Aortic Valve Replacement with Rapid-Deployment Bioprostheses: Long-Term Single-Center Results After 1000 Consecutive Implantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Endpoints
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Endpoints
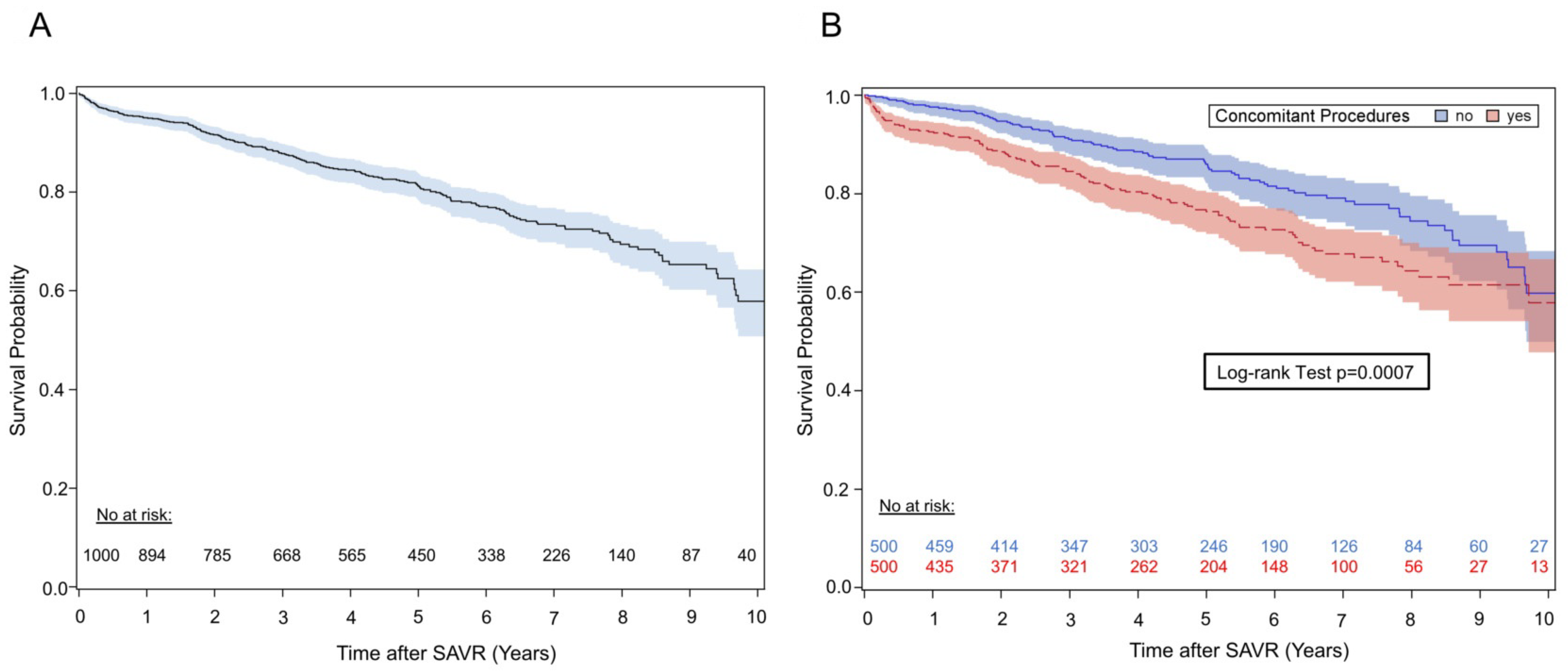
3.2.1. Survival
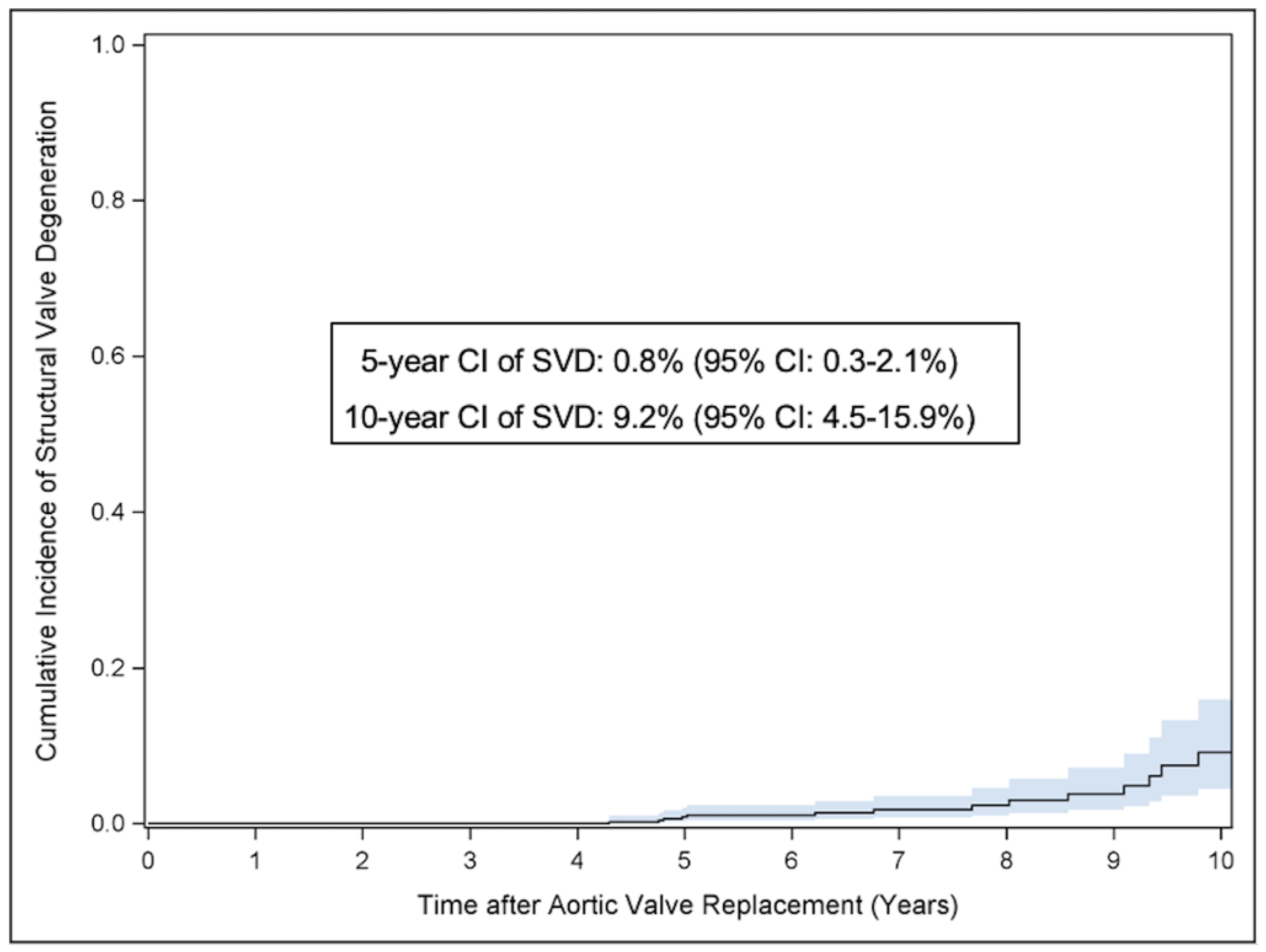
3.2.2. Structural Valve Degeneration
3.3. Secondary Endpoints
3.3.1. Composite Endpoint Reoperation with Valve Explantation
3.3.2. Non-Structural Valve Dysfunction
3.3.3. Valve Endocarditis
3.3.4. Permanent Pacemaker Implantation (PPI)
3.3.5. Composite Endpoint Thromboembolic and Major Bleeding Events
4. Discussion
5. Limitations
6. Conclusions and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Généreux, P.; Sharma, R.P.; Cubeddu, R.J.; Aaron, L.; Abdelfattah, O.M.; Koulogiannis, K.P.; Marcoff, L.; Naguib, M.; Kapadia, S.R.; Makkar, R.R.; et al. The Mortality Burden of Untreated Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 82, 2101–2109. [Google Scholar] [CrossRef]
- Raghav, V.; Okafor, I.; Quach, M.; Dang, L.; Marquez, S.; Yoganathan, A.P. Long-Term Durability of Carpentier-Edwards Magna Ease Valve: A One Billion Cycle In Vitro Study. Ann. Thorac. Surg. 2016, 101, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.R.; Soltesz, E.G.; Vakil, N.; Rajeswaran, J.; Roselli, E.E.; Sabik, J.F., 3rd; Smedira, N.G.; Svensson, L.G.; Lytle, B.W.; Blackstone, E.H. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann. Thorac. Thorac. Surg. 2015, 99, 1239–1247. [Google Scholar] [CrossRef]
- Capelli, C.; Corsini, C.; Biscarini, D.; Ruffini, F.; Migliavacca, F.; Kocher, A. Pledget-Armed Sutures Affect the Haemodynamic Performance of Biologic Aortic Valve Substitutes: A Preliminary Experimental and Computational Study. Cardiovasc. Eng. Technol. 2017, 8, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Coti, I.; Haberl, T.; Scherzer, S.; Werner, P.; Shabanian, S.; Kocher, A.; Laufer, G.; Taylor, A.M.; Schievano, S.; Andreas, M.; et al. Outcome of rapid deployment aortic valves: Long-term experience after 700 implants. Ann. Cardiothorac. Surg. 2020, 9, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Akins, C.W.; Miller, D.C.; Turina, M.I.; Kouchoukos, N.T.; Blackstone, E.H.; Grunkemeier, G.L.; Takkenberg, J.J.; David, T.E.; Butchart, E.G.; Adams, D.H.; et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur. J. Cardio-Thorac. Surg. 