Denosumab in the Management of Glucocorticoid-Induced Osteoporosis: Long-Term Efficacy and Secondary Fracture Outcomes
Abstract
1. Introduction
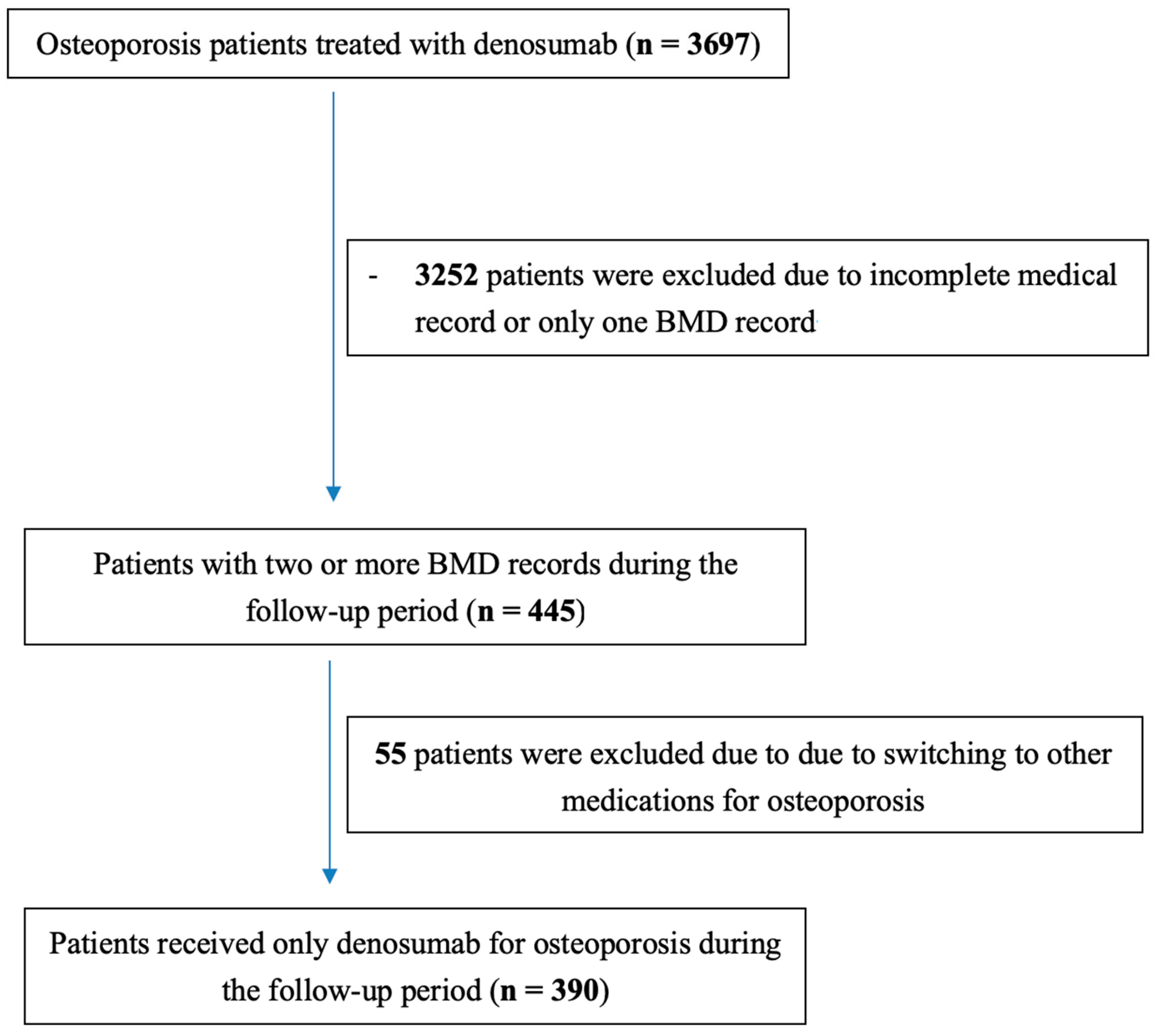
2. Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kok, C.; Sambrook, P.N. Secondary osteoporosis in patients with an osteoporotic fracture. Best Pract. Res. Clin. Rheumatol. 2009, 23, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.P.; Leufkens, H.G.M.; Cooper, C. The epidemiology of corticosteroid-induced osteoporosis: A meta-analysis. Osteoporos. Int. 2002, 13, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallach, S.; Cohen, S.; Reid, D.M.; Hughes, R.A.; Hosking, D.J.; Laan, R.F.; Doherty, S.M.; Maricic, M.; Rosen, C.; Brown, J.; et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif. Tissue Int. 2000, 67, 277–285. [Google Scholar] [CrossRef]
- Reid, D.M.; Devogelaer, J.P.; Saag, K.; Roux, C.; Lau, C.S.; Reginster, J.Y.; Papanastasiou, P.; Ferreira, A.; Hartl, F.; Fashola, T.; et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2009, 373, 1253–1263. [Google Scholar] [CrossRef]
- Thomas, T.; Horlait, S.; Ringe, J.D.; Abelson, A.; Gold, D.T.; Atlan, P.; Lange, J.L. Oral bisphosphonates reduce the risk of clinical fractures in glucocorticoid-induced osteoporosis in clinical practice. Osteoporos. Int. 2013, 24, 263–269. [Google Scholar] [CrossRef]
- Oxlund, H.; Ortoft, G.; Thomsen, J.S.; Danielsen, C.C.; Ejersted, C.; Andreassen, T.T. The anabolic effect of PTH on bone is attenuated by simultaneous glucocorticoid treatment. Bone 2006, 39, 244–252. [Google Scholar] [CrossRef]
- Jilka, R.L.; Weinstein, R.S.; Bellido, T.; Roberson, P.; Parfitt, A.M.; Manolagas, S.C. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Investig. 1999, 104, 439–446. [Google Scholar] [CrossRef]
- Saag, K.G.; Zanchetta, J.R.; Devogelaer, J.P.; Adler, R.A.; Eastell, R.; See, K.; Krege, J.H.; Krohn, K.; Warner, M.R. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: Thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009, 60, 3346–3355. [Google Scholar] [CrossRef]
- Overman, R.A.; Gourlay, M.L.; Deal, C.L.; Farley, J.F.; Brookhart, M.A.; Layton, J.B. Fracture rate associated with quality metric-based anti-osteoporosis treatment in glucocorticoid-induced osteoporosis. Osteoporos. Int. 2015, 26, 1515–1524. [Google Scholar] [CrossRef]
