%) b | <0.001 |
| Stage 2 | 21 (17.9%) | 14 (20.3%) | 7 (14.6%) | ||
| Stage 3 | 15 (12.8%) | 12 (17.4%) | 3 (6.3%) | ||
| Stage 4 | 22 (18.8%) | 15 (21.7%) | 7 (14.6%) | ||
| Stage 5 | 36 (30.8%) | 6 (8.7%) a | 30 (62.5%) b | ||
| H&S | 3 (2–5) | 2 (1–3) | 5 (4–5) | <0.001 | |
| H&S (distribution) | Stage 1 | 17 (14.5%) | 15 (21.7%) a | 2 (4.2%) b | <0.001 |
| Stage 2 | 27 (23.1%) | 25 (36.2%) a | 2 (4.2%) b | ||
| Stage 3 | 18 (15.4%) | 14 (20.3%) | 4 (8.3%) | ||
| Stage 4 | 10 (8.5%) | 4 (5.8%) | 6 (12.5%) | ||
| Stage 5 | 45 (38.4%) | 11 (15.9%) a | 34 (70.9%) b |
| Overall (n = 117) | Survival (n = 69, %59) | Mortality (n = 48, %41) | p | ||
|---|---|---|---|---|---|
| Treatment | Endovascular coil | 6 (5.1%) | 4 (5.8%) | 2 (4.2%) | 0.353 |
| Neurosurgery | 79 (67.5%) | 43 (62.3%) | 36 (75%) | ||
| Conservative | 32 (27.4%) | 22 (31.9%) | 10 (20.8%) | ||
| Complications | Vasospasm | 45 (38.5%) | 22 (31.9%) | 23 (47.9%) | 0.080 |
| Rebleeding | 19 (16.2%) | 8 (11.6%) | 11 (22.9%) | 0.102 | |
| Hydrocephalus | 38 (32.5%) | 14 (20.3%) | 24 (50%) | <0.001 | |
| DCI | 36 (30.8%) | 15 (21.7%) | 21 (43.8%) | 0.011 | |
| Hyponatraemia | 42 (35.9%) | 22 (31.9%) | 20 (41.7%) | 0.278 | |
| Hypernatraemia | 30 (25.6%) | 11 (15.9%) | 19 (39.6%) | 0.004 | |
| Seizure | 12 (10.3%) | 8 (11.6%) | 4 (8.3%) | 0.567 | |
| Pulmonary oedema | 31 (26.5%) | 13 (18.8%) | 18 (37.5%) | 0.024 | |
| Meningitis | 9 (7.7%) | 1 (1.4%) | 8 (16.7%) | 0.002 | |
| VAP | 45 (38.5%) | 14 (20.3%) | 31 (64.6%) | <0.001 | |
| Sepsis/Septic shock | 47 (40.2%) | 15 (21.7%) | 32 (66.7%) | <0.001 | |
| Microbiological culture (+) | 59 (50.4%) | 26 (68.8%) | 33 (37.7%) | <0.001 | |
| Additional treatments | Vasopressor (+) | 50 (42.7%) | 11 (15.9%) | 39 (81.3%) | <0.001 |
| Controlled hypertension (+) | 12 (10.3%) | 5 (7.2%) | 7 (14.6%) | 0.318 | |
