Effects of Global Postural Re-Education Versus Specific Therapeutic Exercises on Pain, Head Posture, and Pain-Related Psychosocial Factors in Women with Chronic Nonspecific Neck Pain: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Randomization and Blinding
2.4. Evaluations
2.5. Outcome Variables
2.5.1. Primary Outcome Variables
2.5.2. Secondary Outcome Variables
- Mechanosensitivity: It was assessed using the PPT, measured with a digital algometer (Force Ten™-Model FDX; Wagner, Greenwich, CT, USA) featuring a 1 cm2 round tip surface. This device [93] was used to quantify PPT (kgf) at specific bilateral assessment sites: the paravertebral muscles adjacent to the second (C2) and sixth (C6) cervical vertebrae (prone position) and the upper trapezius muscle (sitting position) to evaluate peripheral sensitization, as well as the tibialis anterior muscle (supine position) to assess central sensitization [94,95,96,97]. The evaluator progressively increased pressure at each site until the participant reported the onset of pain [98]. PPT measurement via algometry has demonstrated excellent test–retest reliability (ICC = 0.91, 95% CI 0.82–0.97) [99].
- Head Posture: To assess the head position, the craniocervical angle (CCA) was measured using a universal goniometer device (Sammons Preston-Rolyan, Bolingbrook, IL, USA). This angle is obtained from a line connecting C7 with the tragus and the horizontal line in the standing position. The measurement was carried out 3 times considering the mean value. The CCA is a widely used surface-based measurement for assessing head posture, particularly in individuals with cervical dysfunction. Previous studies have demonstrated its acceptable (ICC ≥ 0.71) to good (ICC ≥ 0.85) intra-rater reliability [98,100]. Additionally, research comparing CCA with radiographic indices has reported moderate to high correlations, supporting its validity as a clinical tool for evaluating postural alignment [101].
- Pain-related psychosocial factors: To evaluate attitudes and thoughts related to pain, we assessed the influence of kinesiophobia and pain catastrophizing toward CNSNP:
- Kinesiophobia: This was measured using the Portuguese version of the 13-item Tampa Kinesiophobia Scale (TSK-13-PT) that assesses fear of movement and re-injury, with a very good consistency and test–retest reliability [102]. It consists of 13 items and each item is scored from 1 to 4 points. A higher score indicates higher levels of kinesiophobia, and it is classified into four ranges of intensity: ’subclinical’ (13–22); ’mild’ (23–32); ’moderate’ (33–42); and ’severe’ (43–52) [103].
- Catastrophizing: To measure pain catastrophizing, as a tendency to magnify the threat value of a painful stimulus and to feel helpless in the presence of pain, the Portuguese version of the pain catastrophizing scale (PCS-PT) was used, also with very good internal consistency and test–retest reliability [104,105]. This scale is composed of 13 items, each scored from 0 to 4 points (none to all the time). Participants have to describe the frequency with which they experience different thoughts and feelings associated with pain that are grouped into 3 subscales: rumination (4 items), magnification (3 items), and helplessness (6 items) [106,107].
2.6. Interventions
2.6.1. Global Postural Re-Education (GPR)
2.6.2. Specific Therapeutic Exercise (STE)
2.7. Statistical Analysis
3. Results
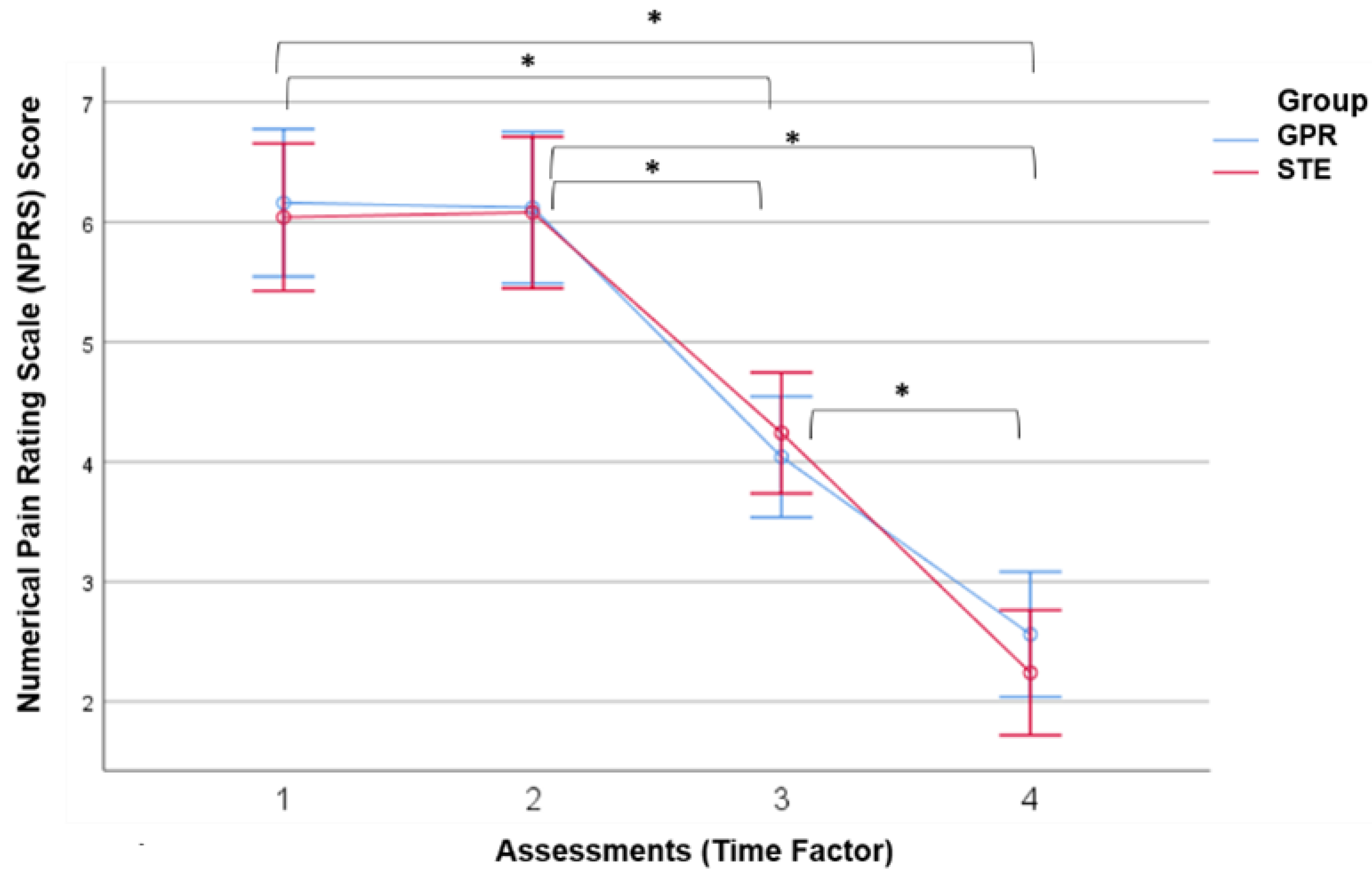
3.1. Results of Neck Pain
3.2. Results of Pressure Pain Threshold (PPT)
3.3. Results of Craniocervical Angle (CCA)
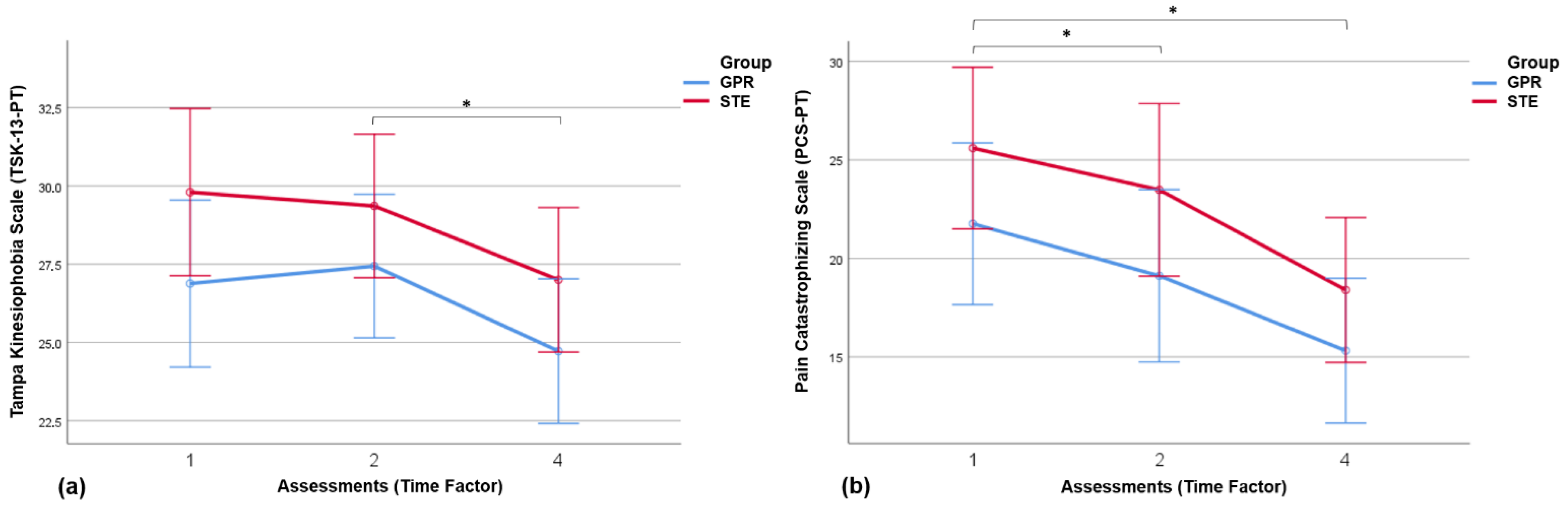
3.4. Results of Pain-Related Psychosocial Factors
4. Discussion
4.1. Neck Pain
4.2. Pressure Pain Threshold (PPT)
4.3. Craniocervical Angle
4.4. Pain-Related Psychosocial Factors
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Trial Registration
References
- Daffner, S.D.; Hilibrand, A.S.; Hanscom, B.S.; Brislin, B.T.; Vaccaro, A.R.; Albert, T.J. Impact of neck and arm pain on overall health status. Spine 2003, 28, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.; Sindhusake, D.; Cameron, I.D.; Rubin, G.; Feyer, A.M.; Walsh, J.; Gold, M.; Schofield, W.N. A prospective cohort study of health outcomes following whiplash associated disorders in an Australian population. Inj. Prev. 2006, 12, 93–98. [Google Scholar] [CrossRef]
- Childs, J.D.; Clelandt, J.A.; Elliot, J.M.; Teyhen, D.S.; Wainner, R.S.; Whitmam, J.M.; Sopky, B.J.; Godges, J.J.; Flynn, T.W.; American Physical Therapy Association. Neck Pain: Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability, and Health from the Orthopaedic Section of the American Physical Therapy Association. J. Orthop. Sports Phys. Ther. 2008, 38, A1–A34. [Google Scholar] [CrossRef]
- Verhagen, A.P. Physiotherapy management of neck pain. J. Physiother. 2021, 67, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Fejer, R.; Kyvik, K.O.; Hartvigsen, J. The prevalence of neck pain in the world population: A systematic critical review of the literature. Eur. Spine J. 2006, 15, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.D.; Fritz, J.M.; Piva, S.R.; Whitman, J.M. Proposal of a classification system for patients with neck pain. J. Orthop. Sports Phys. Ther. 2004, 34, 686–696. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Kamper, S.J.; Rebbeck, T.J.; Maher, C.G.; McAuley, J.H.; Sterling, M. Course and prognostic factors of whiplash: A systematic review and meta-analysis. Pain 2008, 138, 617–629. [Google Scholar] [CrossRef]
- Côté, P.; van der Velde, G.; Cassidy, J.D.; Carroll, L.J.; Hogg-Johnson, S.; Holm, L.W.; Carragee, E.J.; Haldeman, S.; Nordin, M.; Hurwitz, E.L.; et al. The burden and determinants of neck pain in workers: Results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. J Manip. Physiol. Ther. 2009, 32 (Suppl. S2), S70–S86. [Google Scholar] [CrossRef]
