Association Between Contrast Sensitivity and Ganglion Cell–Inner Plexiform Layer Thickness After Resolution of Macular Edema Due to Branch Retinal Vein Occlusion
Abstract
1. Introduction
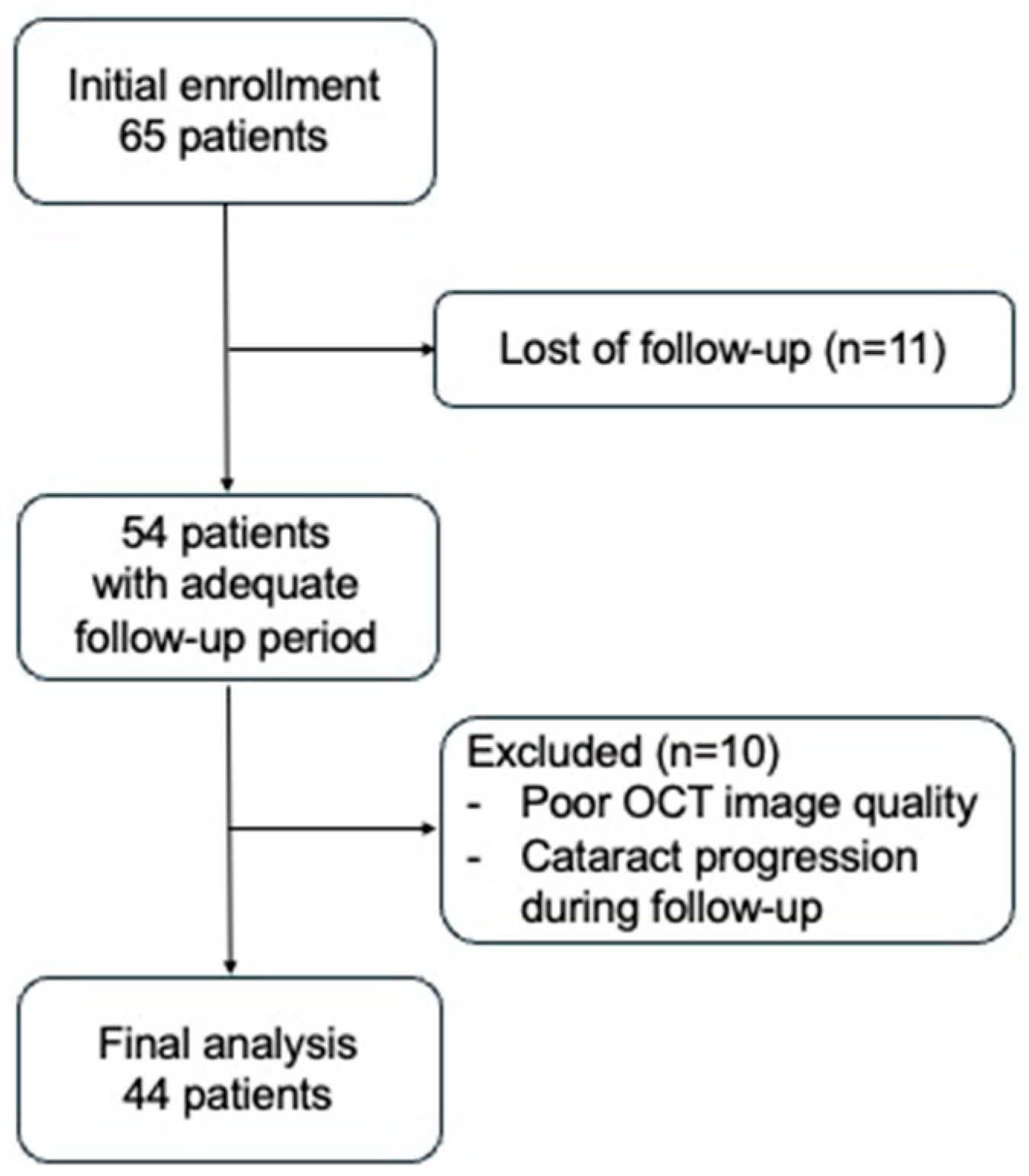
2. Materials and Methods
2.1. Assessments
2.2. Treatment
2.3. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaulim, A.; Ahmed, B.; Khanam, T.; Chatziralli, I.P. Branch retinal vein occlusion: Epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina 2013, 33, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.L.; McIntosh, R.L.; Lim, L.; Mitchell, P.; Cheung, N.; Kowalski, J.W.; Nguyen, H.P.; Wang, J.J.; Wong, T.Y. Natural history of branch retinal vein occlusion: An evidence-based systematic review. Ophthalmology 2010, 117, 1094–1101.e1095. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Brown, D.M.; Awh, C.C.; Lee, S.Y.; Gray, S.; Saroj, N.; Murahashi, W.Y.; Rubio, R.G. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a phase III study. Ophthalmology 2011, 118, 2041–2049. [Google Scholar] [CrossRef]
- Clark, W.L.; Boyer, D.S.; Heier, J.S.; Brown, D.M.; Haller, J.A.; Vitti, R.; Kazmi, H.; Berliner, A.J.; Erickson, K.; Chu, K.W.; et al. Intravitreal Aflibercept for Macular Edema Following Branch Retinal Vein Occlusion: 52-Week Results of the VIBRANT Study. Ophthalmology 2016, 123, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Owsley, C.; Sloane, M.E. Contrast sensitivity, acuity, and the perception of ‘real-world’ targets. Br. J. Ophthalmol. 1987, 71, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.F.; Kasetty, M.; Vingopoulos, F.; Katz, R.; Cho, J.; Lesmes, L.A.; Zacks, D.N.; Kim, L.A.; Miller, J.B. Measuring Contrast Sensitivity Function with Active Learning in Retinal Vein Occlusion: A New Endpoint of Visual Function. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Nixon, D.R.; Flinn, N. Visual Function for Driving in Diabetic Macular Edema and Retinal Vein Occlusion Post-Stabilization with Anti-Vascular Endothelial Growth Factor. Clin. Ophthalmol. 2021, 15, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Okamoto, F.; Murakami, T.; Morikawa, S.; Hiraoka, T.; Oshika, T. Time course of changes in contrast sensitivity following intravitreal ranibizumab injection for branch retinal vein occlusion. Jpn. J. Ophthalmol. 2020, 64, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, Y.; Vingopoulos, F.; Kasetty, M.; Silverman, R.F.; Katz, R.; Kim, L.; Miller, J.B. Disorganisation of retinal inner layers is associated with reduced contrast sensitivity in retinal vein occlusion. Br. J. Ophthalmol. 2022, 106, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Maganti, N.; Squires, N.; Bomdica, P.; Nigam, D.; Shapiro, A.; Gill, M.K.; Lyon, A.T.; Mirza, R.G. Contrast Sensitivity Testing in Retinal Vein Occlusion Using a Novel Stimulus. Transl. Vis. Sci. Technol. 2020, 9, 29. [Google Scholar] [CrossRef]