2008, 33, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; Treede, H.; Sarano, M.E.; Feldman, T.; Wijeysundera, H.C.; et al. Standardized Definition of Structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. Circulation 2018, 137, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.L.; Flynn, C.D.; Mamo, A.A.; Tian, D.H.; Kappert, U.; Wilbring, M.; Folliguet, T.; Fiore, A.; Miceli, A.; D’onofrio, A.; et al. Long-term outcomes of sutureless and rapid-deployment aortic valve replacement: A systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2020, 9, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Laufer, G.; Haverich, A.; Andreas, M.; Mohr, F.W.; Walther, T.; Shrestha, M.; Rahmanian, P.; Holzhey, D.; Roth, M.; Schmitz, C.; et al. Long-term outcomes of a rapid deployment aortic valve: Data up to 5 years. Eur. J. Cardio-Thorac. Surg. 2017, 52, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kocher, A.A.; Laufer, G.; Haverich, A.; Shrestha, M.; Walther, T.; Misfeld, M.; Kempfert, J.; Gilliam, L.; Schmitz, C.; Wahlers, T.C.; et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: A prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J. Thorac. Cardiovasc. Surg. 2013, 145, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, G.R.; Accola, K.D.; Grossi, E.A.; Woo, Y.J.; Mumtaz, M.A.; Sabik, J.F.; Slachman, F.N.; Patel, H.J.; Borger, M.A.; Garrett, H.E.; et al. TRANSFORM (Multicenter Experience With Rapid Deployment Edwards INTUITY Valve System for Aortic Valve Replacement) US clinical trial: Performance of a rapid deployment aortic valve. J. Thorac. Cardiovasc. Surg. 2017, 153, 241–251.e2. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Glaser, N.; Nilsson, J.; Friberg, Ö.; Franco-Cereceda, A.; Sartipy, U. Comparison of Long-term Performance of Bioprosthetic Aortic Valves in Sweden From 2003 to 2018. JAMA Netw. Open 2022, 5, e220962. [Google Scholar] [CrossRef]
- Yongue, C.; Lopez, D.C.; Soltesz, E.G.; Roselli, E.E.; Bakaeen, F.G.; Gillinov, A.M.; Pettersson, G.B.; Semple, M.E.; Rajeswaran, J.; Tong, M.Z.; et al. Durability and Performance of 2298 Trifecta Aortic Valve Prostheses: A Propensity-Matched Analysis. Ann. Thorac. Surg. 2021, 111, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Coti, I.; Kaider, A.; Gritsch, J.; Mach, M.; Kocher, A.; Laufer, G.; Andreas, M. Long-term durability after surgical aortic valve replacement with the Trifecta and the Intuity valve—A comparative analysis. Eur. J. Cardio-Thorac. Surg. 2021, 61, 416–424. [Google Scholar] [CrossRef]