- Falla, D.; Farina, D. Neuromuscular adaptation in experimental and clinical neck pain. J. Electromyogr. Kinesiol. 2008, 18, 255–261. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Falla, D.; Elliott, J.M.; Jull, G. Muscle dysfunction in cervical spine pain: Implications for assessment and management. J. Orthop. Sports Phys. Ther. 2009, 39, 324–333. [Google Scholar] [CrossRef]
- Field, S.; Treleaven, J.; Jull, G. Standing balance: A comparison between idiopathic and whiplash-induced neck pain. Man. Ther. 2008, 13, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Meisingset, I.; Woodhouse, A.; Stensdotter, A.K.; Stavdahl, Ø.; Lorås, H.; Gismervik, S.; Andresen, H.; Austreim, K.; Vasseljen, O. Evidence for a general stiffening motor control pattern in neck pain: A cross sectional study. BMC Musculoskelet. Disord. 2015, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Grimby-Ekman, A.; Verbunt, J.; Simmonds, M.J. Pain-related fear: A critical review of the related measures. Pain Res. Treat. 2011, 2011, 494196. [Google Scholar] [CrossRef] [PubMed]
- Luque-Suarez, A.; Martinez-Calderon, J.; Falla, D. Role of kinesiophobia on pain, disability and quality of life in people suffering from chronic musculoskeletal pain: A systematic review. Br. J. Sports Med. 2019, 53, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Gunay Ucurum, S. The relationship between pain severity, kinesiophobia, and quality of life in patients with non-specific chronic neck pain. J. Back Musculoskelet. Rehabil. 2019, 32, 677–683. [Google Scholar] [CrossRef]
- Bonilla-Barba, L.; Florencio, L.L.; Rodríguez-Jiménez, J.; Falla, D.; Fernández-de-Las-Peñas, C.; Ortega-Santiago, R. Women with mechanical neck pain exhibit increased activation of their superficial neck extensors when performing the cranio-cervical flexion test. Musculoskelet. Sci. Pract. 2020, 49, 102222. [Google Scholar] [CrossRef] [PubMed]
- Meleger, A.L.; Krivickas, L.S. Neck and back pain: Musculoskeletal disorders. Neurol. Clin. 2007, 25, 419–438. [Google Scholar] [CrossRef]
- López-de-Uralde-Villanueva, I.; Beltran-Alacreu, H.; Fernández-Carnero, J.; Gil-Martínez, A.; La Touche, R. Differences in Neural Mechanosensitivity between Patients with Chronic Nonspecific Neck PainWith andWithout Neuropathic Features. A Descriptive Cross-Sectional Study. Pain Med. 2015, 17, 136–148. [Google Scholar]
- Muñoz-Muñoz, S.; Muñoz-García, M.T.; Alburquerque-Sendín, F.; Arroyo-Morales, M.; Fernández-de-las-Peñas, C. Myofascial trigger points, pain, disability, and sleep quality in individuals with mechanical neck pain. J. Manip. Physiol. Ther. 2012, 35, 608–613. [Google Scholar] [CrossRef]
- Chiarotto, A.; Clijsen, R.; Fernandez-de-las-Penas, C.; Barbero, M. The prevalence of myofascial trigger points in spinal disorders: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2016, 97, 316–337. [Google Scholar] [CrossRef] [PubMed]
- Cagnie, B.; Castelein, B.; Pollie, F.; Steelant, L.; Verhoeyen, H.; Cools, A. Evidence for the use of ischemic compression and dry needling in the management of trigger points of the upper trapezius in patients with neck pain. Am. J. Phys. Med. Rehabil. 2015, 94, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.C.; Belgrave, A.; Naden, A.; Fang, H.; Matthews, P.; Parshottam, S. The prevalence of myofascial trigger points in neck and shoulder-related disorders: A systematic review of the literature. BMC Musculoskelet. Disord. 2018, 19, 252. [Google Scholar] [CrossRef]
- Martínez-Merinero, P.; Aneiros Tarancón, F.; Montañez-Aguilera, J.; Nuñez-Nagy, S.; Pecos-Martín, D.; Fernández-Matías, R.; Achalandabaso-Ochoa, A.; Fernández-Carnero, S.; Gallego-Izquierdo, T. Interaction between Pain, Disability, Mechanosensitivity and Cranio-Cervical Angle in Subjects with Cervicogenic Headache: A Cross-Sectional Study. J. Clin. Med. 2021, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.E.A.; Pluma, A.F.; Pecos-Martín, D.; Achalandabaso-Ochoa, A.; Fernández-Matías, R.; Martinez-Merinero, P.; Nuñez-Nagy, S.; Gallego-Izquierdo, T. Relationship between Neuromuscular Mechanosensitivity and Chronic Neck Pain in Guitarists: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 2673. [Google Scholar] [CrossRef]
- Falla, D.L.; Jull, G.A.; Hodges, P.W. Patients with neck pain demonstrate reduced electromyographic activity of the deep cervical flexor muscles during performance of the craniocervical flexion test. Spine 2004, 29, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Jull, G.; Kristjansson, E.; Dall’Alba, P. Impairment in the cervical flexors: A comparison of whiplash and insidious onset neck pain patients. Man. Ther. 2004, 9, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Jull, G.; Noteboom, J.T.; Darnell, R.; Galloway, G.; Gibbon, W.W. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: A magnetic resonance imaging analysis. Spine 2006, 31, 847–855. [Google Scholar] [CrossRef]
- Schomacher, J.; Farina, D.; Lindstroem, R.; Falla, D. Chronic trauma-induced neck pain impairs the neural control of the deep semispinalis cervicis muscle. Clin. Neurophysiol. 2012, 123, 1403–1408. [Google Scholar] [CrossRef]
- Falla, D.; Bilenkij, G.; Jull, G. Patients with chronic neck pain demonstrate altered patterns of muscle activation during performance of a functional upper limb task. Spine 2004, 29, 1436–1440. [Google Scholar] [CrossRef]
- Barton, P.M.; Hayes, K.C. Neck flexor muscle strength, efficiency, and relaxation times in normal subjects and subjects with unilateral neck pain and headache. Arch. Phys. Med. Rehabil. 1996, 77, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.T.; Sing, K.L. Evaluation of cervical range of motion and isometric neck muscle strength: Reliability and validity. Clin. Rehabil. 2002, 16, 851–858. [Google Scholar] [CrossRef]
- Placzek, J.D.; Pagett, B.T.; Roubal, P.J.; Jones, B.A.; McMichael, H.G.; Rozanski, E.A.; Gianotto, K.L. The influence of the cervical spine on chronic headache in women: A pilot study. J. Man. Manip. Ther. 1999, 7, 33–39. [Google Scholar] [CrossRef]