- Pece, A.; Isola, V.; Piermarocchi, S.; Calori, G. Efficacy and safety of anti-vascular endothelial growth factor (VEGF) therapy with intravitreal ranibizumab (Lucentis) for naive retinal vein occlusion: 1-year follow-up. Br. J. Ophthalmol. 2011, 95, 56–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Preti, R.C.; Ramirez, L.M.; Pimentel, S.L.; Nakashima, Y.; Machado, C.G.; Pelayes, D.E.; Monteiro, M.L.; Takahashi, W.Y. Effect of a single intravitreal bevacizumab injection on contrast sensitivity and macular thickness in eyes with macular edema from central retinal vein occlusion: A prospective, nonrandomized, three-month follow-up study. Ophthalmic Res. 2014, 51, 140–145. [Google Scholar] [CrossRef]
- Murakami, T.; Okamoto, F.; Sugiura, Y.; Morikawa, S.; Okamoto, Y.; Hiraoka, T.; Oshika, T. Contrast sensitivity and quality of life following intravitreal ranibizumab injection for central retinal vein occlusion. Br. J. Ophthalmol. 2023, 107, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.; Vermeer, K.A.; van Meurs, J.C.; La Heij, E.C. Visual Acuity Inadequately Reflects Vision-Related Quality of Life in Patients After Macula-Off Retinal Detachment Surgery. Investig. Ophthalmol. Vis. Sci. 2020, 61, 34. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, F.; Okamoto, Y.; Fukuda, S.; Hiraoka, T.; Oshika, T. Vision-related quality of life and visual function after vitrectomy for various vitreoretinal disorders. Investig. Ophthalmol. Vis. Sci. 2010, 51, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Kal, M.; Brzdek, M.; Zarebska-Michaluk, D.; Pinna, A.; Mackiewicz, J.; Odrobina, D.; Winiarczyk, M.; Karska-Basta, I. Optical Coherence Tomography Angiography Assessment of the Optic Nerve Head in Patients Hospitalized due to COVID-19 Bilateral Pneumonia. Medicina 2024, 60, 502. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.P.; Jang, H.; Kim, J.; Kang, S.H.; Kim, J.S.; Lee, J.; Jung, Y.H.; Na, D.L.; Seo, S.W.; et al. Optical coherence tomography angiography as a potential screening tool for cerebral small vessel diseases. Alzheimers Res. Ther. 2020, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, R.A.; Barteselli, G.; You, Q.; Goud, A.; Jabeen, A.; Rao, H.L.; Jabeen, A.; Chhablani, J. In vivo evaluation of retinal ganglion cells degeneration in eyes with branch retinal vein occlusion. Br. J. Ophthalmol. 2016, 100, 1506–1510. [Google Scholar] [CrossRef]
