The Impact of Pleural Effusion on Long-Term Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Study Design, and Data Collection
2.2. Inclusion and Exclusion Criteria
2.3. Imaging and Assessments
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Prognostic Impact of Pleural Effusion in Patients Who Underwent TAVI
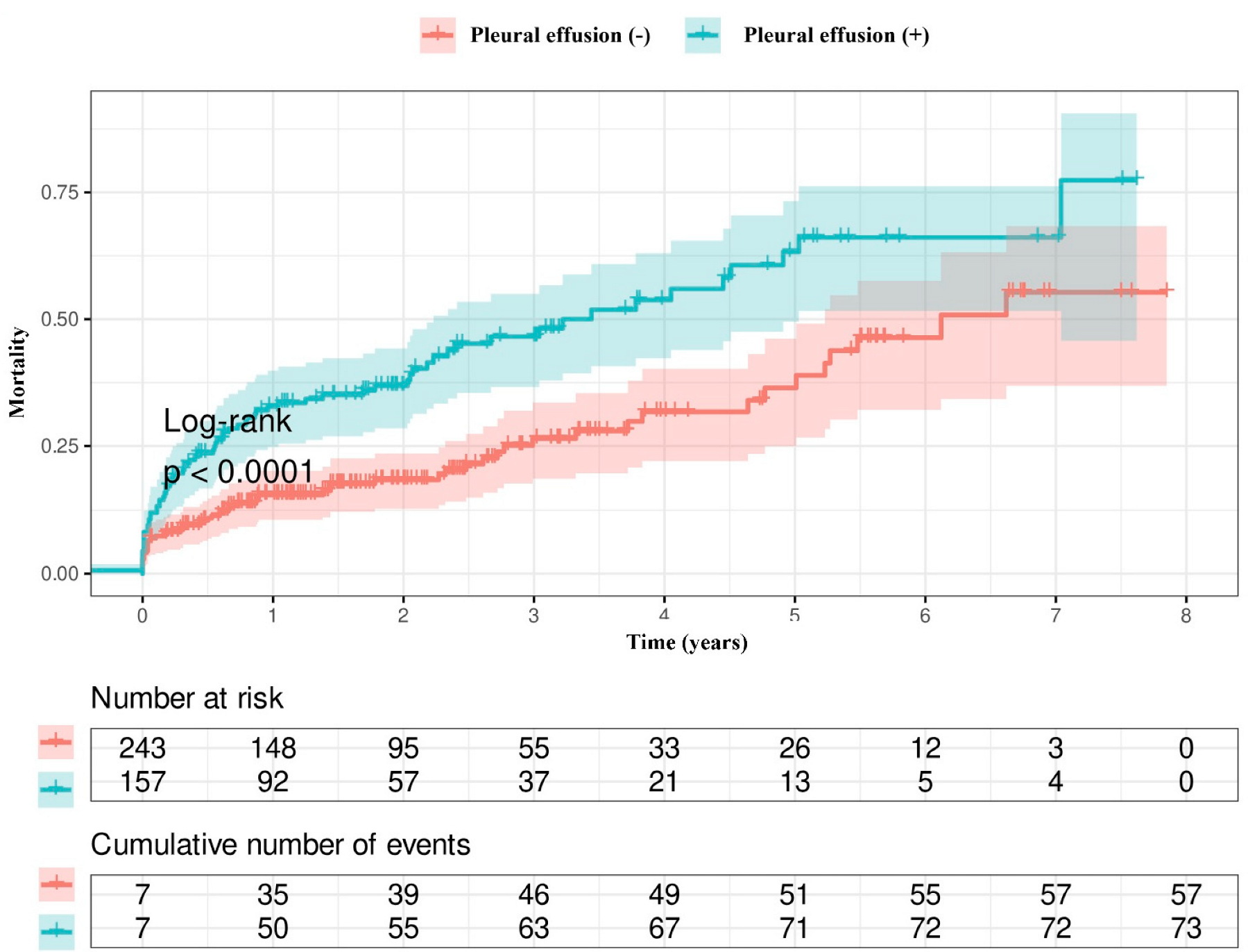
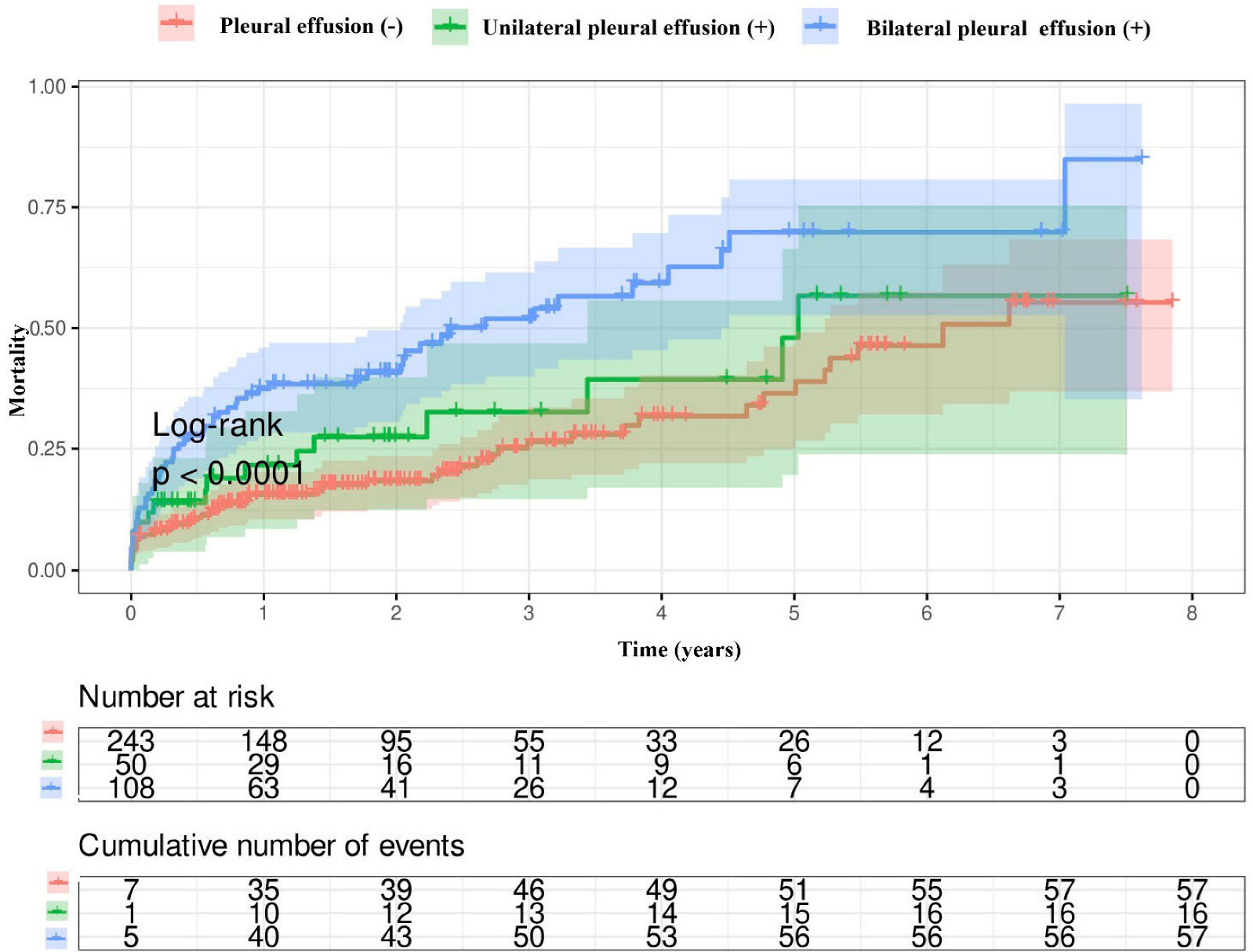
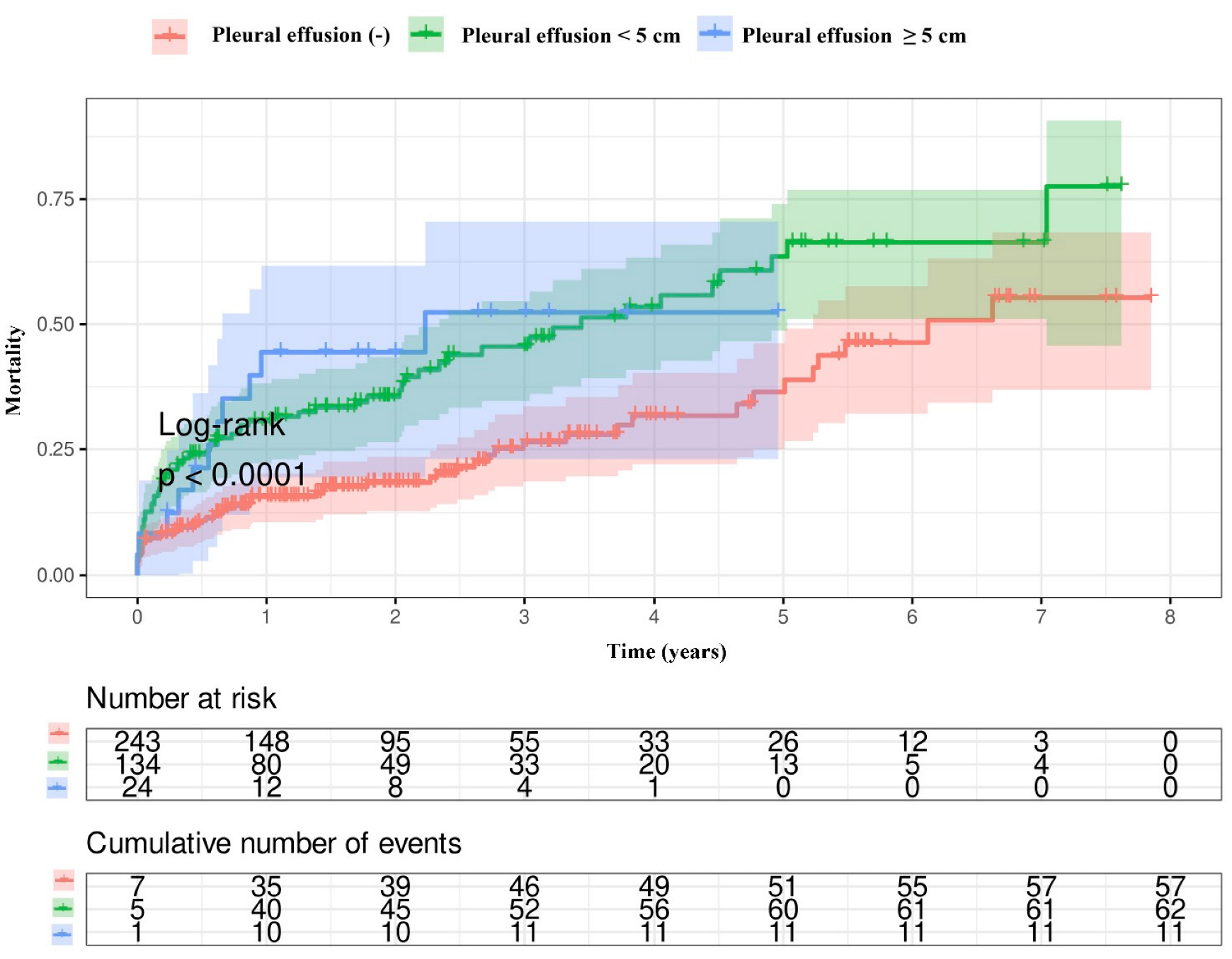
3.3. Survival Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Dweck, M.R.; Lancellotti, P.; Généreux, P.; Piérard, L.A.; O’Gara, P.T.; Bonow, R.O. Management of asymptomatic severe aortic stenosis: Evolving concepts in timing of valve replacement. J. Am. Coll. Cardiol. 2020, 75, 295–307. [Google Scholar]
- Balci, A.; Balci, T.B. Pleural effusion: Pathophysiology and clinical implications in cardiac patients. Curr. Cardiol. Rev. 2021, 17, 134–143. [Google Scholar]
- Wang, H.E.; Shrive, F.M.; Johnson, J.A. Association of renal function with cardiovascular outcomes after transcatheter aortic valve implantation. Am. J. Cardiol. 2017, 119, 145–151. [Google Scholar]
- Kass, D.A.; Maughan, W.L.; Yamazaki, T. Mechanisms of increased pulmonary vascular pressures in heart failure: Role of left ventricular filling pressures. Circulation 1993, 88, 2435–2442. [Google Scholar]
- Nicholson, J.P.; Wolmarans, M.R.; Park, G.R. The role of albumin in critical illness. Br. J. Anaesth. 2000, 85, 599–610. [Google Scholar] [CrossRef]
- Imazio, M.; Mayosi, B.M.; Brucato, A. Medical therapy versus pericardial drainage in chronic large pericardial effusion: A randomized trial. Ann. Intern. Med. 2014, 160, 743–751. [Google Scholar]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Schiefenhövel, F.; Poncette, A.-S.; Boyle, E.M.; von Heymann, C.; Menk, M.; Vorderwülbecke, G.; Grubitzsch, H.; Treskatsch, S.; Balzer, F. Pleural effusions are associated with adverse outcomes after cardiac surgery: A propensity-matched analysis. J. Cardiothorac. Surg. 2022, 17, 298. [Google Scholar] [PubMed]