- Ylinen, J.; Salo, P.; Nykanen, M.; Kautiainen, H.; Hakkinen, A. Decreased isometric neck strength in women with chronic neck pain and the repeatability of neck strength measurements. Arch. Phys. Med. Rehabil. 2004, 85, 1303–1308. [Google Scholar] [CrossRef]
- Treleaven, J.; Jull, G.; Sterling, M. Dizziness and unsteadiness following whiplash injury: Characteristic features and relationship with cervical joint position error. J. Rehabil. Med. 2003, 35, 36–43. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Ischebeck, B.K.; Voogt, L.P.; van der Geest, J.N.; Janssen, M.; Frens, M.A.; Kleinrensink, G.J. Joint position sense error in people with neck pain: A systematic review. Man. Ther. 2015, 20, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.R.; Leake, H.B.; Chalmers, K.J.; Moseley, G.L. Evidence of impaired proprioception in chronic, idiopathic neck pain: Systematic review and meta-analysis. Phys. Ther. 2016, 96, 876–887. [Google Scholar] [CrossRef]
- Armstrong, B.; McNair, P.; Taylor, D. Head and Neck Position Sense. Sports Med. 2008, 38, 101–117. [Google Scholar] [CrossRef]
- Subbarayalu, A.V.; Ameer, M.A. Relationships among head posture, pain intensity, disability and deep cervical flexor muscle performance in subjects with postural neck pain. J. Taibah Univ. Med. Sci. 2017, 12, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Lee, M. Consistency of cervical and cervicothoracic posture in standing. Aust. J. Physiother. 1994, 40, 235–240. [Google Scholar]
- Martinez-Merinero, P.; Nuñez-Nagy, S.; Achalandabaso-Ochoa, A.; Fernandez-Matias, R.; Pecos-Martin, D.; Gallego-Izquierdo, T. Relationship between Forward Head Posture and Tissue Mechanosensitivity: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Alfonso, V.; Roselló-Sastre, E. Hypothesis: Anterior knee pain in the young patient-what causes the pain? ”Neural model” Acta Orthop. Scand. 2003, 74, 697–703. [Google Scholar] [CrossRef]
- Woodhouse, A.; Vasseljen, O. Altered motor control patterns in whiplash and chronic neck pain. BMC Musculoskelet. Disord. 2008, 9, 90. [Google Scholar] [CrossRef]
- Meisingset, I.; Stensdotter, A.K.; Woodhouse, A.; Vasseljen, O. Neck motion, motor control, pain and disability: A longitudinal study of associations in neck pain patients in physiotherapy treatment. Man. Ther. 2016, 22, 94–100. [Google Scholar] [CrossRef]
- Scott, D.; Jull, G.; Sterling, M. Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but not chronic idiopathic neck pain. Clin. J. Pain 2005, 21, 175–181. [Google Scholar] [CrossRef]
- Sterling, M.; Hodkinson, E.; Pettiford, C.; Souvlis, T.; Curatolo, M. Psychologic factors are related to some sensory pain thresholds but not nociceptive flexion reflex threshold in chronic whiplash. Clin. J. Pain 2008, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Salom-Moreno, J.; Ortega-Santiago, R.; Cleland, J.A.; Palacios-Ceña, M.; Truyols-Domínguez, S.; Fernández-de-las-Peñas, C. Immediate changes in neck pain intensity and widespread pressure pain sensitivity in patients with bilateral chronic mechanical neck pain: A randomized controlled trial of thoracic thrust manipulation vs. non-thrust mobilization. J. Manip. Physiol. Ther. 2014, 37, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Segura, R.; De-la-Llave-Rincón, A.I.; Ortega-Santiago, R.; Cleland, J.A.; Fernández-de-Las-Peñas, C. Immediate changes in widespread pressure pain sensitivity, neck pain, and cervical range of motion after cervical or thoracic thrust manipulation in patients with bilateral chronic mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2012, 42, 806–814. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152 (Suppl. S3), S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Edwards, I.; Gifford, L. Conceptual models for implementing biopsychosocial theory in clinical practice. Man. Ther. 2002, 7, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Walton, D.M.; Kwok, T.S.; Mehta, S.; Loh, E.; Smith, A.; Elliott, J.; Kamper, S.J.; Kasch, H.; Sterling, M. Cluster analysis of an international pressure pain threshold database identifies 4 meaningful subgroups of adults with mechanical neck pain. Clin. J. Pain 2017, 33, 422–428. [Google Scholar] [CrossRef]
- Malfliet, A.; Kregel, J.; Cagnie, B.; Kuipers, M.; Dolphens, M.; Roussel, N.; Meeus, M.; Danneels, L.; Bramer, W.M.; Nijs, J. Lack of evidence for central sensitization in idiopathic, non-traumatic neck pain: A systematic review. Pain Physician 2015, 18, 223–236. [Google Scholar]
- Ceballos-Laita, L.; Medrano-de-la-Fuente, R.; Mingo-Gómez, M.T.; Hernando-Garijo, I.; Estébanez-de-Miguel, E.; Jiménez-Del-Barrio, S. Effects of dry needling on pain, disability, kinesiophobia, pain catastrophizing and psychological distress in patients with chronic neck pain: A randomized controlled pilot study. J. Back Musculoskelet. Rehabil. 2021, 35, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.P.; Urmston, M.; Oldham, J.A.; Woby, S.R. The association between cognitive factors, pain and disability in patients with idiopathic chronic neck pain. Disabil. Rehabil. 2010, 32, 1758–1767. [Google Scholar] [CrossRef]
- Dimitriadis, Z.; Kapreli, E.; Strimpakos, N.; Oldham, J. Do psychological states associate with pain and disability in chronic neck pain patients? J. Back Musculoskelet. Rehabil. 2015, 28, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Gross, A.; D’Sylva, J.; Burnie, S.J.; Goldsmith, C.H.; Graham, N.; Haines, T.; Brønfort, G.; Hoving, J.L. Manual therapy and exercise for neck pain: A systematic review. Man. Ther. 2010, 15, 334–354. [Google Scholar] [CrossRef]
- Schroeder, J.; Kaplan, L.; Fischer, D.J.; Skelly, A.C. The outcomes of manipulation or mobilization therapy compared with physical therapy or exercise for neck pain: A systematic review. Evid.-Based Spine-Care J. 2013, 4, 30–41. [Google Scholar] [PubMed]