- Fatehi, N.; Nowroozizadeh, S.; Henry, S.; Coleman, A.L.; Caprioli, J.; Nouri-Mahdavi, K. Association of Structural and Functional Measures with Contrast Sensitivity in Glaucoma. Am. J. Ophthalmol. 2017, 178, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Cheng, H.; Tang, R.A.; Frishman, L.J. Multifocal visual evoked potentials and contrast sensitivity correlate with ganglion cell-inner plexiform layer thickness in multiple sclerosis. Clin. Neurophysiol. 2019, 130, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Satue, M.; Rodrigo, M.J.; Otin, S.; Bambo, M.P.; Fuertes, M.I.; Ara, J.R.; Martin, J.; Polo, V.; Larrosa, J.M.; Pablo, L.; et al. Relationship between Visual Dysfunction and Retinal Changes in Patients with Multiple Sclerosis. PLoS ONE 2016, 11, e0157293. [Google Scholar] [CrossRef]
- Shamsi, F.; Liu, R.; Owsley, C.; Kwon, M. Identifying the Retinal Layers Linked to Human Contrast Sensitivity via Deep Learning. Investig. Ophthalmol. Vis. Sci. 2022, 63, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gu, X.; Song, S.; Yu, X.; Zhang, P.; Dai, H. Structural and Visual Changes in Branch Retinal Vein Occlusion Patients with Retinal Atrophy. J. Ophthalmol. 2022, 2022, 8945467. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Tsujikawa, A.; Ota, M.; Yamaike, N.; Kotera, Y.; Miyamoto, K.; Kita, M.; Yoshimura, N. Evaluation of potential visual acuity in eyes with macular oedema secondary to retinal vein occlusion. Clin. Exp. Ophthalmol. 2009, 37, 208–216. [Google Scholar] [CrossRef]
- Chylack, L.T., Jr.; Padhye, N.; Khu, P.M.; Wehner, C.; Wolfe, J.; McCarthy, D.; Rosner, B.; Friend, J. Loss of contrast sensitivity in diabetic patients with LOCS II classified cataracts. Br. J. Ophthalmol. 1993, 77, 7–11. [Google Scholar] [CrossRef][Green Version]
- Iijima, H. Mechanisms of vision loss in eyes with macular edema associated with retinal vein occlusion. Jpn. J. Ophthalmol. 2018, 62, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Lee, W.H.; Ryu, C.K.; Kim, T.Y.; Lim, H.B.; Lee, Y.H.; Kim, J.Y. Effects of Prolonged Type 2 Diabetes on the Inner Retinal Layer and Macular Microvasculature: An Optical Coherence Tomography Angiography Study. J. Clin. Med. 2020, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, M.; Hasegawa, T.; Ikeda, H.O.; Inoue, Y.; Iwai, S.; Iida, K.; Tsujikawa, A. Involvement of endothelins in neuroprotection of valosin-containing protein modulators against retinal ganglion cell damage. Sci. Rep. 2022, 12, 16156. [Google Scholar] [CrossRef]
| Baseline | At the Time the ME Was Resolved | p | |
|---|---|---|---|
| Age, years | 66.4 ± 10.9 | ||
| Sex (male/female) | 20/24 | ||
| Lens status (clear lens/mild cataract/IOL) | 11/28/5 | ||
| AULCSF | 0.75 ± 0.22 | 1.13 ± 0.22 | <0.001 * |
| BCVA (logMAR) | 0.39 ± 0.29 | 0.03 ± 0.13 | <0.001 * |
| CFT (µm) | 518 ± 210 | 190 ± 36 | <0.001 * |