- Yanbeiy, Z.A.; Hansen, K.E. Denosumab in the treatment of glucocorticoid-induced osteoporosis: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2019, 13, 2843–2852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kendler, D.L.; Roux, C.; Benhamou, C.L.; Brown, J.P.; Lillestol, M.; Siddhanti, S.; Man, H.S.; Martin, J.S.; Bone, H.G. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J. Bone Miner. Res. 2010, 25, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hans, D.; McDermott, M.; Huang, S.; Kim, M.; Shevroja, E.; McClung, M. Long-term effect of denosumab on bone microarchitecture as assessed by tissue thickness-adjusted trabecular bone score in postmenopausal women with osteoporosis: Results from FREEDOM and its open-label extension. Osteoporos. Int. 2023, 34, 1075–1084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiba, K.; Iwamoto, N.; Watanabe, K.; Shiraishi, K.; Saito, K.; Okubo, N.; Kawakami, A.; Osaki, M. Denosumab improves bone mineral density microarchitecture in rheumatoid arthritis: Randomized controlled trial byHR-pQCT. J. Bone Miner. Metab. 2023, 41, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Papapoulos, S.; Chapurlat, R.; Libanati, C.; Brandi, M.L.; Brown, J.P.; Czerwiński, E.; Krieg, M.-A.; Man, Z.; Mellström, D.; Radominski, S.C.; et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: Results from the first two years of the FREEDOM extension. J. Bone Miner. Res. 2012, 27, 694–701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simon, J.A.; Recknor, C.; Moffett, A.H., Jr.; Adachi, J.D.; Franek, E.; Lewiecki, E.M.; McClung, M.R.; Mautalen, C.A.; Ragi-Eis, S.; Nicholson, G.C.; et al. Impact of denosumab on the peripheral skeleton of postmenopausal women with osteoporosis: Bone density, mass, and strength of the radius, and wrist fracture. Menopause 2013, 20, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Hofbauer, L.C.; Ho, P.R.; Wark, J.D.; Zillikens, M.C.; Fahrleitner-Pammer, A.; Hawkins, F.; Micaelo, M.; Minisola, S.; Papaioannou, N.; et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: Efficacy and safety results from a randomized open-label study. Bone 2014, 58, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Suresh, E.; Abrahamsen, B. Denosumab: A novel antiresorptive drug for osteoporosis. Cleve Clin. J. Med. 2015, 82, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chavassieux, P.; Portero-Muzy, N.; Roux, J.P.; Horlait, S.; Dempster, D.W.; Wang, A.; Wagman, R.B.; Chapurlat, R. Reduction of cortical bone turnover and erosion depth after 2 and 3 years of denosumab: Iliac bone histomorphometry in the FREEDOM Trial. J. Bone Miner. Res. 2019, 34, 626–631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bilezikian, J.P.; Lin, C.J.F.; Brown, J.P.; Wang, A.T.; Yin, X.; Ebeling, P.R.; Fahrleitner-Pammer, A.; Franek, E.; Gilchrist, N.; Miller, P.D.; et al. Long-term denosumab treatment restores cortical bone loss and reduces fracture risk at the forearm and humerus: Analyses from the FREEDOM Extension cross-over group. Osteoporos. Int. 2019, 30, 1855–1864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobza, A.O.; Herman, D.; Papaioannou, A.; Lau, A.N.; Adachi, J.D. Understanding and managing corticosteroid-induced osteoporosis. Open Access Rheumatol. 2021, 13, 177–190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Heckmann, B.L.; Yang, X.; Long, H. Osteoblast autophagy in glucocorticoid-induced osteoporosis. J. Cell Physiol. 2019, 234, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, M.; Ono, N.; Bringhurst, F.R.; Kronenberg, H.M.; Guo, J. Loss of wnt/β-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J. Bone Miner. Res. 2012, 27, 2344–2358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weinstein, R.S. Glucocorticoids, osteocytes, and skeletal fragility: The role of bone vascularity. Bone 2010, 46, 564–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wijenayaka, A.R.; Kogawa, M.; Lim, H.P.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE 2011, 6, e25900. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jia, D.; O’Brien, C.A.; Stewart, S.A.; Manolagas, S.C.; Weinstein, R.S. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology 2006, 147, 5592–5599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.J. New understanding of glucocorticoid action in bone cells. BMB Rep. 2010, 43, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Erratum to: Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2015, 26, 2045–2047. [Google Scholar] [CrossRef]
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017, 69, 1521–1537. [Google Scholar] [CrossRef]
- Mok, C.C.; Ho, L.Y.; Ma, K.M. Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: A 12-month randomized controlled trial. Bone 2015, 75, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woitge, H.W.; Seibel, M.J. Markers of Bone and Cartilage Turnover. Exp. Clin. Endocrinol. Diabetes 2017, 125, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Iseri, K.; Iyoda, M.; Watanabe, M.; Matsumoto, K.; Sanada, D.; Inoue, T.; Tachibana, S.; Shibata, T. The