- Hidalgo, B.; Hall, T.; Bossert, J.; Dugeny, A.; Cagnie, B.; Pitance, L. The efficacy of manual therapy and exercise for treating non-specific neck pain: A systematic review. J. Back Musculoskelet. Rehabil. 2017, 30, 1149–1169. [Google Scholar] [CrossRef]
- Treleaven, J.; Peterson, G.; Ludvigsson, M.L.; Kammerlind, A.S.; Peolsson, A. Balance, dizziness and proprioception in patients with chronic whiplash associated disorders complaining of dizziness: A prospective randomized study comparing three exercise programs. Man. Ther. 2016, 22, 122–130. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Woolf, A.; Blyth, F.; Brooks, P.; Smith, E.; Vos, T.; Barendregt, J.; Blore, J.; Murray, C.; et al. The global burden of neck pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1309–1315. [Google Scholar] [CrossRef]
- Manafnezhad, J.; Salahzadeh, Z.; Salimi, M.; Ghaderi, F.; Ghojazadeh, M. The effects of shock wave and dry needling on active trigger points of upper trapezius muscle in patients with non-specific neck pain: A randomized clinical trial. J. Back Musculoskelet. Rehabil. 2019, 32, 811–818. [Google Scholar] [CrossRef]
- Gallego-Sendarrubias, G.M.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Martín, J.L. Efficacy of dry needling as an adjunct to manual therapy for patients with chronic mechanical neck pain: A randomised clinical trial. Acupunct. Med. 2020, 38, 244–254. [Google Scholar] [CrossRef]
- Beltran-Alacreu, H.; López-de-Uralde-Villanueva, I.; Fernández-Carnero, J.; La Touche, R. Manual Therapy, Therapeutic Patient Education, and Therapeutic Exercise, an Effective Multimodal Treatment of Nonspecific Chronic Neck Pain: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2015, 94 (Suppl. S1), 887–897. [Google Scholar] [CrossRef]
- Javdaneh, N.; Saeterbakken, A.H.; Shams, A.; Barati, A.H. Pain Neuroscience Education Combined with Therapeutic Exercises Provides Added Benefit in the Treatment of Chronic Neck Pain. Int. J. Environ. Res. Public Health 2021, 18, 8848. [Google Scholar] [CrossRef]
- Jull, G.; Trott, P.; Potter, H.; Zito, G.; Niere, K.; Shirley, D.; Emberson, J.; Marschner, I.; Richardson, C. A randomized controlled trial of exercise and manipulative therapy for cervicogenic headache. Spine 2002, 27, 1835–1843. [Google Scholar] [CrossRef]
- Gross, A.R.; Paquin, J.P.; Dupont, G.; Blanchette, S.; Lalonde, P.; Cristie, T.; Graham, N.; Kay, T.; Burnie, S.; Gelley, G.; et al. Exercises for mechanical neck disorders: A Cochrane review update. Man. Ther. 2016, 24, 25–45. [Google Scholar] [CrossRef]
- Falla, D.; Gizzi, L.; Parsa, H.; Dieterich, A.; Petzke, F. People with chronic neck pain walk with a stiffer spine. J. Orthop. Sports Phys. Ther. 2017, 47, 268–277. [Google Scholar] [CrossRef]
- Fortin, C.; Feldman, D.E.; Tanaka, C.; Houde, M.; Labelle, H. Inter-rater reliability of the evaluation of muscular chains associated with posture alterations in scoliosis. BMC Musculoskelet. Disord. 2012, 13, 80. [Google Scholar] [CrossRef]
- Bonetti, F.; Curti, S.; Mattioli, S.; Mugnai, R.; Vanti, C.; Violante, F.S.; Pillastrini, P. Effectiveness of a ‘Global Postural Reeducation’ program for persistent low back pain: A non-randomized controlled trial. BMC Musculoskelet. Disord. 2010, 11, 285. [Google Scholar] [CrossRef]
- Pillastrini, P.; Banchelli, F.; Guccione, A.; Di Ciaccio, E.; Violante, F.S.; Brugnettini, M.; Vanti, C. Global Postural Reeducation in patients with chronic nonspecific neck pain: Cross-over analysis of a randomized controlled trial. Med. Lav. 2018, 109, 16–30. [Google Scholar]
- Dupuis, S.; Fortin, C.; Caouette, C.; Leclair, I.; Aubin, C.É. Global postural re-education in pediatric idiopathic scoliosis: A biomechanical modeling and analysis of curve reduction during active and assisted self-correction. BMC Musculoskelet. Disord. 2018, 19, 200. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.E.; Barreto, R.G.; Robinson, C.C.; Plentz, R.D.; Silva, M.F. Global Postural Reeducation for patients with musculoskeletal conditions: A systematic review of randomized controlled trials. Braz. J. Phys. Ther. 2016, 20, 194–205. [Google Scholar] [CrossRef]
- Souchard, P. Rééducation Posturale Globale: RPG—La Méthode; Elsevier Masson: Paris, France, 2011. [Google Scholar]
- Fernández-de-las-Peñas, C.; Alonso-Blanco, C.; Morales-Cabezas, M.; Miangolarra-Page, J.C. Two exercise interventions for the management of patients with ankylosing spondylitis: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2005, 84, 407–419. [Google Scholar] [CrossRef]
- Guastala, F.A.M.; Guerini, M.H.; Klein, P.F.; Leite, V.C.; Cappellazzo, R.; Facci, L.M. Effect of global postural re-education and isostretching in patients with nonspecific chronic low back pain: A randomized clinical trial. Fisioter. Mov. 2016, 29, 515–525. [Google Scholar] [CrossRef]
- do Rosário, J.L.P.; Sousa, A.; Cabral, C.M.N.; João, S.M.A.; Marques, A.P. Global posture reeducation and static muscle stretching on improving flexibility, muscle strength, and range of motion: A comparative study. Fisioter. Pesqui. 2008, 15, 12–18. [Google Scholar]
- Adorno, M.L.; Brasil-Neto, J.P. Assessment of the quality of life through the SF-36 questionnaire in patients with chronic nonspecific low back pain. Acta Ortop. Bras. 2013, 21, 202–207. [Google Scholar] [CrossRef]
- Teodori, R.M.; Negri, J.R.; Cruz, M.C.; Marques, A.P. Global Postural Re-education: A literature review. Rev. Bras. Fisioter. 2011, 15, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, G. Effectiveness of Global Postural Reeducation Compared to Segmental Stretching on Pain, Disability, and QOL of Subjects with Neck and Shoulder Pain. J. Korean Phys. Ther. 2017, 29, 7–15. [Google Scholar] [CrossRef]
- Lomas-Vega, R.; Garrido-Jaut, M.V.; Rus, A.; Del-Pino-Casado, R. Effectiveness of Global Postural Re-education for Treatment of Spinal Disorders: A Meta-analysis. Am. J. Phys. Med. Rehabil. 2017, 96, 124–130. [Google Scholar] [CrossRef] [PubMed]