- Walker, S.P.; Morley, A.J.; Stadon, L.; De Fonseka, D.; Arnold, D.T.; Medford, A.R.; Maskell, N.A. Non-malignant Pleural Effusions: A Prospective Study of 356 Consecutive Unselected Patients. Thorax 2017, 72, 449–455. [Google Scholar]
- Wang, T.; Godinjak, A.; Iglica, A.; Burekovic, A.; Jusufovic, S.; Ajanovic, A.; Tancica, I.; Kukuljac, A. Hyperglycemia and pleural fluid accumulation in critically ill patients. Crit. Care Med. 2018, 46, e112–e118. [Google Scholar]
- Das, A.; Khosla, R. Pleural Effusion Characteristics and Mortality. Chest 2016, 150, 1534–1541. [Google Scholar] [CrossRef]
- Eid, A.A.; Keddissi, J.I.; Kinasewitz, G.T. Hypoalbuminemia as a cause of pleural effusions. Chest 1999, 115, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Light, R.W.; Macgregor, M.I.; Luchsinger, P.C.; Ball, W.C., Jr. Systemic inflammation and pleural effusions: Markers and mechanisms. Chest 1973, 64, 528–532. [Google Scholar]
- Prais, D.; Kuzmenko, E.; Amir, J.; Harel, L. Association of Hypoalbuminemia with the Presence and Size of Pleural Effusion in Children with Pneumonia. Pediatrics 2008, 121, e533–e538. [Google Scholar] [CrossRef]
- Porcel, J.M.; Light, R.W. Pleural effusions due to pulmonary embolism. Curr. Opin. Pulm. Med. 2008, 14, 337–342. [Google Scholar] [CrossRef]
- Kookoolis, A.S.; Puchalski, J.T.; Murphy, T.E.; Araujo, K.L.; Pisani, M.A. Mortality of Hospitalized Patients with Pleural Effusions. J. Pulm. Respir. Med. 2014, 4, 184. [Google Scholar] [PubMed]
- Xiong, M.; Zhang, Z.; Hu, K.; Dong, M.; Hu, W. Recurrent, late-onset pleural effusions in elderly patients receiving pacemaker therapy. Medicine 2018, 97, e12915. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Pieragnoli, P.; Haugaa, K.H.; Potpara, T.S.; Rasero, L.; Ramacciati, N.; Ricciardi, G.; Solimene, F.; Mascia, G.; Mascioli, G.; et al. The influence of age on the psychological profile of patients with cardiac implantable electronic devices: Results from the Italian population in a multicenter study conducted by the European Heart Rhythm Association. Aging Clin. Exp. Res. 2019, 31, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Bediwy, A.S.; Al-Biltagi, M.; Saeed, N.K.; Bediwy, H.A.; Elbeltagi, R. Pleural effusion in critically ill patients and intensive care setting. World J. Clin. Cases 2023, 11, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Breuss, A.; Porsch, M.; Aschmann, A.; Weber, L.; Appert, S.; Haager, P.K.; Weilenmann, D.; Wildermuth, S.; Rickli, H.; Maeder, M.T. Pleural effusion in severe aortic stenosis: Marker of an adverse haemodynamic constellation and poor prognosis. ESC Heart Fail. 2024, 11, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, X.; Lin, C.; He, Y.; Cai, X.; Xu, Q.; Hu, P.; Gao, F.; Jiang, J.; Lin, X.; et al. Meta-Analysis of Outcomes and Evolution of Pulmonary Hypertension Before and After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2017, 9, 91–99. [Google Scholar] [CrossRef]
- D’Errigo, P.; Moretti, C.; D’Ascenzo, F.; Rosato, S.; Biancari, F.; Barbanti, M.; Santini, F.; Ranucci, M.; Miceli, A.; Tamburino, C.; et al. OBSERVANT Research Group. Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement for Severe Aortic Stenosis in Patients With Chronic Kidney Disease Stages 3b to 5. Ann. Thorac. Surg. 2016, 102, 540–547. [Google Scholar] [CrossRef]
- Kjønås, D.; Schirmer, H.; Aakhus, S.; Eidet, J.; Malm, S.; Aaberge, L.; Busund, R.; Rösner, A. Clinical and echocardiographic parameters predicting 1- and 2-year mortality after transcatheter aortic valve implantation. Front. Cardiovasc. Med. 2021, 8, 739710. [Google Scholar] [CrossRef] [PubMed]