effects of denosumab and alendronate on glucocorticoid-induced osteoporosis in patients with glomerular disease: A randomized, controlled trial. PLoS ONE 2018, 13, e0193846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saag, K.G.; Wagman, R.B.; Geusens, P.; Adachi, J.D.; Messina, O.D.; Emkey, R.; Chapurlat, R.; Wang, A.; Pannacciulli, N.; Lems, W.F.; et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: A multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol. 2018, 6, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Q.; Yan, G.; Jin, X. Denosumab compared to bisphosphonates to treat postmenopausal osteoporosis: A meta-analysis. J. Orthop. Surg. Res. 2018, 13, 194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, L.; Dong, J.; Wei, J.; Liu, L. Comparison of denosumab and oral bisphosphonates for the treatment of glucocorticoid-induced osteoporosis: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2022, 23, 1027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, A.D.; Saag, K.G. Anabolics in the management of glucocorticoid-induced osteoporosis: An evidence-based review of long-term safety, efficacy and place in therapy. Core Evid. 2019, 14, 41–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiodini, I.; Merlotti, D.; Falchetti, A.; Gennari, L. Treatment options for glucocorticoid-induced osteoporosis. Expert. Opin. Pharmacother. 2020, 21, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Popp, A.W.; Zysset, P.K.; Lippuner, K. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos. Int. 2016, 27, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Sung, Y.K. Update on glucocorticoid induced osteoporosis. Endocrinol. Metab. 2021, 36, 536–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beaudoin, C.; Jean, S.; Bessette, L.; Ste-Marie, L.G.; Moore, L.; Brown, J.P. Denosumab compared to other treatments to prevent or treat osteoporosis in individuals at risk of fracture: A systematic review and meta-analysis. Osteoporos. Int. 2016, 27, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.K.; Chuang, A.T.M.; Liao, T.C.; Shao, S.C.; Liu, P.P.S.; Tu, Y.K.; Lai, E.C.C. Denosumab and the risk of diabetes in patients treated for osteoporosis. JAMA Netw. Open 2024, 7, e2354734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Himič, V.; Syrmos, N.; Ligarotti, G.K.I.; Kato, S.; Fehlings, M.G.; Ganau, M. The role of genetic and epigenetic factors in determining the risk of spinal fragility fractures: New insights in the management of spinal osteoporosis. Quant. Imaging Med. Surg. 2023, 13, 7632–7645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Without Glucocorticoid (n = 268) | With Glucocorticoid (n = 122) | p Value | |||
