Effect of Homoarginine on Coronary Artery Complexity and Atherosclerotic Burden in Patients with STEMI
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Coronary Intervention
2.2. SYNTAX Score and Angiographic Analysis
2.3. Homoarginine Detection and Sample Preparation Method
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SYNTAX | The SYNergy between percutaneous coronary intervention (PCI) with TAXUS and cardiac surgery |
| CAD | Coronary artery disease |
| STEMI | ST-segment elevation myocardial infarction |
| NO | Nitric oxide |
| ACS | Acute coronary syndrome |
| MI | Myocardial infarction |
| SS | SYNTAX score |
| CABG | Coronary artery by-pass grafting |
| CVD | Cardiovascular disease |
| MACE | Major adverse cardiac events |
| COPD | Chronic obstructive pulmonary disease |
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The forgotten majority: Unfinished business in cardiovascular risk reduction. J. Am. Coll. Cardiol. 2005, 46, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Meinitzer, A.; Tomaschitz, A.; Drechsler, C.; Ritz, E.; Krane, V.; Wanner, C.; Boehm, B.O.; März, W. Low homoarginine concentration is a novel risk factor for heart disease. Heart 2011, 97, 1222–1227. [Google Scholar] [CrossRef]
- Henningsson, R.; Lundquist, I. Arginine-induced insulin release is decreased and glucagon increased in parallel with islet NO production. Am. J. Physiol. 1998, 275, E500e6. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. USA 1990, 87, 5193–5197. [Google Scholar] [CrossRef]
- Karetnikova, E.S.; Jarzebska, N.; Markov, A.G.; Weiss, N.; Lentz, S.R.; Rodionov, R.N. Is Homoarginine a Protective Cardiovascular Risk Factor? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Paliogiannis, P.; Carru, C.; Mangoni, A.A. Homoarginine and All-Cause Mortality: A Systematic Review and Meta-Analysis. Eur. J. Clin. Investig. 2018, 48, e12960. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.U.; Atzler, D.; Wild, P.S.; Carter, A.M.; Böger, R.H.; Ojeda, F.; Simova, O.; Stockebrand, M.; Lackner, K.; Nabuurs, C.; et al. Homoarginine levels are regulated by L-arginine: Glycine amidinotransferase and affect stroke outcome: Results from human and murine studies. Circulation 2013, 128, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Baum, C.; Ojeda, F.; Keller, T.; Cordts, K.; Schnabel, R.B.; Choe, C.U.; Lackner, K.J.; Münzel, T.; Böger, R.H.; et al. Low Homoarginine Levels in the Prognosis of Patients With Acute Chest Pain. J. Am. Heart Assoc. 2016, 5, e002565. [Google Scholar] [CrossRef]
- Atzler, D.; Rosenberg, M.; Anderssohn, M.; Choe, C.U.; Lutz, M.; Zugck, C.; Böger, R.H.; Frey, N.; Schwedhelm, E. Homoarginine—An independent marker of mortality in heart failure. Int. J. Cardiol. 2013, 168, 4907–4909. [Google Scholar] [CrossRef] [PubMed]
- Sianos, G.; Morel, M.-A.; Kappetein, A.P.; Morice, M.-C.; Colombo, A.; Dawkins, K.; Brand, M.V.D.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005, 1, 219–227. [Google Scholar] [PubMed]
- Kaya, A.; Keskin, M.; Tatlisu, M.A.; Cinar, T. Association of SYNTAX score with abdom inal aortic intima–media thickness in non-ST elevation myocardial infarction. Angiology 2018, 70, 569–570. [Google Scholar] [CrossRef]
- Biondi-Zoccai, G.; Lotrionte, M.; Sheiban, I. Management of multivessel coronary disease after ST-elevation myocardial infarction treated by primary coronary angioplasty. Am. Heart J. 2010, 160 (Suppl. 6), S28–S35. [Google Scholar] [CrossRef]
- Brown, A.J.; McCormick, L.M.; Gajendragadkar, P.R.; Hoole, S.P.; West, N.E. Initial SYNTAX score predicts major adverse cardiac events after primary percutaneous coronary intervention. Angiology 2013, 65, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Tada, T.; Fusaro, M.; Cassese, S.; King, L.; Hadamitzky, M.; Haase, H.-U.; Schömig, A.; Kastrati, A.; Pache, J. Association of coronary atherosclerotic burden with clinical presentation and prognosis in patients with stable and unstable coronary artery disease. Clin. Res. Cardiol. 2012, 101, 1003–1011. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, J.L.; Du, H.; Lan, X.B.; Yin, Y.H. Coronary score adds prognostic information for patients with acute coronary syndrome. Circ. J. 2010, 74, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Sinning, C.; Lillpopp, L.; Appelbaum, S.; Ojeda, F.; Zeller, T.; Schnabel, R.; Lubos, E.; Jagodzinski, A.; Keller, T.; Munzel, T.; et al. Angiographic score assessment improves car- diovascular risk prediction: The clinical value of SYNTAX and Gensini application. Clin. Res. Cardiol. 2013, 102, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Hsieh, M.J.; Chen, C.C.; Chang, S.H.; Wang, C.Y.; Lee, C.H.; Hsieh, I.C. SYNTAX score: An independent predictor of long-term cardiac mortality in patients with acute ST-elevation myocardial infarction. Coron. Artery Dis. 2012, 23, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.K.; Gibson, C.M. The SYNTAX score: Usefulness, limitations, and future directions. J. Invasive Cardiol. 2011, 23, 511–512. [Google Scholar] [PubMed]
- Girasis, C.; Garg, S.; Räber, L.; Sarno, G.; Morel, M.-A.; Garcia-Garcia, H.M.; Lüscher, T.F.; Serruys, P.W.; Windecker, S. SYNTAX score and Clinical SYNTAX score as predictors of very long-term clinical outcomes in patients undergoing percutaneous coronary interventions: A substudy of SIRolimus-eluting stent compared with pacliTAXel-eluting stent for coronary revascularization (SIRTAX) trial. Eur. Heart J. 2011, 32, 3115–3127. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sarno, G.; Serruys, P.W.; Rodriguez, A.E.; Bolognese, L.; Anselmi, M.; De Cesare, N.; Colangelo, S.; Moreno, R.; Gambetti, S.; et al. STRATEGY and MULTISTRATEGY Investigators. Prediction of 1-year clinical outcomes using the SYNTAX score in patients with acute ST-segment elevation myocardial infarction undergoing primary percuta- neous coronary intervention: A substudy of the STRATEGY (single high-dose bolus tirofiban and sirolimus-eluting stent versus abciximab and bare-metal stent in acute myocardial infarction) and MULTISTRATEGY (multicenter evaluation of single high-dose bolus tirofiban versus abciximab with sirolimus-eluting stent or bare-metal stent in acute myocardial infarction study) trials. JACC Cardiovasc. Interv. 2011, 4, 66–75. [Google Scholar]