- Borger, M.A.; Moustafine, V.; Conradi, L.; Knosalla, C.; Richter, M.; Merk, D.R.; Doenst, T.; Hammerschmidt, R.; Treede, H.; Dohmen, P.; et al. A Randomized Multicenter Trial of Minimally Invasive Rapid Deployment Versus Conventional Full Sternotomy Aortic Valve Replacement. Ann. Thorac. Surg. 2015, 99, 17–25. [Google Scholar] [CrossRef]
- Andreas, M.; Berretta, P.; Solinas, M.; Santarpino, G.; Kappert, U.; Fiore, A.; Glauber, M.; Misfeld, M.; Savini, C.; Mikus, E.; et al. Minimally invasive access type related to outcomes of sutureless and rapid deployment valves. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020, 58, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, S.; Fujita, B.; Bauer, T.; Möllmann, H.; Beckmann, A.; Bekeredjian, R.; Bleiziffer, S.; Landwehr, S.; Hamm, C.W.; Mohr, F.W.; et al. Rapid Deployment Versus Conventional Bioprosthetic Valve Replacement for Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Coti, I.; Schukro, C.; Drevinja, F.; Haberl, T.; Kaider, A.; Kocher, A.; Laufer, G.; Andreas, M. Conduction disturbances following surgical aortic valve replacement with a rapid-deployment bioprosthesis. J. Thorac. Cardiovasc. Surg. 2020, 162, 803–811. [Google Scholar] [CrossRef]
- Paolisso, P.; Belmonte, M.; Gallinoro, E.; Scarsini, R.; Bergamaschi, L.; Portolan, L.; Armillotta, M.; Esposito, G.; Moscarella, E.; Benfari, G.; et al. SGLT2-inhibitors in diabetic patients with severe aortic stenosis and cardiac damage undergoing transcatheter aortic valve implantation (TAVI). Cardiovasc. Diabetol. 2024, 23, 420. [Google Scholar] [CrossRef]

| Preoperative Patient Characteristics | N = 1000 |
|---|---|
| Age (years, SD) | 73.4 ± 7.2 |
| Male (n, %) | 548 (54.8) |
| BMI (SD) kg/m2 | 28.1 ± 5.0 |
| BSA (SD) m2 | 1.9 ± 0.2 |
| EuroSCORE II (%), IQR | 2.7 (1.4–5.5) |
| STS Score (%), IQR | 1.9 (1.3–3.1) |
| NYHA Class III/IV (n, %) | 680 (68) |
| Arterial hypertension (n, %) | 876 (87.6) |
| Dyslipidemia (n, %) | 664 (66.4) |
| Creatinine (mg/dl, IQR) | 1.0 (0.8–1.1) |
| Coronary artery disease (n, %) | 444 (44.4) |