- De Lima, E.S.R.F.; Vanti, C.; Banchelli, F.; Trani Brandao, J.G.; Oliveira Amorim, J.B.; Villafañe, J.H.; Guccione, A.A.; Pillastrini, P. The effect of Global Postural Reeducation on body weight distribution in sitting posture and on musculoskeletal pain. A pilot study. Med. Lav. 2017, 108, 187–196. [Google Scholar] [CrossRef]
- Vanti, C.; Monticone, M.; Ceron, D.; Bonetti, F.; Piccarreta, R.; Guccione, A.A.; Pillastrini, P. Italian version of the physical therapy patient satisfaction questionnaire: Cross-cultural adaptation and psychometric properties. Phys. Ther. 2013, 93, 911–922. [Google Scholar] [CrossRef]
- Oliveri, M.; Caltagirone, C.; Loriga, R.; Pompa, M.N.; Versace, V.; Souchard, P. Fast increase of motor cortical inhibition following postural changes in healthy subjects. Neurosci. Lett. 2012, 530, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Apuzzo, D.; Giotti, C.; Pasqualetti, P.; Ferrazza, P.; Soldati, P.; Zucco, G.M. An observational retrospective/horizontal study to compare oxygen-ozone therapy and/or global postural re-education in complicated chronic low back pain. Funct. Neurol. 2014, 29, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Lopez, V.; Medina-Porqueres, I. Intervention using global postural reeducation method in patients with musculoskeletal diseases: Systematic review. Phys. Med. Rehabil. Res. 2016, 1, 34–40. [Google Scholar] [CrossRef]
- Pillastrini, P.; de Lima E Sá Resende, F.; Banchelli, F.; Burioli, A.; Di Ciaccio, E.; Guccione, A.A. Effectiveness of Global Postural Re-education in Patients With Chronic Nonspecific Neck Pain: Randomized Controlled Trial. Phys. Ther. 2016, 96, 1408–1416. [Google Scholar] [CrossRef]
- Beer, A.; Treleaven, J.; Jull, G. Can a functional postural exercise improve performance in the cranio-cervical flexion test? a preliminary study. Man. Ther. 2012, 17, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 Explanation and Elaboration: Guidance for Protocols of Clinical Trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar]
- Dworkin, R.H.; Turk, D.C.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Katz, N.P.; Kerns, R.D.; Stucki, G.; Allen, R.R.; Bellamy, N.; et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005, 113, 9–19. [Google Scholar] [CrossRef]
- Cleland, J.A.; Childs, J.D.; Whitman, J.M. Psychometric properties of the Neck Disability Index and numeric pain rating scale in patients with mechanical neck pain. Arch. Phys. Med. Rehabil. 2008, 89, 69–74. [Google Scholar] [CrossRef]
- Jørgensen, R.; Ris, I.; Falla, D.; Juul-Kristensen, B. Reliability, construct and discriminative validity of clinical testing in subjects with and without chronic neck pain. BMC Musculoskelet. Disord. 2014, 15, 408. [Google Scholar] [CrossRef]
- Fischer, A. Algometry in diagnosis of musculoskeletal pain and evaluation of treatment outcome: An update. J. Muscoskel Pain. 1998, 6, 5–32. [Google Scholar] [CrossRef]
- Azevedo, D.C.; de Lima Pires, T.; de Souza Andrade, F.; McDonnell, M.K. Influence of scapular position on the pressure pain threshold of the upper trapezius muscle region. Eur. J. Pain 2008, 12, 226–232. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, V.M.; Alburquerque-Sendín, F.; Bérzin, F.; Stefanelli, V.C.; de Souza, D.P.; Fernández-de-las-Peñas, C. Immediate effects on electromyographic activity and pressure pain thresholds after a cervical manipulation in mechanical neck pain: A randomized controlled trial. J. Manip. Physiol. Ther. 2011, 34, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Walton, D.M.; Macdermid, J.C.; Nielson, W.; Teasell, R.W.; Chiasson, M.; Brown, L. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J. Orthop. Sports Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef]
- Silva, A.G.; Punt, T.D.; Sharples, P.; Vilas-Boas, J.P.; Johnson, M.I. Head Posture and Neck Pain of Chronic Nontraumatic Origin: A Comparison Between Patients and Pain-Free Persons. Arch. Phys. Med. Rehabil. 2009, 90, 669–674. [Google Scholar] [CrossRef]
- Chesterton, L.S.; Sim, J.; Wright, C.C.; Foster, N.E. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin. J. Pain 2007, 23, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Aliaa, R.Y. Photogrammetric quantification of forward head posture is side dependent in healthy participants and patients with mechanical neck pain. Int. J. Physiother. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Oakley, P.A.; Moustafa, I.M.; Haas, J.W.; Betz, J.W.; Harrison, D.E. Two Methods of Forward Head Posture Assessment: Radiography vs. Posture and Their Clinical Comparison. J. Clin. Med. 2024, 13, 2149. [Google Scholar] [CrossRef]
- Cordeiro, N.; Pezarat-Correia, P.; Gil, J.; Cabri, J. Portuguese language version of the Tampa Scale for Kinesiophobia [13 items]. J. Musculoskelet. Pain 2013, 21, 58–63. [Google Scholar] [CrossRef]
- Neblett, R.; Hartzell, M.; Mayer, T.; Bradford, E.; Gatchel, R. Establishing clinically meaningful severity levels for the Tampa Scale for Kinesiophobia (TSK-13). Eur. J. Pain 2016, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Jácome, C.; Cruz, E. Adaptação Cultural e Contributo para a Validação da Pain Catastrophizing Scale (PCS). Bachelor’s Thesis, Escola Superior de Saúde-Instituto Politécnico de Setúbal, Setubal, Portugal, 2004. [Google Scholar]
- Sehn, F.; Chachamovich, E.; Vidor, L.P.; Dall-Agnol, L.; de Souza, I.C.; Torres, I.L.; Fregni, F.; Caumo, W. Cross-cultural adaptation and validation of the Brazilian Portuguese version of the pain catastrophizing scale. Pain Med. 2012, 13, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Bishop, S.; Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Adams, H.; Sullivan, M.E. Communicative dimensions of pain catastrophizing: Social cueing effects on pain behaviour and coping. Pain 2004, 107, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Fernandes, T.; Puente-González, A.S.; Márquez-Vera, M.A.; Vila-Chã, C.; Méndez-Sánchez, R. Effects of Global Postural Reeducation versus Specific Therapeutic Neck Exercises on Pain, Disability, Postural Control, and Neuromuscular Efficiency in Women with Chronic Nonspecific Neck Pain: Study Protocol for a Randomized, Parallel, Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 10704. [Google Scholar] [CrossRef]