- Casiglia, E.; Tikhonoff, V. Inflammatory and coagulative markers of atherosclerosis. Eur. Heart J. 2007, 28, 271–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Incalcaterra, E.; Accardi, G.; Balistreri, C.R.; Caimi, G.; Candore, G.; Caruso, M.; Caruso, C. Pro-inflammatory genetic markers of atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 329. [Google Scholar] [CrossRef] [PubMed]
- Marz, W.; Meinitzer, A.; Drechsler, C.; Pilz, S.; Krane, V.; Kleber, M.E.; Fischer, J.; Winkelmann, B.R.; Bohm, B.O.; Ritz, E.; et al. Homoarginine, cardiovascular risk, and mortality. Circulation 2010, 122, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, J.; Widder, J.D. Endothelial dysfunction in heart failure. Pharmacol. Rep. 2008, 60, 119–126. [Google Scholar] [PubMed]
- Michel, T. R is for arginine: Metabolism of arginine takes off again, in new directions. Circulation 2013, 128, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Isenovic, E.R.; Soskic, S.; Dungen, H.-D.; Dobutovic, B.; Elvis, T.; Simone, I.; Marche, P. Regulation of endothelial nitric oxide synthase in pathophysiological conditions. Cardiovasc. Hematol. Disord. Targets 2011, 11, 109–118. [Google Scholar] [CrossRef]
- Atzler, D.; Gore, M.O.; Ayers, C.R.; Choe, C.U.; Böger, R.H.; de Lemos, J.A.; McGuire, D.K.; Schwedhelm, E. Homoarginine and cardiovascular outcome in the population-based Dallas Heart Study. Arter. Thromb. Vasc. Biol. 2014, 34, 2501–2507. [Google Scholar] [CrossRef]
- Kleist, C.J.; Choe, C.-U.; Atzler, D.; Schönhoff, M.; Böger, R.; Schwedhelm, E.; Wicha, S.G. Population kinetics of homoarginine and optimized supplementation for cardiovascular risk reduction. Amino Acids 2022, 54, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Myers, A.; Farhat, M.; Cathapermal, S.; Ramwell, P.W. Effect of N-substituted arginine compounds on blood pressure in anesthetized rats. J. Pharmacol. Exp. Ther. 1992, 261, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Song, R.J.; Vasan, R.S.; van den Heuvel, E.R.; Hannemann, J.; Xanthakis, V.; Böger, R. Association of Lower Plasma Homoarginine Concentrations with Greater Risk of All-Cause Mortality in the Community: The Framingham Offspring Study. J. Clin. Med. 2020, 9, 2016. [Google Scholar] [CrossRef] [PubMed]