|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | ||
| Age | 70.5 | ±10.3 | 67.2 | ±10.6 | 0.006 ** |
| Sex, n (%) | 0.329 | ||||
| Female | 226 | (84.3%) | 98 | (80.3%) | |
| Male | 42 | (15.7%) | 24 | (19.7%) | |
| Albumin | 4.1 | ±0.5 | 4.0 | ±0.5 | 0.044 |
| ALP | 110.3 | ±72.0 | 124.6 | ±140.7 | 0.731 |
| Serum Calcium | 9.0 | ±0.6 | 9.0 | ±0.6 | 0.920 |
| Serum Creatinine | 1.2 | ±1.7 | 1.5 | ±2.3 | 0.003 ** |
| Serum iPTH | 201.1 | ±578.0 | 340.0 | ±735.9 | 0.131 |
| Serum Phosphate | 3.8 | ±0.8 | 4.0 | ±1.2 | 0.580 |
| Baseline BMD and TBS | |||||
| BMD, L spine | 0.85 | ±0.15 | 0.88 | ±0.15 | 0.009 ** |
| BMD, left femoral neck | 0.67 | ±0.11 | 0.68 | ±0.10 | 0.229 |
| BMD, right femoral neck | 0.67 | ±0.09 | 0.66 | ±0.10 | 0.831 |
| T-score, L spine | −2.36 | ±1.25 | −2.02 | ±1.17 | 0.003 ** |
| T-score, left femoral neck | −2.38 | ±0.80 | −2.30 | ±0.81 | 0.254 |
| T-score, right femoral neck | −2.37 | ±0.84 | −2.43 | ±0.81 | 0.650 |
| TBS BMD | 1.24 | ±0.10 | 1.26 | ±0.10 | 0.489 |
| TBS T-score | −2.34 | ±1.17 | −2.23 | ±1.10 | 0.832 |
| Without Glucocorticoid (n = 107) | With Glucocorticoid (n = 107) | p Value | |||
|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | ||
| Age | 68.6 | ±8.3 | 68.6 | ±8.3 | 0.997 |
| Sex, n (%) | 0.579 | ||||
| Female | 91 | (85.0%) | 88 | (82.2%) | |
| Male | 16 | (15.0%) | 19 | (17.8%) | |
| Albumin | 4.1 | ±0.4 | 4.0 | ±0.5 | 0.207 |
| ALP | 112.0 | ±71.1 | 109.9 | ±87.9 | 0.559 |
| Serum Calcium | 9.1 | ±0.6 | 9.0 | ±0.6 | 0.449 |
| Serum Creatinine | 1.3 | ±2.1 | 1.4 | ±2.2 | 0.039 * |
| Serum iPTH | 181.6 | ±361.6 | 296.3 | ±694.9 | 0.314 |
| Serum Phosphate | 3.9 | ±0.6 | 4.0 | ±1.2 | 0.429 |
| Baseline BMD and TBS | |||||
| BMD, L spine | 0.83 | ±0.15 | 0.87 | ±0.15 | 0.015 * |
| BMD, left femoral neck | 0.66 | ±0.11 | 0.67 | ±0.10 | 0.610 |
| BMD, right femoral neck | 0.67 | ±0.09 | 0.65 | ±0.10 | 0.093 |
| T-score, L spine | −2.52 | ±1.25 | −2.05 | ±1.20 | 0.005 * |
| T-score, left femoral neck | −2.34 | ±0.74 | −2.34 | ±0.79 | 0.662 |
| T-score, right femoral neck | −2.33 | ±0.76 | −2.48 | ±0.80 | 0.117 |
| TBS BMD | 1.22 | ±0.10 | 1.25 | ±0.10 | 0.366 |