- Lozano-Quijada, C.; Poveda-Pagán, E.J.; Segura-Heras, J.V.; Hernández-Sánchez, S.; Prieto-Castelló, M.J. Changes in Postural Sway After a Single Global Postural Reeducation Session in University Students: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2017, 40, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.F.; Antonino, G.B.; Monte-Silva, K.K.D.; Guerino, M.R.; Ferreira, A.P.L.; das Graças Rodrigues de Araújo, M. The ‘Global Postural Re-education’ in non-specific neck and low back pain treatment: A pilot study. J. Back Musculoskelet. Rehabil. 2019, 33, 823–828. [Google Scholar] [CrossRef]
- Cunha, A.C.; Burke, T.N.; França, F.J.; Marques, A.P. Effect of global posture reeducation and of static stretching on pain, range of motion, and quality of life in women with chronic neck pain: A randomized clinical trial. Clinics 2008, 63, 763–770. [Google Scholar] [CrossRef]
- Amorim, C.S.; Gracitelli, M.E.; Marques, A.P.; Alves, V.L. Effectiveness of global postural reeducation compared to segmental exercises on function, pain, and quality of life of patients with scapular dyskinesis associated with neck pain: A preliminary clinical trial. J. Manip. Physiol. Ther. 2014, 37, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Falla, D.; Lindstrøm, R.; Rechter, L.; Boudreau, S.; Petzke, F. Effectiveness of an 8-week exercise programme on pain and specificity of neck muscle activity in patients with chronic neck pain: A randomized controlled study. Eur. J. Pain 2013, 17, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Falla, D.; Jull, G.; Russell, T.; Vicenzino, B.; Hodges, P. Effect of neck exercise on sitting posture in patients with chronic neck pain. Phys. Ther. 2007, 87, 408–417. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, S.; Falla, D.; Hodges, P.W.; Jull, G.; Vicenzino, B. Specific therapeutic exercise of the neck induces immediate local hypoalgesia. J. Pain 2007, 8, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Pool, J.J.M.; Ostelo, R.W.J.G.; Hoving, J.L.; Bouter, L.M.; de Vet, H.C.W. Minimal clinically important change of the neck disability index and the numerical rating scale for patients with neck pain. Spine 2007, 32, 3047–3051. [Google Scholar] [CrossRef]
- Young, I.; Dunning, J.; Butts, R.; Mourad, F.; Cleland, J.A. Reliability, construct validity, and responsiveness of the neck disability index and numeric pain rating scale in patients with mechanical neck pain without upper extremity symptoms. Physiother. Theory Pract. 2018, 35, 1328–1335. [Google Scholar] [CrossRef]
- Russell, I.J. The reliability of algometry in the assessment of patients with fibromyalgia syndrome. J. Musculoskelet. Pain 2010, 6, 139–152. [Google Scholar] [CrossRef]
- Fischer, A.A. Algometry in the daily practice of pain management. J. Back Musculoskelet. Rehabil. 1997, 8, 151–163. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Yarnitsky, D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J. Pain 2009, 10, 556–572. [Google Scholar] [CrossRef]
- Eisenberg, E.; Midbari, A.; Haddad, M.; Pud, D. Predicting the analgesic effect to oxycodone by ‘static’and ‘dynamic’quantitative sensory testing in healthy subjects. Pain 2010, 151, 104–109. [Google Scholar] [CrossRef]
- Marcuzzi, A.; Wrigley, P.J.; Dean, C.M.; Adams, R.; Hush, J.M. The longterm reliability of static and dynamic quantitative sensory testing in healthy individuals. Pain 2017, 158, 1217–1223. [Google Scholar] [CrossRef]
- Walton, D.M.; Levesque, L.; Payne, M.; Schick, J. Clinical pressure pain threshold testing in neck pain: Comparing protocols, responsiveness, and association with psychological variables. Phys. Ther. 2014, 94, 827–837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodríguez-Sanz, J.; Malo-Urriés, M.; Corral-de-Toro, J.; López-de-Celis, C.; Lucha-López, M.O.; Tricás-Moreno, J.M.; Lorente, A.I.; Hidalgo-García, C. Does the Addition of Manual Therapy Approach to a Cervical Exercise Program Improve Clinical Outcomes for Patients with Chronic Neck Pain in Short- and Mid-Term? A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 6601. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Iqbal, A.; Alghadir, A.H. Controlled intervention to compare the efficacies of manual pressure release and the muscle energy technique for treating mechanical neck pain due to upper trapezius trigger points. J. Pain Res. 2018, 11, 3151–3160. [Google Scholar] [CrossRef]
- Bernal-Utrera, C.; Gonzalez-Gerez, J.J.; Anarte-Lazo, E.; Rodriguez-Blanco, C. Manual therapy versus therapeutic exercise in non-specific chronic neck pain: A randomized controlled trial. Trials 2020, 21, 682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Segura, R.; Fernández-de-las-Peñas, C.; Ruiz-Sáez, M.; López-Jiménez, C.; Rodríguez-Blanco, C. Immediate effects on neck pain and active range of motion after a single cervical high-velocity low-amplitude manipulation in subjects presenting with mechanical neck pain: A randomized controlled trial. J. Manip. Physiol. Ther. 2006, 29, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Espí-López, G.V.; Aguilar-Rodríguez, M.; Zarzoso, M.; Serra-Añó, P.; Martínez DE LA Fuente, J.M.; Inglés, M.; Marques-Sule, E. Efficacy of a proprioceptive exercise program in patients with nonspecific neck pain: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2021, 57, 397–405. [Google Scholar] [CrossRef]