| Cerebrovascular disease (n, %) | 189 (18.9) |
| Peripheral vascular disease (n, %) | 86 (8.6) |
| Chronic lung disease (n, %) | 178 (17.8) |
| Prior atrial fibrillation (n, %) | 203 (20.3) |
| Previous cardiac surgery (n, %) | 40 (4) |
| LVEF (%, SD) | 57.6 ± 10.6 |
| Mean transvalvular gradient (mmHg, SD) | 52.6 ± 18.1 |
| Peak transvalvular gradient (mmHg, SD) | 85.2 ± 29.0 |
| Effective orifice area (cm2) | 0.73 ± 0.22 |
| Variables | N = 1000 |
|---|---|
| Elective procedure (n, %) | 911 (91.1) |
| Concomitant procedures (n, %) | 500 (50.0%) |
| Aortic surgery (n, %) | 67 (6.7) |
| CABG (n, %) | 319 (31.9) |
| Mitral valve surgery (n, %) | 64 (6.4) |
| Tricuspid valve surgery (n, %) | 41 (4.1) |
| Atrial fibrillation surgery (n, %) | 52 (5.2) |
| Access (n, %) - Full sternotomy - Upper sternotomy - Thoracotomy | 515 (51.5) 252 (25.2) 233 (23.3) |
| MIS isolated AVR (n, %) | 415 (83) |
| CPB time (min, IQR) | 111 (91–140) |
| XCT time (min, IQR) | 77 (60–98) |
| >1 valve positioning attempt (n, %) | 23 (2.3) |
| Revision for bleeding (n, %) | 91 (9.1) |
| ECMO (n, %) | 14 (1.4) |
| Dialysis (n, %) | 18 (1.8) |
| Early PPI (14-days), (n, %) | 91 (9.1) |
| Perioperative AF (n, %) | 257 (25.7) |
| Perioperative neurological event (<72 h) (n, %) | 16 (1.6) |
| Wound infections (n, %) | 27 (2.7) |
| Univariate Analyses | Multivariable Analysis | |||
|---|---|---|---|---|
| Prognostic Factors | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Gender (male) | 1.12 (0.86–1.47) | 0.401 | 1.07 (0.74–1.54) | 0.733 |
| Age (years) * | 1.98 (1.61–2.43) | <0.001 | 1.91 (1.54–2.36) | <0.001 |
| Arterial hypertension | 1.17 (0.77–1.77) | 0.467 | 1.07 (0.69–1.68) | 0.757 |
| Dyslipidemia | 0.96 (0.72–1.26) | 0.749 | 0.86 (0.64–1.61) | 0.325 |
| Diabetis mellitus | 1.42 (1.07–1.89) | 0.016 | 1.35 (1.00–1.83) | 0.049 |
| COPD | 1.47 (1.08–2.00) | 0.013 | 1.48 (1.08–2.02) | 0.015 |
| Atrial fibrillation | 1.50 (1.10–2.05) | 0.010 | 1.12 (0.81–1.55) | 0.498 |
| Concomitant procedures | <0.001 | 0.007 | ||
| 3.36 (1.83–6.17) | 2.68 (1.44–4.99) | ||
| 1.18 (0.84–1.64) | 1.06 (0.75–1.50) | ||
| Elective procedures | <0.001 | <0.001 | ||
| 4.79 (2.81–8.15) | 3.38 (1.88–6.07) | ||
| 1.54 (0.94–2.54) | 1.23 (0.72–2.11) | ||
| BMI | 0.97 (0.94–1.00) | 0.049 | 1.01 (0.97–1.05) | 0.765 |