- Stortecky, S.; Windecker, S. Optimizing outcomes in patients undergoing transcatheter aortic valve implantation: The role of comorbidities. Eur. Heart J. 2015, 36, 1304–1307. [Google Scholar]
- Nombela-Franco, L.; Ribeiro, H.B.; Urena, M.; Allende, R.; Amat-Santos, I.; De Larochellière, R.; Dumont, E.; Doyle, D.; De Larochellière, H.; Laflamme, J.; et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: A comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J. Am. Coll. Cardiol. 2014, 63, 2643–2658. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.P.; Jilaihawi, H.; Kapadia, S.; Pichard, A.D.; Douglas, P.S.; Thourani, V.H.; Babaliaros, V.C.; Webb, J.G.; Herrmann, H.C.; et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N. Engl. J. Med. 2012, 366, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Panoulas, V.; Jabbour, R.J.; Ruparelia, N.; Malik, I.S.; Hadjiloizou, N.; Frame, A.; Sen, S.; Sutaria, N.; Mikhail, G.W.; et al. Left ventricular speckle tracking echocardiographic evaluation before and after TAVI. Echo Res. Pract. 2020, 7, 29–38. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Lombardo, M.; Baravelli, M.; Trotta, G.; Sommese, C.; Anzà, C. Exercise stress echocardiography with tissue Doppler imaging in risk stratification of mild to moderate aortic stenosis. Int. J. Cardiovasc. Imaging 2015, 31, 1519–1527. [Google Scholar] [CrossRef]
- Stolz, L.; Schmid, S.; Steffen, J.; Doldi, P.M.; Weckbach, L.T.; Stocker, T.J.; Löw, K.; Fröhlich, C.; Fischer, J.; Haum, M.; et al. Prognostic impact of left and right atrial strain in patients undergoing transcatheter aortic valve replacement. Eur. Heart J. Cardiovasc. Imaging 2024. epub ahead of print. [Google Scholar] [CrossRef]
- Goublaire, C.; Melissopoulou, M.; Lobo, D.; Kubota, N.; Verdonk, C.; Cimadevilla, C.; Codogno, I.; Brochet, E.; Vahanian, A.; Messika-Zeitoun, D. Prognostic Value of Exercise-Stress Echocardiography in Asymptomatic Patients with Aortic Valve Stenosis. JACC Cardiovasc. Imaging 2018, 11, 787–795. [Google Scholar] [CrossRef] [PubMed]