- Ylinen, J.; Takala, E.P.; Kautiainen, H.; Nykänen, M.; Häkkinen, A.; Pohjolainen, T.; Karppi, S.L.; Airaksinen, O. Effect of long-term neck muscle training on pressure pain threshold: A randomized controlled trial. Eur. J. Pain 2005, 9, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sacristán, L.; Calvo-Lobo, C.; Pecos-Martín, D.; Fernández-Carnero, J.; Alonso-Pérez, J.L. Dry needling in active or latent trigger point in patients with neck pain: A randomized clinical trial. Sci. Rep. 2022, 12, 3188. [Google Scholar] [CrossRef] [PubMed]
- Ziaeifar, M.; Arab, A.; Mosallanezhad, Z.; Nourbakhsh, M. Dry needling versus trigger point compression of the upper trapezius: A randomized clinical trial with two-week and three-month follow-up. J. Man. Manip. Ther. 2019, 27, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Celenay, S.T.; Akbayrak, T.; Kaya, D.O. A Comparison of the Effects of Stabilization Exercises Plus Manual Therapy to Those of Stabilization Exercises Alone in Patients With Nonspecific Mechanical Neck Pain: A Randomized Clinical Trial. J. Orthop. Sports Phys. Ther. 2016, 46, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Lytras, D.E.; Sykaras, E.I.; Christoulas, K.I.; Myrogiannis, I.S.; Kellis, E. Effects of Exercise and an Integrated Neuromuscular Inhibition Technique Program in the Management of Chronic Mechanical Neck Pain: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43, 100–113. [Google Scholar] [CrossRef]
- Pacheco, J.; Raimundo, J.; Santos, F.; Ferreira, M.; Lopes, T.; Ramos, L.; Silva, A.G. Forward head posture is associated with pressure pain threshold and neck pain duration in university students with subclinical neck pain. Somatosens. Mot. Res. 2018, 35, 103–108. [Google Scholar] [CrossRef]
- Szeto, G.P.Y.; Straker, L.M.; O’Sullivan, P.B. A comparison of symptomatic and asymptomatic office workers performing monotonous keyboard work—1: Neck and shoulder muscle recruitment patterns. Man. Ther. 2005, 10, 270–280. [Google Scholar] [CrossRef]
- Patwardhan, A.G.; Khayatzadeh, S.; Havey, R.M.; Voronov, L.I.; Smith, Z.A.; Kalmanson, O.; Ghanayem, A.J.; Sears, W. Cervical sagittal balance: A biomechanical perspective can help clinical practice. Eur. Spine J. 2018, 27, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Jull, G.; Bullock-Saxton, J.; Darnell, R.; Lander, C. Cervical musculoskeletal impairment in frequent intermittent headache. Part 2: Subjects with concurrent headache types. Cephalalgia 2007, 27, 891–898. [Google Scholar] [CrossRef]
- Miller, M.B.; Roumanis, M.J.; Kakinami, L.; Dover, G.C. Chronic pain patients’ kinesiophobia and catastrophizing are associated with activity intensity at different times of the day. J. Pain Res. 2020, 13, 273. [Google Scholar] [CrossRef]
- Reddy, R.S.; Meziat-Filho, N.; Ferreira, A.S.; Tedla, J.S.; Kandakurti, P.K.; Kakaraparthi, V.N. Comparison of neck extensor muscle endurance and cervical proprioception between asymptomatic individuals and patients with chronic neck pain. J. Bodyw. Mov. Ther. 2020, 26, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kandakurti, P.K.; Reddy, R.S.; Kakarparthy, V.N.; Rengaramanujam, K.; Tedla, J.S.; Dixit, S.; Gautam, A.P.; Silvian, P.; Gular, K.; Eapen, C. Comparison and Association of Neck Extensor Muscles’ Endurance and Postural Function in Subjects with and without Chronic Neck Pain–A Cross-Sectional Study. Phys. Med. Rehabil. Kurortmed. 2021, 31, 295–301. [Google Scholar] [CrossRef]
- Asiri, F.; Reddy, R.S.; Tedla, J.S.; ALMohiza, M.A.; Alshahrani, M.S.; Govindappa, S.C.; Sangadala, D.R. Kinesiophobia and its correlations with pain, proprioception, and functional performance among individuals with chronic neck pain. PLoS ONE 2021, 16, e0254262. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Linton, S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000, 85, 317–332. [Google Scholar] [CrossRef]
- Tejera, D.M.; Beltran-Alacreu, H.; Cano-de-la-Cuerda, R.; Leon Hernández, J.V.; Martín-Pintado-Zugasti, A.; Calvo-Lobo, C.; Gil-Martínez, A.; Fernández-Carnero, J. Effects of Virtual Reality versus Exercise on Pain, Functional, Somatosensory and Psychosocial Outcomes in Patients with Non-specific Chronic Neck Pain: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 5950. [Google Scholar] [CrossRef]
- Peterson, G.E.; Landén Ludvigsson, M.H.; O’Leary, S.P.; Dedering, Å.M.; Wallman, T.; Jönsson, M.I.; Peolsson, A.L. The effect of 3 different exercise approaches on neck muscle endurance, kinesiophobia, exercise compliance, and patient satisfaction in chronic whiplash. J. Manip. Physiol. Ther. 2015, 38, 465–476.e4. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, M.; Goossens, M.E.J.B.; Linton, S.J.; Crombez, G.; Boersma, K.; Vlaeyen, J.W.S. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J. Behav. Med. 2007, 30, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Voogt, L.; de Vries, J.; Meeus, M.; Struyf, F.; Meuffels, D.; Nijs, J. Analgesic effects of manual therapy in patients with musculoskeletal pain: A systematic review. Man. Ther. 2015, 20, 250–256. [Google Scholar] [CrossRef]
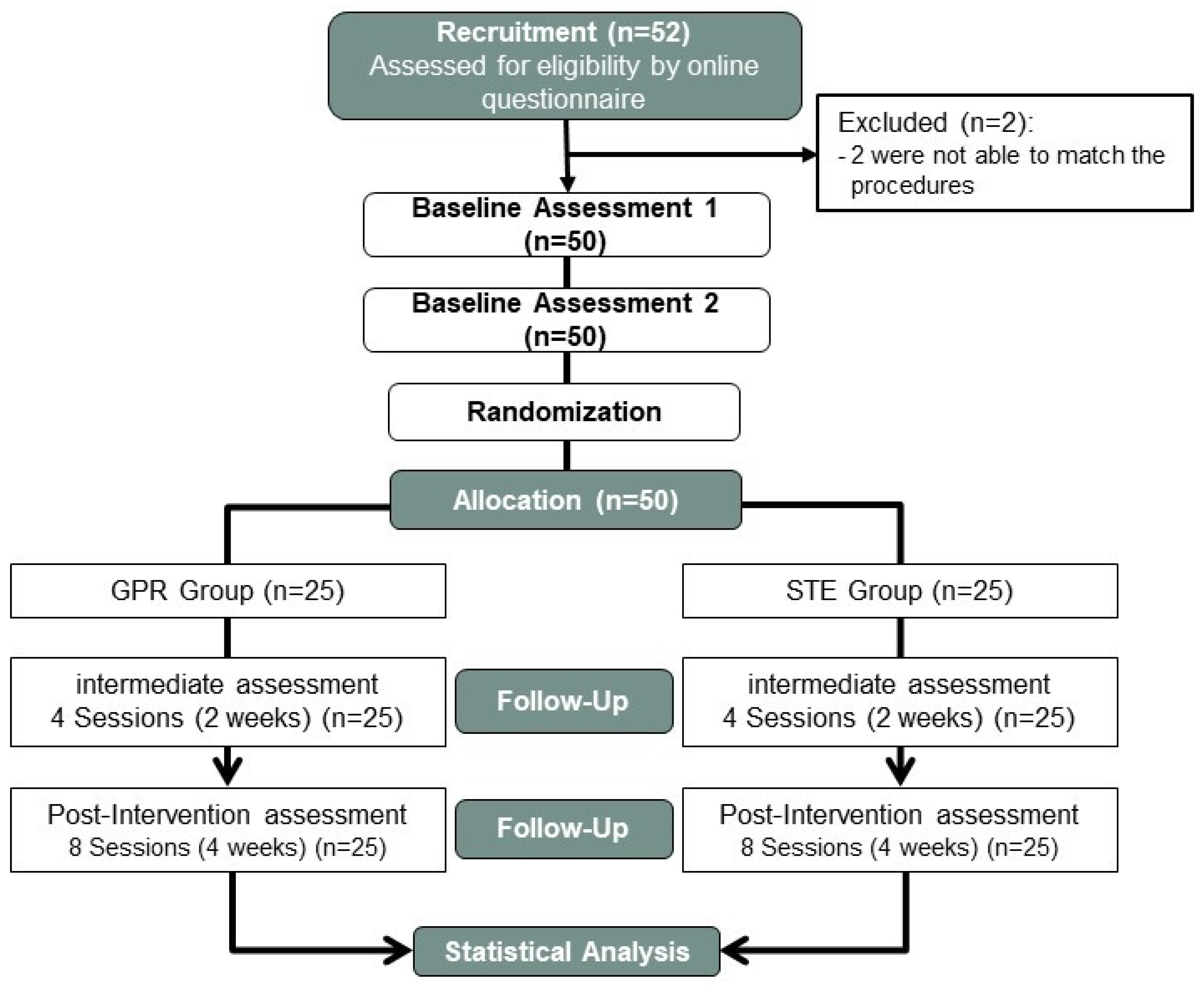
| Variables | Intervention Groups (Mean ± Standard Deviation) | |||
|---|---|---|---|---|
| GPR Group (n = 25) | STE Group (n = 25) | Group Differences GPR-STE (p-Value) | ||
| Age (years) | 47.8 ± 8.9 | 53.8 ± 7.7 | −5.9 | (0.015) * |
| Weight (kg) | 61.8 ± 6.9 | 62.9 ± 10.9 | −1.1 | (0.682) |
| Height (m) | 1.61 ± 0.04 | 1.59 ± 0.06 | 0.01 | (0.418) |
| BMI (kg/m2) | 24 ± 3 | 24.6 ± 4.1 | −0.75 | (0.459) |
| NPRS | 6.2 ± 1.4 | 6 ± 1.7 | 0.1 | (0.783) |
| DNI | 15.5 ± 5.4 | 16.1 ± 5.4 | −0.6 | (0.715) |
| CCA | 47.4 ± 4.5 | 46.9 ± 7 | 0.5 | (0.739) |
| TSK | 26.9 ± 6 | 29.8 ± 7.2 | −2.9 | (0.126) |
| PCS | 21.8 ± 10.1 | 25.6 ± 10.3 | −3.8 | (0.189) |
| PPT upper right Trapezius (kgf) | 1.64 ± 0.75 | 1.75 ± 0.73 | −0.11 | (0.594) |
| PPT upper left Trapezius (kgf) | 1.50 ± 0.60 | 1.44 ± 0.45 | 0.06 | (0.650) |
| PPT right Tibialis Anterior (kgf) | 2.66 ± 1.12 | 2.40 ± 0.88 | 0.26 | (0.360) |
| PPT left Tibialis Anterior (kgf) | 2.28 ± 0.93 | 2.34 ± 0.73 | −0.06 | (0.800) |
| PPT right C2 (kgf) | 1.52 ± 0.43 | 1.56 ± 0.53 | −0.04 | (0.770) |