| Drainage system | EVD | 62 (53%) | 43 (62.3%) a | 19 (39.6%) b | 0.002 |
| LD | 47 (40.2%) | 19 (27.5%) a | 28 (58.3%) b | ||
| Yok | 8 (6.8%) | 7 (10.1%) | 1 (2.1%) | ||
| Decompressive craniectomy | 32 (27.4%) | 10 (14.5%) | 22 (45.8%) | <0.001 | |
| Duration of mechanical ventilation, day | 14.1 ± 22 | 7.4 ± 15.7 | 23.6 ± 26 | <0.001 | |
| Length of stay in the ICU, day | 17.8 ± 22.3 | 12.5 ± 16.6 | 25.3 ± 27 | 0.002 |
| Variables | Univariate | ||
|---|---|---|---|
| OR | 95% CI | p | |
| Age | 1.02 | 0.998–1.049 | 0.074 |
| Gender | 1.31 | 0.41–4.17 | 0.640 |
| CCI | 1.52 | 1.04–2.23 | 0.029 |
| GCS (<8) | 0.10 | 0.02–0.54 | 0.007 |
| Loss of consciousness | 5.97 | 1.76–20.20 | 0.004 |
| Modified Fisher Scale | 0.49 | 0.09–2.59 | 0.404 |
| H&S (>2) | 5.32 | 1.07–26.43 | 0.040 |
| WFNS | 0.46 | 0.07–2.74 | 0.400 |
| DCI | 2.10 | 0.58–7.64 | 0.256 |
| Hydrocephalus | 1.73 | 0.50–6.00 | 0.382 |
| Vasospasm | 1.965 | 0.920–4.201 | 0.081 |
| Meningitis | 13.6 | 1.640–112.764 | 0.016 |
| Sepsis/Septic shock | 5.24 | 1.36–20.19 | 0.015 |
| Rebleeding | 2.39 | 0.52–10.9 | 0.258 |
| Decompressive craniectomy | 0.83 | 0.20–3.31 | 0.793 |
| Variables | Multivariate Model † | p | ||
|---|---|---|---|---|
| Nagelkerke R2 = 0.637 | ||||
| OR | 95% CI | |||
| Lower Level | Upper Level | |||
| CCI | 1.294 | 1.173 | 1.427 | <0.001 |
| GCS (<8) | ||||
| 3–8 | 7.289 | 3.590 | 14.802 | <0.001 |
| 9–12 | 1.853 | 0.947 | 3.626 | 0.072 |
| 13–15 (ref) | ||||
| Loss of consciousness | ||||
| Yes | 4.438 | 2.718 | 7.246 | <0.001 |
| No (ref) | ||||
| H&S (>2) | ||||
| Score >2 | 4.324 | 2.227 | 8.395 | <0.001 |
| Score ≤2 (ref) | ||||
| Meningitis | ||||
| Yes | 27.452 | 7.297 | 103.279 | <0.001 |
| No (ref) | ||||
| Sepsis/Septic shock | ||||
| Yes | 3.442 | 2.224 | 5.327 | <0.001 |
| No (ref) | ||||
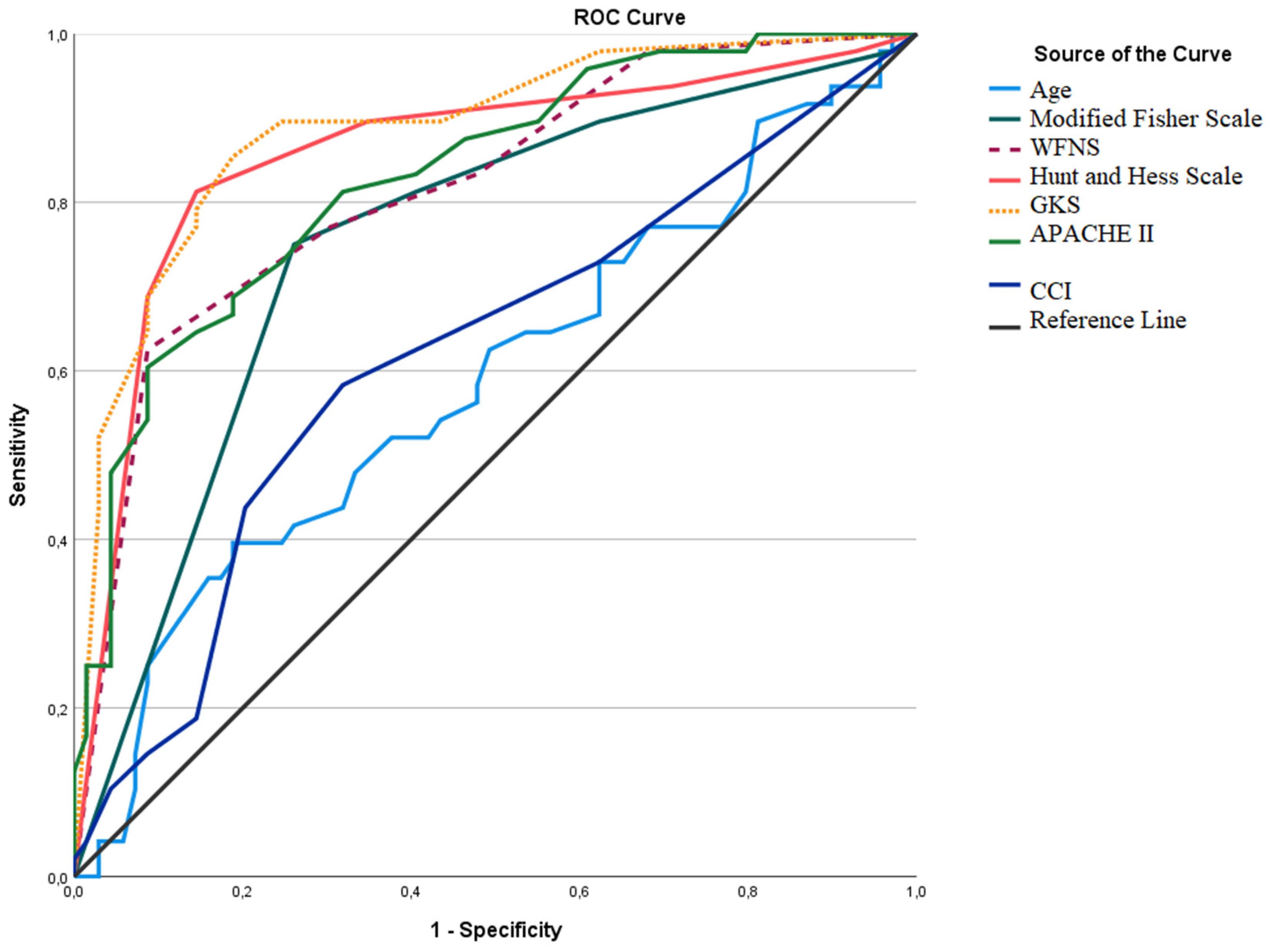
