Molecular and Neuroimaging Profile Associated with the Recurrence of Different Types of Strokes: Contribution from Real-World Data
Abstract
:1. Introduction
2. Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consent
2.2. Study Design
2.3. Clinical Variables and Neuroimaging Studies
2.4. Biomarker Determination
2.5. Statistical Analysis
3. Results
Multivariable Logistic Regression for Stroke Recurrence Etiology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Béjot, Y.; Christensen, L.M.; De Marchis, G.M.; Dichgans, M.; Hagberg, G.; Heldner, M.R.; Milionis, H.; Li, L.; Pezzella, F.R.; et al. European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack. Eur. Stroke J. 2022, 7, I–II. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Hankey, G.J. Primary and Secondary Prevention of Ischemic Stroke and Cerebral Hemorrhage: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 1804–1818. [Google Scholar] [CrossRef]
- Flach, C.; Muruet, W.; Wolfe, C.D.A.; Bhalla, A.; Douiri, A. Risk and Secondary Prevention of Stroke Recurrence: A Population-Base Cohort Study. Stroke 2020, 51, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.-J.; Chen, S.; Ganesh, A.; Hill, M.D. Long-term neurological, vascular, and mortality outcomes after stroke. Int. J. Stroke 2018, 13, 787–796. [Google Scholar] [CrossRef]
- Rodríguez-Castro, E.; López-Dequit, I.; Santamaría-Cadavid, M.; Arias-Rivas, S.; Rodríguez-Yáñez, M.; Pumar, J.M.; Hervella, P.; López-Arias, E.; da Silva-Candal, A.; Estany, A.; et al. Trends in stroke outcome in the last ten years in a European tertiary hospital. BMC Neurol. 2018, 18, 164. [Google Scholar] [CrossRef]
- Hilkens, N.A.; Casolla, B.; Leung, T.W.; de Leeuw, F.-E. Stroke. Lancet 2024, 403, 2820–2836. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C.; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline from the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Khanevski, A.N.; Bjerkreim, A.T.; Novotny, V.; Næss, H.; Thomassen, L.; Logallo, N.; Kvistad, C.E.; NOR-STROKE Study Group. Recurrent ischemic stroke: Incidence, predictors, and impact on mortality. Acta Neurol. Scand. 2019, 140, 3–8. [Google Scholar] [CrossRef]
- Muruet, W.; Rudd, A.; Wolfe, C.D.A.; Douiri, A. Long-Term Survival After Intravenous Thrombolysis for Ischemic Stroke: A Propensity Score-Matched Cohort with up to 10-Year Follow-Up. Stroke 2018, 49, 607–613. [Google Scholar] [CrossRef]
- Iglesias-Rey, R.; Rodríguez-Yáñez, M.; Rodríguez-Castro, E.; Pumar, J.M.; Arias, S.; Santamaría, M.; López-Dequidt, I.; Hervella, P.; Correa-Paz, C.; Sobrino, T.; et al. Worse outcome in stroke patients treated with tPA without early reperfusion: Associated factors. Transl. Stroke Res. 2018, 9, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- McCabe, J.J.; Walsh, C.; Gorey, S.; Arnold, M.; DeMarchis, G.M.; Harris, K.; Hervella, P.; Iglesias-Rey, R.; Jern, C.; Katan, M.; et al. Interleukin-6, C-Reactive Protein, and Recurrence After Stroke: A Time-Course Analysis of Individual-Participant Data. Stroke 2024, 55, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Candal, A.; Pérez-Mato, M.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Ávila-Gómez, P.; Sobrino, T.; Campos, F.; Castillo, J.; Hervella, P.; et al. The presence of leukoaraiosis enhances the association between sTWEAK and hemorrhagic transformation. Ann. Clin. Transl. Neurol. 2020, 7, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Lowe, G.D.; Chalmers, J.; Campbell, D.J.; Rumley, A.; Neal, B.C.; MacMahon, S.W.; Woodward, M. Associations of proinflammatory cytokines with the risk of recurrent stroke. Stroke 2008, 39, 2226–2230. [Google Scholar] [CrossRef]
- Rodríguez-Yáñez, M.; Sobrino, T.; Blanco, M.; de la Ossa, N.P.; Brea, D.; Rodríguez-González, R.; Leira, R.; Dávalos, A.; Castillo, J. High serum levels of pro-brain natriuretic peptide (pro BNP) identify cardioembolic origin in undetermined stroke. Dis. Markers 2009, 26, 189–195. [Google Scholar] [CrossRef]
- Hervella, P.; Pérez-Mato, M.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Sobrino, T.; Campos, F.; Castillo, J.; da Silva-Candal, A.; Iglesias-Rey, R. sTWEAK as Predictor of Stroke Recurrence in Ischemic Stroke Patients Treated with Reperfusion Therapies. Front. Neurol. 2021, 12, 652867. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Real-World Evidence. 2022. Available online: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (accessed on 16 December 2024).