| Figure | Bivariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| Number | Mean ± SD | p | β | 95% Confidence Interval (Lower, Upper) | p | |
| ELM disruption (+) | 8 | 1.08 ± 0.18 | 0.514 | |||
| (−) | 36 | 1.14 ± 0.23 | ||||
| EZ disruption (+) | 20 | 1.09 ± 0.20 | 0.098 | |||
| (−) | 24 | 1.17 ± 0.23 | ||||
| Clear lens | 11 | 1.28 ± 0.16 | 0.010 * | Reference | Reference | |
| Mild cataract | 28 | 1.06 ± 0.23 | −0.182 | −0.286, −0.078 | 0.001 ‡‡ | |
| IOL | 5 | 1.09 ± 0.05 | ||||
| r | p value | |||||
| GCIPL thickness | 0.500 | <0.001 †† | 0.008 | 0.002, 0.014 | 0.011 ‡ | |
| CFT | 0.479 | <0.001 †† | 0.003 | 0.001, 0.004 | 0.001 ‡‡ | |
| Age | −0.346 | 0.021 † | ||||
| Bivariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Number | Mean ± SD | p | β | 95% Confidence Interval (Lower, Upper) | p | |
| ELM disruption (+) | 8 | 0.10 ± 0.13 | 0.093 | |||
| (−) | 36 | 0.01 ± 0.13 | ||||
| EZ disruption (+) | 20 | 0.08 ± 0.11 | 0.014 * | |||
| (−) | 24 | −0.02 ± 0.14 | ||||
| Clear lens | 11 | −0.01 ± 0.13 | 0.243 | Reference | Reference | |
| Mild cataract | 28 | 0.05 ± 0.14 | 0.079 | 0.008, 0.150 | 0.029 ‡ | |
| IOL | 5 | −0.03 ± 0.11 | ||||
| r | p | |||||
| GCIPL thickness | −0.427 | 0.004 † | ||||
| CFT | −0.483 | <0.001 †† | −0.002 | −0.003, −0.001 | <0.001 ‡‡ | |
| Age | 0.214 | 0.162 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, T.; Okamoto, F.; Matsueda, T.; Sugiura, Y.; Morikawa, S.; Okamoto, Y.; Hiraoka, T.; Oshika, T. Association Between Contrast Sensitivity and Ganglion Cell–Inner Plexiform Layer Thickness After Resolution of Macular Edema Due to Branch Retinal Vein Occlusion. J. Clin. Med. 2025, 14, 1507. https://doi.org/10.3390/jcm14051507
Murakami T, Okamoto F, Matsueda T, Sugiura Y, Morikawa S, Okamoto Y, Hiraoka T, Oshika T. Association Between Contrast Sensitivity and Ganglion Cell–Inner Plexiform Layer Thickness After Resolution of Macular Edema Due to Branch Retinal Vein Occlusion. Journal of Clinical Medicine. 2025; 14(5):1507. https://doi.org/10.3390/jcm14051507
Chicago/Turabian StyleMurakami, Tomoya, Fumiki Okamoto, Takeshi Matsueda, Yoshimi Sugiura, Shohei Morikawa, Yoshifumi Okamoto, Takahiro Hiraoka, and Tetsuro Oshika. 2025. "Association Between Contrast Sensitivity and Ganglion Cell–Inner Plexiform Layer Thickness After Resolution of Macular Edema Due to Branch Retinal Vein Occlusion" Journal of Clinical Medicine 14, no. 5: 1507. https://doi.org/10.3390/jcm14051507
APA StyleMurakami, T., Okamoto, F., Matsueda, T., Sugiura, Y., Morikawa, S., Okamoto, Y., Hiraoka, T., & Oshika, T. (2025). Association Between Contrast Sensitivity and Ganglion Cell–Inner Plexiform Layer Thickness After Resolution of Macular Edema Due to Branch Retinal Vein Occlusion. Journal of Clinical Medicine, 14(5), 1507. https://doi.org/10.3390/jcm14051507