| AUC (%95 CI) | Cut-Off | p | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Age | 0.589 (0.482–0.696) | 63.5 | 0.104 | 39.6% | 81.2% |
| Modified Fisher Scale | 0.752 (0.660–0.843) | ≥3.5 | <0.001 | 75.0% | 73.9% |
| WFNS | 0.818 (0.739–0.896) | ≥4.5 | <0.001 | 62.5% | 91.3% |
| H&S | 0.859 (0.783–0.934) | ≥3.5 | <0.001 | 81.3% | 85.5% |
| GCS | 0.887 (0.824–0.950) | ≤10.5 | <0.001 | 85.4% | 81.2% |
| APACHE II | 0.831 (0.757–0.906) | ≥18.5 | <0.001 | 60.4% | 91.3% |
| CCI | 0.620 (0.515–0.726) | ≥1.5 | 0.027 | 58.3% | 68.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetinkaya, O.; Arslan, U.; Temel, H.; Kavakli, A.S.; Cakin, H.; Cengiz, M.; Yilmaz, M.; Barcin, N.E.; Ikiz, F. Factors Influencing the Mortality of Patients with Subarachnoid Haemorrhage in the Intensive Care Unit: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 1650. https://doi.org/10.3390/jcm14051650
Cetinkaya O, Arslan U, Temel H, Kavakli AS, Cakin H, Cengiz M, Yilmaz M, Barcin NE, Ikiz F. Factors Influencing the Mortality of Patients with Subarachnoid Haemorrhage in the Intensive Care Unit: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(5):1650. https://doi.org/10.3390/jcm14051650
Chicago/Turabian StyleCetinkaya, Onur, Ulku Arslan, Hakan Temel, Ali Sait Kavakli, Hakan Cakin, Melike Cengiz, Murat Yilmaz, Nur Ebru Barcin, and Fatih Ikiz. 2025. "Factors Influencing the Mortality of Patients with Subarachnoid Haemorrhage in the Intensive Care Unit: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 5: 1650. https://doi.org/10.3390/jcm14051650
APA StyleCetinkaya, O., Arslan, U., Temel, H., Kavakli, A. S., Cakin, H., Cengiz, M., Yilmaz, M., Barcin, N. E., & Ikiz, F. (2025). Factors Influencing the Mortality of Patients with Subarachnoid Haemorrhage in the Intensive Care Unit: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(5), 1650. https://doi.org/10.3390/jcm14051650