| BSA | 0.56 (0.29–1.08) | 0.084 | 0.47 (0.16–1.42) | 0.181 |
| Log2 creatinine ** | 1.94 (1.64–2.31) | <0.001 | 1.89 (1.54–2.34) | <0.001 |
| LVEF * | 0.76 (0.67–0.85) | <0.001 | 0.89 (0.78–1.01) | 0.070 |
| NYHA | 1.26 (1.00–1.58) | 0.046 | 0.90 (0.71–1.14) | 0.381 |
| Variables | N = 1000 CI at 5 Years | N = 1000 CI at 10 Years |
|---|---|---|
| Severe SVD (95% CI) | 0.8% (0.3 to 2.1%) | 9.2% (4.5 to 15.9%) |
| Re-operation due to SVD (95% CI) | 0.6% (0.2 to 1.7%) | 3.6% (1.6 to 7.0%) |
| Re-operation due to NSVD (95% CI) | 1.8% (1.1 to 2.8%) | 2.4% (1.4 to 3.9%) |
| Prosthesis endocarditis (95% CI) | 1.8% (1.0 to 2.9%) | 2.9% (1.7 to 4.6%) |
| Re-operation due to endocarditis (95% CI) | 1.0% (0.5 to 2.0%) | 1.7% (0.8 to 3.1%) |
| Composite aortic valve re-op (95% CI) | 3.5% (2.4 to 5.0%) | 7.7% (5.0 to 11.2%) |
| Pacemaker implantation (95% CI) | 13.4 (11.2 to 15.7%) | 15.4% (12.5 to 18.6%) |
| Composite thromboembolic– major bleeding event (95% CI) | 7.1% (5.5 to 9.0%) | 8.1% (6.2 to 10.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coti, I.; Werner, P.; Kaider, A.; El-Nashar, J.; Kocher, A.; Laufer, G.; Zimpfer, D.; Andreas, M. Aortic Valve Replacement with Rapid-Deployment Bioprostheses: Long-Term Single-Center Results After 1000 Consecutive Implantations. J. Clin. Med. 2025, 14, 1552. https://doi.org/10.3390/jcm14051552
Coti I, Werner P, Kaider A, El-Nashar J, Kocher A, Laufer G, Zimpfer D, Andreas M. Aortic Valve Replacement with Rapid-Deployment Bioprostheses: Long-Term Single-Center Results After 1000 Consecutive Implantations. Journal of Clinical Medicine. 2025; 14(5):1552. https://doi.org/10.3390/jcm14051552
Chicago/Turabian StyleCoti, Iuliana, Paul Werner, Alexandra Kaider, Jasmine El-Nashar, Alfred Kocher, Guenther Laufer, Daniel Zimpfer, and Martin Andreas. 2025. "Aortic Valve Replacement with Rapid-Deployment Bioprostheses: Long-Term Single-Center Results After 1000 Consecutive Implantations" Journal of Clinical Medicine 14, no. 5: 1552. https://doi.org/10.3390/jcm14051552
APA StyleCoti, I., Werner, P., Kaider, A., El-Nashar, J., Kocher, A., Laufer, G., Zimpfer, D., & Andreas, M. (2025). Aortic Valve Replacement with Rapid-Deployment Bioprostheses: Long-Term Single-Center Results After 1000 Consecutive Implantations. Journal of Clinical Medicine, 14(5), 1552. https://doi.org/10.3390/jcm14051552