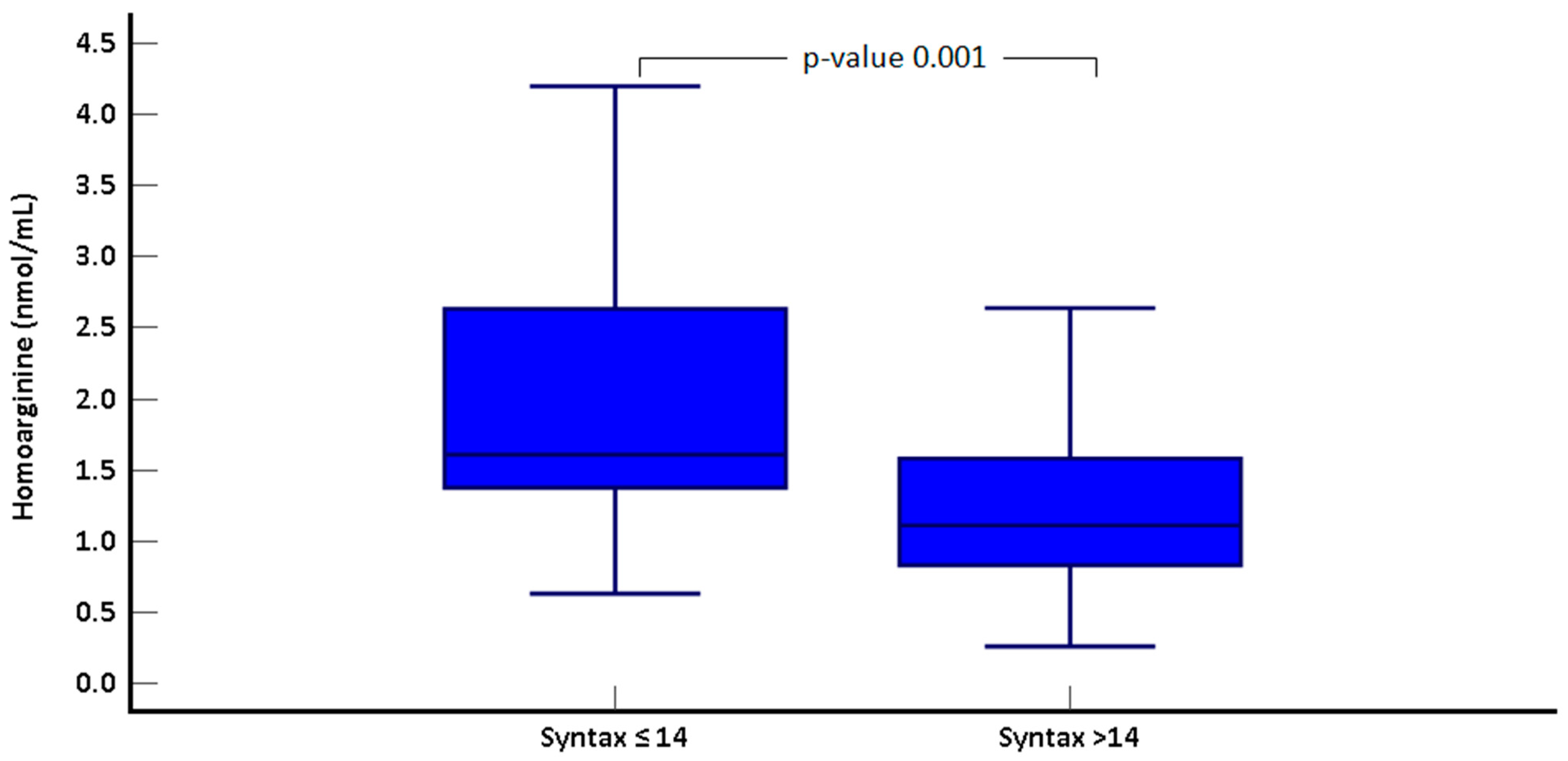

| Syntax ≤ 14 (n = 35) | Syntax > 14 (n = 32) | p-Value | |
|---|---|---|---|
| Age (year) | 57.2 ± 8.8 | 59.6 ± 10.6 | 0.322 |
| Female Gender (n, %) | 3 (8.6) | 3 (9.4) | 0.999 |
| Male Gender (n, %) | 32 (91.4) | 29 (90.6) | 0.908 |
| Hypertension (n, %) | 11 (31.4) | 18 (56.3) | 0.051 |
| Diabetes (n, %) | 4 (11.4) | 11 (34.4) | 0.039 * |
| Current smoker (n, %) | 22 (62.9) | 16 (50) | 0.331 |
| Height (cm) | 168.6 ± 6.8 | 169.3 ± 6.9 | 0.697 |
| Weight (kg) | 73.6 ± 9.4 | 71.9 ± 6.9 | 0.408 |
| Body mass index (kg/m2) | 25.2 ± 2.6 | 24.5 ± 2 | 0.239 |
| Serum fasting glucose (mg/dL) | 110 ± 28.4 | 144.9 ± 67.1 | 0.007 * |
| Hemoglobin (g/dL) | 13.5 ± 2.2 | 12.8 ± 2.8 | 0.216 |
| WBC count (103 μL) | 10.8 ± 4.5 | 11.6 ± 4.6 | 0.482 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.93 ± 0.3 | 0.615 |
| GFR (mL/min per 1.73 m2) | 90.5 ± 17.2 | 83 ± 24.3 | 0.153 |
| ALT (U/L) | 25.4 ± 14.8 | 36.6 ± 35 | 0.090 |
| AST (U/L) | 52.1 ± 77.7 | 63.3 ± 61 | 0.519 |
| Total cholesterol (mg/dL) | 182.9 ± 45.9 | 176.7 ± 48.6 | 0.605 |
| LDL cholesterol (mg/dL) | 116.1 ± 41 | 109.4 ± 34.3 | 0.478 |
| HDL cholesterol (mg/dL) | 37.6 ± 7.9 | 39 ± 9.8 | 0.533 |
| Triglyceride (mg/dL) | 171.1 ± 71.1 | 166.4 ± 93.2 | 0.820 |
| Troponin-T (ng/L) (basal) | 52.3 ± 85.6 | 265.8 ± 581.1 | 0.035 * |
| Troponin-T (ng/L) (peak) | 1905.6 ± 2240 | 2598.5 ± 2660.5 | 0.252 |
| Urea mg/dL | 33.03 ± 10.9 | 43 ± 16.7 | 0.006 * |
| Homoarginine(nmol/mL) | 2 ± 0.9 | 1.3 ± 0.7 | 0.001 * |
| Syntax ≤ 14 (n = 35) | Syntax > 14 (n = 32) | p-Value | |
|---|---|---|---|
| Single vessel | 18 (51.4) | 3 (9.4) | <0.001 * |
| Two-vessel | 15 (42.9) | 14 (43.8) | <0.001 * |
| Three- vessel | 2 (5.7) | 15 (46.9) | <0.001 * |
| LA (cm) | 3.7 ± 0.5 | 3.8 ± 0.4 | 0.299 |
| LVEDd (cm) | 4.9 ± 0.4 | 4.8 ± 0.4 | 0.383 |
| LVESd (cm) | 3.4 ± 0.5 | 3.4 ± 0.6 | 0.948 |
| RVEDd (cm) | 2.8 ± 0.5 | 2.8 ± 0.4 | 0.972 |
| RA (cm) | 3.4 ± 0.3 | 3.5 ± 0.4 | 0.195 |
| TAPSE (cm) | 2 ± 0.1 | 2 ± 0.2 | 0.270 |
| LVEF (%) | 51.1 ± 8.9 | 49.4 ± 10.9 | 0.514 |