| PPT left C2 (kgf) | 1.39 ± 0.40 | 1.64 ± 0.53 | −0.25 | (0.066) |
| PPT right C6 (kgf) | 1.59 ± 0.45 | 1.73 ± 0.64 | −0.14 | (0.378) |
| PPT left C6 (kgf) | 1.71 ± 0.68 | 1.74 ± 0.61 | −0.03 | (0.879) |
| Variables | GPR Group 1st–2nd Assessments | STE Group 1st–2nd Assessments | ||
|---|---|---|---|---|
| Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | |
| NPRS | 0.04 (−0.45, 0.53) | 1.000 | −0.04 (−0.53, 0.45) | 1.000 |
| DNI | 1.56 (−0.08, 3.20) | 0.067 | 0.72 (−0.92, 2.36) | 0.632 |
| CCA | 0.32 (−0.86, 1.50) | 0.975 | 0.24 (−0.94, 1.42) | 0.994 |
| TSK | −0.56 (−2.45,1.33) | 0.848 | 0.44 (−1.45, 2.33) | 0.918 |
| PCS | 2.64 (0.22, 5.06) | 0.028 * | 2.12 (−0.30, 4.54) | 0.102 |
| PPT Right Upper Trapezius | 0.37 (0.09, 0.65) | 0.004 † | 0.31 (0.02, 0.60) | 0.030 * |
| PPT Left Upper Trapezius | 0.26 (0.01, 0.51) | 0.041 * | 0.15 (−0.12, 0.40) | 0.540 |
| PPT Right Tibialis Anterior | 0.68 (0.25, 1.11) | 0.000 † | 0.22 (−0.21, 0.66) | 0.650 |
| PPT Left Tibialis Anterior | 0.46 (0.13, 0.79) | 0.003 † | 0.26 (−0.07, 0.59) | 0.207 |
| PPT C2 (Right) | 0.23 (0.05, 0.41) | 0.006 † | 0.19 (0.01, 0.37) | 0.039 * |
| PPT C2 (Left) | 0.15 (−0.01, 0.32) | 0.081 | 0.26 (0.09, 0.42) | 0.001 † |
| PPT C6 (Right) | 0.22 (0.02, 0.42) | 0.026 * | 0.30 (0.10, 0.51) | 0.001 † |
| PPT C6 (Left) | 0.33 (0.09, 0.57) | 0.003 † | 0.33 (0.09,0.58) | 0.003 † |
| ANOVA (Time Factor) | Intra-Group Pairwise Differences (Post Hoc Sidak Test) | |||||||
|---|---|---|---|---|---|---|---|---|
| Location (PPT) | Within-Subject Effects | Group | 2nd–3rd Assessments | 2nd–4th Assessments | 3rd–4th Assessments | |||
| (p-Value) ES (ŋp2) | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | ||
| Right Upper Trapezius | (0.000) (0.493) | GPR | −0.50 (−0.75, −0.26) | 0.000 † | −1.08 (−1.42, −0.74) | 0.000 † | −0.58 (−0.87, −0.29) | 0.000 † |
| STE | −0.37 (−0.62, −0.12) | 0.001 † | −0.862 (−1.21, −0.51) | 0.000 † | −0.49 (−0.79, −0.20) | 0.000 † | ||
| Left Upper Trapezius | (0.000) (0.550) | GPR | −0.43 (−0.65, −0.212) | 0.000 † | −0.90 (−1.20, −0.61) | 0.000 † | −0.47 (−0.76, −0.19) | 0.000 † |
| STE | −0.35 (−0.58, −0.13) | 0.001 † | −0.93 (−1.23, −0.63) | 0.000 † | −0.58 (−0.87, −0.29) | 0.000 † | ||
| Right Tibialis Anterior | (0.000) (0.425) | GPR | −0.70 (−1.11, −0.28) | 0.000 † | −1.18 (−1.60, −0.77) | 0.000 † | −0.49 (−0.86, −0.11) | 0.005 † |
| STE | −0.69 (−1.11, −0.27) | 0.000 † | −1.20 (−1.62, −0.78) | 0.000 † | −0.51 (−0.89, −0.14) | 0.003 † | ||
| Left Tibialis Anterior | (0.000) (0.543) | GPR | −0.81 (−1.17, −0.45) | 0.000 † | −1.44 (−1.87, −1.02) | 0.000 † | −0.63 (−1.02, −0.24) | 0.000 † |
| STE | −0.60 (−0.96, −0.24) | 0.000 † | −1.276 (−1.70, −0.86) | 0.000 † | −0.68 (−1.07, −0.28) | 0.000 † | ||
| C2 (Right) | (0.000) (0.559) | GPR | −0.42 (−0.62, −0.22) | 0.000 † | −0.80 (−1.05, −0.56) | 0.000 † | −0.38 (−0.61, −0.15) | 0.000 † |
| STE | −0.28 (−0.48, −0.08) | 0.002 † | −0.70 (−0.95, −0.46) | 0.000 † | −0.42 (−0.65, −0.20) | 0.000 † | ||
| C2 (Left) | (0.000) (0.527) | GPR | −0.46 (−0.66, −0.27) | 0.000 † | −0.74 (−0.98, −0.51) | 0.000 † | −0.28 (−0.52, −0.04) | 0.016 * |
| STE | −0.36 (−0.56, −0.17) | 0.000 † | −0.58 (−0.82, −0.32) | 0.000 † | −0.22 (−0.46, −0.02) | 0.094 | ||
| C6 (Right) | (0.000) (0.541) | GPR | −0.37 (−0.61, −0.13) | 0.001 † | −0.96 (−1.28, −0.65) | 0.000 † | −0.59 (−0.88, −0.30) | 0.000 † |
| STE | −0.42 (−0.66, −0.18) | 0.000 † | −0.85 (−1.17, −0.53) | 0.000 † | −0.43 (−0.72, −0.14) | 0.001 † | ||
| C6 (Left) | (0.000) (0.492) | GPR | −0.34 (−0.60, −0.09) | 0.003 † | −0.78 (−1.02, −0.532) | 0.000 † | −0.43 (−0.67, −0.20) | 0.000 † |
| STE | −0.41 (−0.66, −0.16) | 0.000 † | −0.88 (−1.12, −0.63) | 0.000 † | −0.46 (−0.70, −0.23) | 0.000 † | ||
| ANOVA (Time Factor) | Intra-Group Pairwise Differences (Post Hoc Sidak Test) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Within-Subject Effects | Group | 2nd–3rd Assessments | 2nd–4th Assessments | 3rd–4th Assessments | |||
| (p-Value) ES (ŋp2) | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | ||
| CCA | (0.000) (0.440) | GPR | −1.5 (−2.7, −0.3) | 0.008 * | −4.4 (−6.2, −2.7) | 0.000 † | −3 (−4.6, −1.3) | 0.000 † |
| STE | −0.8 (−2, 0.3) | 0.299 | −3.2 (−4.9, −1.4) | 0.000 † | −2.3 (−4, −0.7) | 0.002 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, T.; Vila-Chã, C.; Polo-Ferrero, L.; Martín-Vallejo, J.; Puente-González, A.S.; Méndez-Sánchez, R. Effects of Global Postural Re-Education Versus Specific Therapeutic Exercises on Pain, Head Posture, and Pain-Related Psychosocial Factors in Women with Chronic Nonspecific Neck Pain: A Randomized Clinical Trial. J. Clin. Med. 2025, 14, 1581. https://doi.org/10.3390/jcm14051581
Fernandes T, Vila-Chã C, Polo-Ferrero L, Martín-Vallejo J, Puente-González AS, Méndez-Sánchez R. Effects of Global Postural Re-Education Versus Specific Therapeutic Exercises on Pain, Head Posture, and Pain-Related Psychosocial Factors in Women with Chronic Nonspecific Neck Pain: A Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(5):1581. https://doi.org/10.3390/jcm14051581
Chicago/Turabian StyleFernandes, Tânia, Carolina Vila-Chã, Luis Polo-Ferrero, Javier Martín-Vallejo, Ana Silvia Puente-González, and Roberto Méndez-Sánchez. 2025. "Effects of Global Postural Re-Education Versus Specific Therapeutic Exercises on Pain, Head Posture, and Pain-Related Psychosocial Factors in Women with Chronic Nonspecific Neck Pain: A Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 5: 1581. https://doi.org/10.3390/jcm14051581
APA StyleFernandes, T., Vila-Chã, C., Polo-Ferrero, L., Martín-Vallejo, J., Puente-González, A. S., & Méndez-Sánchez, R. (2025). Effects of Global Postural Re-Education Versus Specific Therapeutic Exercises on Pain, Head Posture, and Pain-Related Psychosocial Factors in Women with Chronic Nonspecific Neck Pain: A Randomized Clinical Trial. Journal of Clinical Medicine, 14(5), 1581. https://doi.org/10.3390/jcm14051581