| TBS T-score | −2.47 | ±1.09 | −2.34 | ±1.05 | 0.609 |
| Without Glucocorticoid (n = 107) | With Glucocorticoid (n = 107) | p Value | |||
|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | ||
| Follow-up BMD and TBS | |||||
| BMD, L spine | 0.86 | ±0.14 | 0.91 | ±0.16 | 0.048 * |
| BMD, left femoral neck | 0.69 | ±0.09 | 0.68 | ±0.10 | 0.903 |
| BMD, right femoral neck | 0.68 | ±0.09 | 0.66 | ±0.09 | 0.378 |
| T-score, L spine | −2.35 | ±1.18 | −1.94 | ±1.33 | 0.055 |
| T-score, left femoral neck | −2.31 | ±0.70 | −2.34 | ±0.81 | 0.878 |
| T-score, right femoral neck | −2.38 | ±0.73 | −2.47 | ±0.74 | 0.403 |
| TBS BMD | 1.24 | ±0.09 | 1.25 | ±0.10 | 0.601 |
| TBS T-score | −2.44 | ±1.05 | −2.19 | ±1.46 | 0.584 |
| Without Glucocorticoid | With Glucocorticoid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p Value | Before | After | p Value | |||||
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | |||
| BMD | ||||||||||
| L spine | 0.82 | ±0.14 | 0.86 | ±0.14 | <0.001 ** | 0.87 | ±0.14 | 0.90 | ±0.15 | <0.001 ** |
| Left femoral neck | 0.67 | ±0.09 | 0.69 | ±0.09 | <0.001 ** | 0.68 | ±0.09 | 0.68 | ±0.10 | 0.972 |
| Right femoral neck | 0.67 | ±0.09 | 0.68 | ±0.09 | 0.161 | 0.66 | ±0.10 | 0.67 | ±0.09 | 0.010 * |
| T-score | ||||||||||
| L spine | −2.6 | ±1.2 | −2.4 | ±1.2 | 0.003 ** | −2.1 | ±1.2 | −2.0 | ±1.3 | 0.002 ** |
| Left femoral neck | −2.3 | ±0.7 | −2.3 | ±0.7 | 0.118 | −2.3 | ±0.7 | −2.3 | ±0.8 | 0.039 * |
| Right femoral neck | −2.3 | ±0.8 | −2.4 | ±0.7 | 0.540 | −2.4 | ±0.8 | −2.5 | ±0.7 | 0.804 |
| TBS | ||||||||||
| BMD | 1.22 | ±0.11 | 1.22 | ±0.09 | 0.991 | 1.25 | ±0.10 | 1.27 | ±0.11 | 0.086 |
| T-score | −2.5 | ±1.1 | −2.5 | ±0.9 | 0.809 | −2.3 | ±1.1 | −1.9 | ±1.6 | 0.085 |
| Without Glucocorticoid (n = 107) | With Glucocorticoid (n = 107) | p Value | |||
|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | ||
| Re-fracture, n (%) | |||||
| Total | 35 | (32.7%) | 59 | (55.1%) | <0.001 ** |
| Femur | 16 | (45.7%) | 30 | (50.8%) | 0.630 |
| Radius | 3 | (8.6%) | 1 | (1.7%) | 0.144 |
| Spine | 17 | (48.6%) | 29 | (49.2%) | 0.957 |
| Non-Fracture (n = 120) | Fracture (n = 94) | p Value | |||
|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | ||
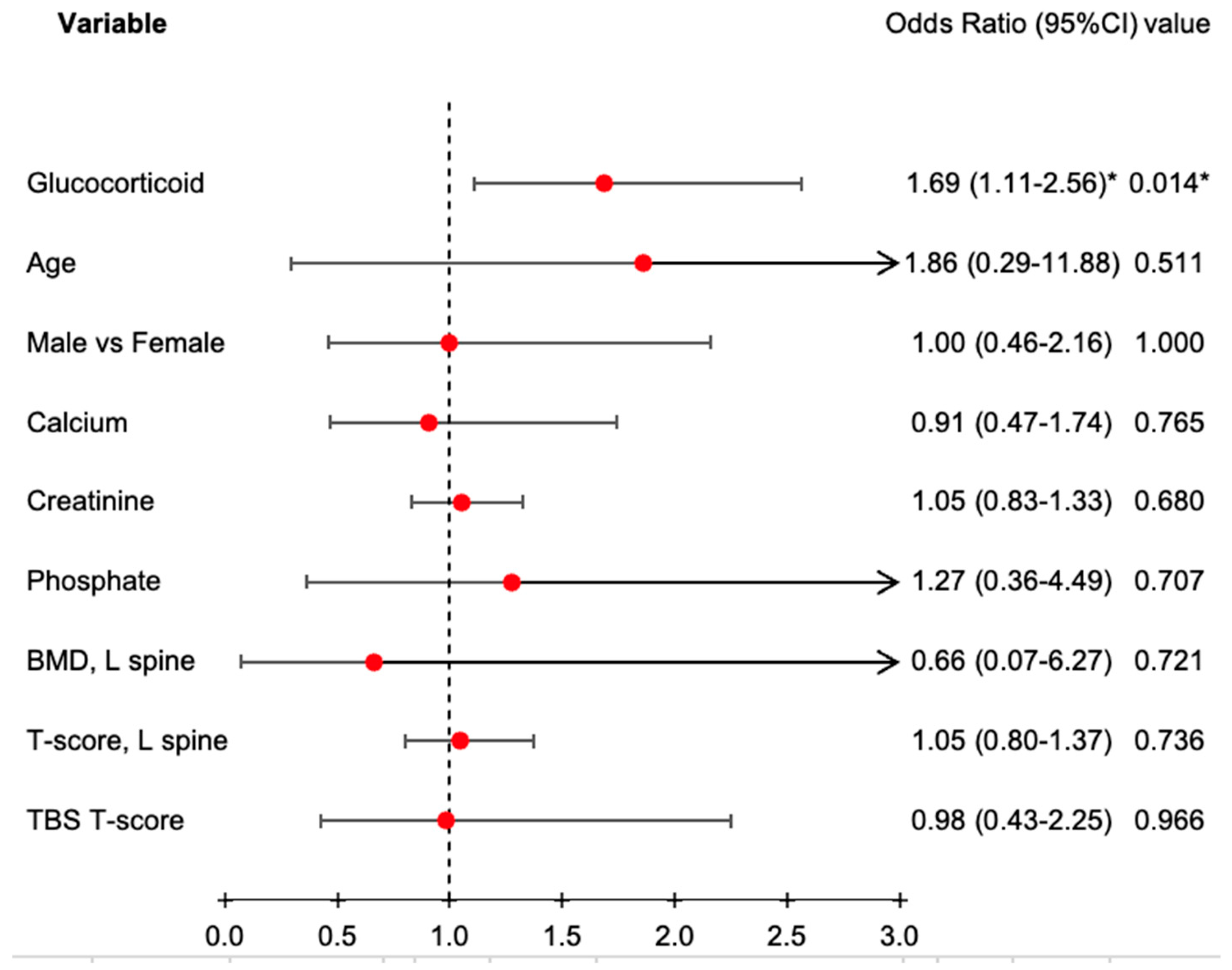
| Patients receive glucocorticoid, n (%) | 48 | (40.0%) | 59 | (62.8%) | 0.001 ** |
| Age | 66.7 | ±8.1 | 71.2 | ±7.8 | <0.001 ** |
| Sex, n (%) | 0.816 | ||||
| Female | 101 | (84.2%) | 78 | (83.0%) | |