| Characteristics (n, %) | No Pleural Effusion (n = 243) | Pleural Effusion Present (n = 158) | p-Value |
|---|---|---|---|
| Age (years) | 76.1 ± 7.7 | 77.3 ± 8.4 | 0.118 |
| Gender (male) | 109 (55.3%) | 88 (44.7%) | 0.034 |
| DM | 87 (64.4%) | 48 (35.6%) | 0.262 |
| HT | 167 (62.3%) | 101 (37.7%) | 0.318 |
| Dyslipidemia | 156 (58.0%) | 113 (42.0%) | 0.127 |
| Carotid artery disease | 3 (1.2%) | 4 (2.5%) | 0.332 |
| Smoking | 7 (2.9%) | 6 (3.8%) | 0.612 |
| CAD | 200 (82.3%) | 133 (84.2%) | 0.625 |
| History of heart valve surgery | 7 (2.9%) | 2 (1.3%) | 0.286 |
| PAD | 28 (11.5%) | 28 (17.7%) | 0.080 |
| Heart failure | 46 (18.9%) | 62 (39.2%) | <0.001 |
| COPD | 23 (9.5%) | 12 (7.6%) | 0.517 |
| Previous stroke | 17 (7.0%) | 12 (7.6%) | 0.821 |
| CKD, n (%) | 47 (19) | 61 (39) | <0.001 |
| Previous AF | 58 (23.9%) | 44 (27.8%) | 0.371 |
| PCI before TAVI | 21 (8.6%) | 19 (12.0%) | 0.269 |
| LVEF (%) | 56.6 ± 8.6 | 49.7 ± 12.5 | <0.001 |
| MR (moderate/severe) | 81 (33.3%) | 89 (56.3%) | <0.001 |
| TR (moderate/severe) | 53 (21.8%) | 70 (44.3%) | <0.001 |
| Valve type, n (%) | 0.278 | ||
| Balloon-expandable | 86 (36) | 48 (31) | |
| Self-expanding | 154 (64) | 109 (69) | |
| TPM post-TAVI | 98 (40.3%) | 68 (43.0%) | 0.389 |
| PPM post-TAVI | 40 (16.5%) | 30 (19.0%) | 0.257 |
| Complications after TAVI, n (%) | 0.409 | ||
| Pseudoaneurysm | 2 (1) | 1(1) | |
| Stent placement for arterial injury | 10 (4) | 9 (6) | |
| Surgical repair of arterial injury | 6 (3) | 8 (5) | |
| Cardiac tamponade after TAVI | 3 (3) | 5 (3) | |
| Long-term mortality, n (%) | 57 (24) | 73 (46) | <0.001 |
| Parameters | No Pleural Effusion (n = 243) | Pleural Effusion Present (n = 158) | p-Value |
|---|---|---|---|
| HGB (g/dL) | 11.3 ± 1.7 | 10.8 ± 1.8 | 0.005 |
| PLT (×103/μL) | 217.8 ± 81.3 | 218.0 ± 87.1 | 0.681 |
| GFR (mL/min) | 62.29 ± 17.25 | 54.40 ± 20.21 | <0.001 |