| sPAP (mmHg) | 23.2 ± 6.2 | 24.8 ± 6.7 | 0.339 |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | 95% CI | 95% CI | ||||||
| OR | p-Value | Lower | Upper | OR | p-Value | Lower | Upper | |
| Female sex | 1.103 | 0.908 | 0.206 | 5.905 | ||||
| Age | 1.026 | 0.318 | 0.975 | 1.080 | ||||
| Smoking | 0.591 | 0.290 | 0.223 | 1.566 | ||||
| Hypertension | 2.805 | 0.043 * | 1.033 | 7.614 | 1.153 | 0.821 | 0.335 | 3.963 |
| Diabetes | 4.060 | 0.031 * | 1.138 | 14.475 | 0.565 | 0.548 | 0.088 | 3.633 |
| Family history | 1.493 | 0.434 | 0.547 | 4.072 | ||||
| Urea | 1.066 | 0.010 * | 1.016 | 1.119 | 1.073 | 0.049 * | 1.000 | 1.151 |
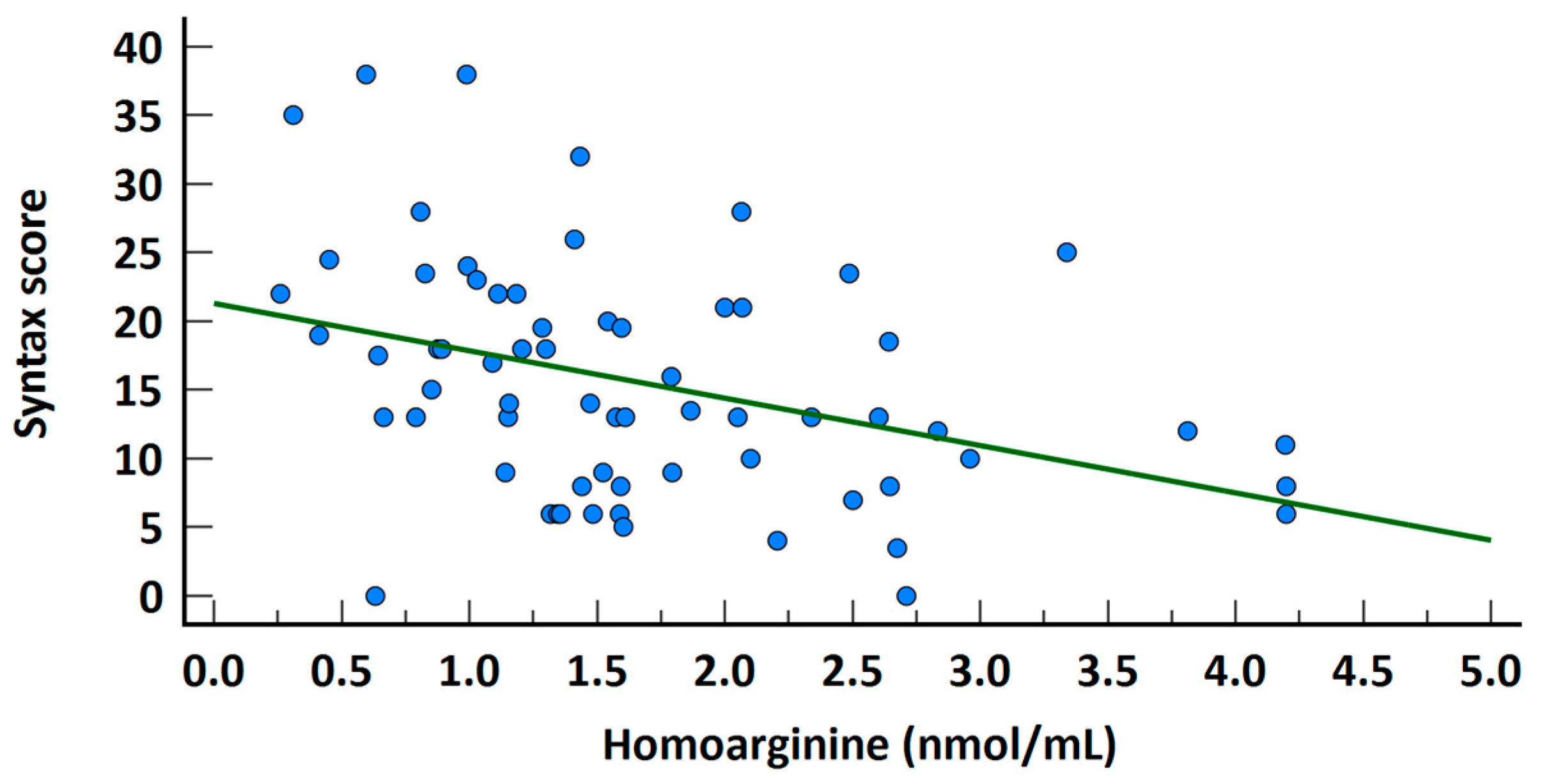
| Homoarginine | 0.313 | 0.003 * | 0.146 | 0.671 | 0.346 | 0.012 * | 0.151 | 0.792 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bingöl, G.; Huraıbat, A.; Ayduk Gövdeli, E.; Ser, Ö.S.; Ünlü, S.; Çelik, M.; Bulut, L.; Özden, Ö.; Özmen, E.; Kılıçkesmez, K. Effect of Homoarginine on Coronary Artery Complexity and Atherosclerotic Burden in Patients with STEMI. J. Clin. Med. 2025, 14, 1501. https://doi.org/10.3390/jcm14051501
Bingöl G, Huraıbat A, Ayduk Gövdeli E, Ser ÖS, Ünlü S, Çelik M, Bulut L, Özden Ö, Özmen E, Kılıçkesmez K. Effect of Homoarginine on Coronary Artery Complexity and Atherosclerotic Burden in Patients with STEMI. Journal of Clinical Medicine. 2025; 14(5):1501. https://doi.org/10.3390/jcm14051501
Chicago/Turabian StyleBingöl, Gülsüm, Ahmad Huraıbat, Elif Ayduk Gövdeli, Özgür Selim Ser, Serkan Ünlü, Murat Çelik, Leyla Bulut, Özge Özden, Emre Özmen, and Kadriye Kılıçkesmez. 2025. "Effect of Homoarginine on Coronary Artery Complexity and Atherosclerotic Burden in Patients with STEMI" Journal of Clinical Medicine 14, no. 5: 1501. https://doi.org/10.3390/jcm14051501
APA StyleBingöl, G., Huraıbat, A., Ayduk Gövdeli, E., Ser, Ö. S., Ünlü, S., Çelik, M., Bulut, L., Özden, Ö., Özmen, E., & Kılıçkesmez, K. (2025). Effect of Homoarginine on Coronary Artery Complexity and Atherosclerotic Burden in Patients with STEMI. Journal of Clinical Medicine, 14(5), 1501. https://doi.org/10.3390/jcm14051501