| Male | 19 | (15.8%) | 16 | (17.0%) | |
| Albumin | 4.1 | ±0.4 | 3.9 | ±0.5 | 0.002 ** |
| ALP | 112.8 | ±77.9 | 108.1 | ±85.2 | 0.357 |
| CALCIUM | 9.1 | ±0.6 | 8.9 | ±0.6 | 0.236 |
| Creatinine | 1.5 | ±2.3 | 1.2 | ±1.8 | 0.808 |
| iPTH | 228.2 | ±391.7 | 272.1 | ±809.5 | 0.644 |
| Phosphate | 3.9 | ±0.8 | 4.1 | ±1.3 | 0.534 |
| Baseline | |||||
| BMD, L spine | 0.8 | ±0.2 | 0.9 | ±0.1 | 0.751 |
| BMD, left femoral neck | 0.7 | ±0.1 | 0.7 | ±0.1 | 0.229 |
| BMD, right femoral neck | 0.7 | ±0.1 | 0.6 | ±0.1 | 0.037 * |
| T-score, L spine | −2.3 | ±1.3 | −2.2 | ±1.2 | 0.203 |
| T-score, left femoral neck | −2.3 | ±0.7 | −2.4 | ±0.8 | 0.847 |
| T-score, right femoral neck | −2.4 | ±0.7 | −2.5 | ±0.9 | 0.476 |
| TBS BMD | 1.2 | ±0.1 | 1.3 | ±0.1 | 0.310 |
| TBS T-score | −2.5 | ±1.0 | −2.1 | ±1.3 | 0.362 |
| After | |||||
| BMD, L spine | 0.9 | ±0.1 | 0.9 | ±0.2 | 0.872 |
| BMD, left femoral neck | 0.7 | ±0.1 | 0.7 | ±0.1 | 0.605 |
| BMD, right femoral neck | 0.7 | ±0.1 | 0.7 | ±0.1 | 0.028 * |
| T-score, L spine | −2.2 | ±1.2 | −2.1 | ±1.3 | 0.329 |
| T-score, left femoral neck | −2.3 | ±0.7 | −2.3 | ±0.8 | 0.374 |
| T-score, right femoral neck | −2.4 | ±0.7 | −2.5 | ±0.8 | 0.344 |
| TBS BMD | 1.2 | ±0.1 | 1.3 | ±0.1 | 0.626 |
| TBS T-score | −2.4 | ±1.1 | −2.0 | ±1.7 | 0.399 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.-S.; Deng, Y.-L.; Hsu, C.-Y.; Lee, H.-T.; Li, C.-R.; Yang, C.-C. Denosumab in the Management of Glucocorticoid-Induced Osteoporosis: Long-Term Efficacy and Secondary Fracture Outcomes. J. Clin. Med. 2025, 14, 1633. https://doi.org/10.3390/jcm14051633
Liao S-S, Deng Y-L, Hsu C-Y, Lee H-T, Li C-R, Yang C-C. Denosumab in the Management of Glucocorticoid-Induced Osteoporosis: Long-Term Efficacy and Secondary Fracture Outcomes. Journal of Clinical Medicine. 2025; 14(5):1633. https://doi.org/10.3390/jcm14051633
Chicago/Turabian StyleLiao, Sian-Siang, Ya-Lian Deng, Chiann-Yi Hsu, Hsu-Tung Lee, Chi-Ruei Li, and Chih-Chan Yang. 2025. "Denosumab in the Management of Glucocorticoid-Induced Osteoporosis: Long-Term Efficacy and Secondary Fracture Outcomes" Journal of Clinical Medicine 14, no. 5: 1633. https://doi.org/10.3390/jcm14051633
APA StyleLiao, S.-S., Deng, Y.-L., Hsu, C.-Y., Lee, H.-T., Li, C.-R., & Yang, C.-C. (2025). Denosumab in the Management of Glucocorticoid-Induced Osteoporosis: Long-Term Efficacy and Secondary Fracture Outcomes. Journal of Clinical Medicine, 14(5), 1633. https://doi.org/10.3390/jcm14051633