| Albumin (g/dL) | 36.76 ± 6.52 | 33.94 ± 5.01 | 0.037 |
| WBC (×103/μL) | 7.85 ± 3.31 | 8.38 ± 3.48 | 0.13 |
| BMI (kg/m2) | 30.38 ± 5.16 | 27.31 ± 6.01 | 0.214 |
| AV PG (mmHg) | 76.33 ± 21.85 | 75.79 ± 24.32 | 0.817 |
| AV MG (mmHg) | 46.45 ± 14.36 | 45.86 ± 15.99 | 0.702 |
| SPAP (mmHg) | 36.0 ± 12.4 | 44.3 ± 15.6 | <0.001 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.043 | 1.017–1.067 | 0.001 | 1.031 | 1.004–1.058 | 0.026 |
| GFR | 0.979 | 0.970–0.988 | <0.001 | 0.986 | 0.976–0.996 | 0.009 |
| MR (moderate/severe) | 1.468 | 1.038–2.077 | 0.030 | |||
| TR (moderate/severe) | 1.471 | 1.031–2.101 | 0.034 | |||
| Balloon-expandable vs. self-expanding | 0.632 | 0.388–1.029 | 0.065 | |||
| SPAP | 1.022 | 1.010–1.034 | <0.001 | 1.019 | 1.003–1.035 | 0.026 |
| Pleural effusion | 2.104 | 1.485–2.979 | <0.001 | 1.568 | 1.065–2.308 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esin, F.; Bozkurt, H.; Palac, B.; Akar, B.; Kiris, T.; Özdemir, E.; Karaca, M. The Impact of Pleural Effusion on Long-Term Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2025, 14, 1596. https://doi.org/10.3390/jcm14051596
Esin F, Bozkurt H, Palac B, Akar B, Kiris T, Özdemir E, Karaca M. The Impact of Pleural Effusion on Long-Term Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine. 2025; 14(5):1596. https://doi.org/10.3390/jcm14051596
Chicago/Turabian StyleEsin, Fatma, Hakan Bozkurt, Berkay Palac, Bahadır Akar, Tuncay Kiris, Emre Özdemir, and Mustafa Karaca. 2025. "The Impact of Pleural Effusion on Long-Term Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation" Journal of Clinical Medicine 14, no. 5: 1596. https://doi.org/10.3390/jcm14051596
APA StyleEsin, F., Bozkurt, H., Palac, B., Akar, B., Kiris, T., Özdemir, E., & Karaca, M. (2025). The Impact of Pleural Effusion on Long-Term Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine, 14(5), 1596. https://doi.org/10.3390/jcm14051596