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Mohan, K.M.; Wolfe, C.D.A.; Rudd, A.G.; Heuschmann, P.U.; Kolominsky-Rabas, P.L.; Grieve, A.P. Risk and cumulative risk of stroke recurrence: A systematic review and meta-analysis. Stroke 2011, 42, 1489–1494. [Google Scholar] [CrossRef]
- Smith, E.E. Leukoaraiosis and Stroke. Stroke 2010, 41, S139–S143. [Google Scholar] [CrossRef] [PubMed]
- Kongbunkiat, K.; Wilson, D.; Kasemsap, N.; Tiamkao, S.; Jichi, F.; Palumbo, V.; Hill, M.D.; Buchan, A.M.; Jung, S.; Mattle, H.P.; et al. Leukoaraiosis, intracerebral hemorrhage, and functional outcome after acute stroke thrombolysis. Neurology 2017, 88, 638–645. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Candal, A.; Custodia, A.; López-Dequidt, I.; Rodríguez-Yáñez, M.; Alonso-Alonso, M.L.; Ávila-Gómez, P.; Pumar, J.M.; Castillo, J.; Sobrino, T.; Campos, F.; et al. sTWEAK is a leukoaraiosis biomarker associated with neurovascular angiopathy. Ann. Clin. Transl. Neurol. 2022, 9, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Dimaras, T.; Merkouris, E.; Tsiptsios, D.; Christidi, F.; Sousanidou, A.; Orgianelis, I.; Polatidou, E.; Kamenidis, I.; Karatzetzou, S.; Gkantzios, A.; et al. Leukoaraiosis as a Promising Biomarker of Stroke Recurrence among Stroke Survivors: A Systematic Review. Neurol. Int. 2023, 15, 994–1013. [Google Scholar] [CrossRef]
- Hijazi, Z.; Oldgren, J.; Wallentin, L.; Andersson, U.; Connolly, S.J.; Yusuf, S.; Ezekowitz, M.D.; Hohnloser, S.H.; Reilly, P.A.; Vinereanu, D.; et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: A Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012, 125, 1605–1616. [Google Scholar] [CrossRef]
- Rodríguez-Castro, E.; Hervella, P.; López-Dequidt, I.; Arias-Rivas, S.; Santamaría-Cadavid, M.; López-Loureiro, I.; da Silva-Candal, A.; Pérez-Mato, M.; Sobrino, T.; Campos, F.; et al. NT-pro-BNP: A novel predictor of stroke risk after transient ischemic attack. Int. J. Cardiol. 2020, 298, 93–97. [Google Scholar] [CrossRef]
- Rodríguez-Yáñez, M.; Arias-Rivas, S.; Santamaría-Cadavid, M.; Sobrino, T.; Castillo, J.; Blanco, M. High pro-BNP levels predict the occurrence of atrial fibrillation after cryptogenic stroke. Neurology 2013, 81, 444–447. [Google Scholar] [CrossRef]
- Llombart, V.; Antolin-Fontes, A.; Bustamante, A.; Giralt, D.; Rost, N.S.; Furie, K.; Shibazaki, K.; Montaner, J. B-type natriuretic peptides help in cardioembolic stroke diagnosis: Pooled data meta-analysis. Stroke 2015, 46, 1187–1195. [Google Scholar] [CrossRef]
- McCabe, J.J.; Walsh, C.; Gorey, S.; Harris, K.; Hervella, P.; Iglesias-Rey, R.; Jern, C.; Kelly, P.J. C-Reactive Protein, Interleukin-6, and Vascular Recurrence According to Stroke Subtype: An Individual Participant Data Meta-Analysis. Neurology 2024, 102, e208016. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Raimondo, D.; di Sciacca, R.; Pinto, A.; Licata, G. Inflammatory cytokines in acute ischemic stroke. Curr. Pharm. Des. 2008, 14, 3574–3589. [Google Scholar] [CrossRef]
- Croft, M.; Siegel, R.M. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Yepes, M. TWEAK and Fn14 in the Neurovascular Unit. Front. Immunol. 2013, 4, 367. [Google Scholar] [CrossRef] [PubMed]
- Boulamery, A.; Desplat-Jégo, S. Regulation of neuroinflammation: What role for the tumor necrosis factor-like weak inducer of apoptosis/Fn14 pathway? Front. Immunol. 2017, 8, 1534. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.; Sbai, O.; Wen, J.; Couraud, P.-O.; Putterman, C.; Khrestchatisky, M.; Desplat-Jégo, S. TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier. J. Neuroinflamm. 2013, 10, 9. [Google Scholar] [CrossRef]
- Rodríguez-Yáñez, M.; Castellanos, M.; Blanco, M.; Millán, M.; Nombela, F.; Sobrino, T.; Lizasoain, I.; Leira, R.; Serena, J.; Dávalos, A.; et al. Micro- and macroalbuminuria predict hemorrhagic transformation in acute ischemic stroke. Neurology 2006, 67, 1172–1177. [Google Scholar] [CrossRef]
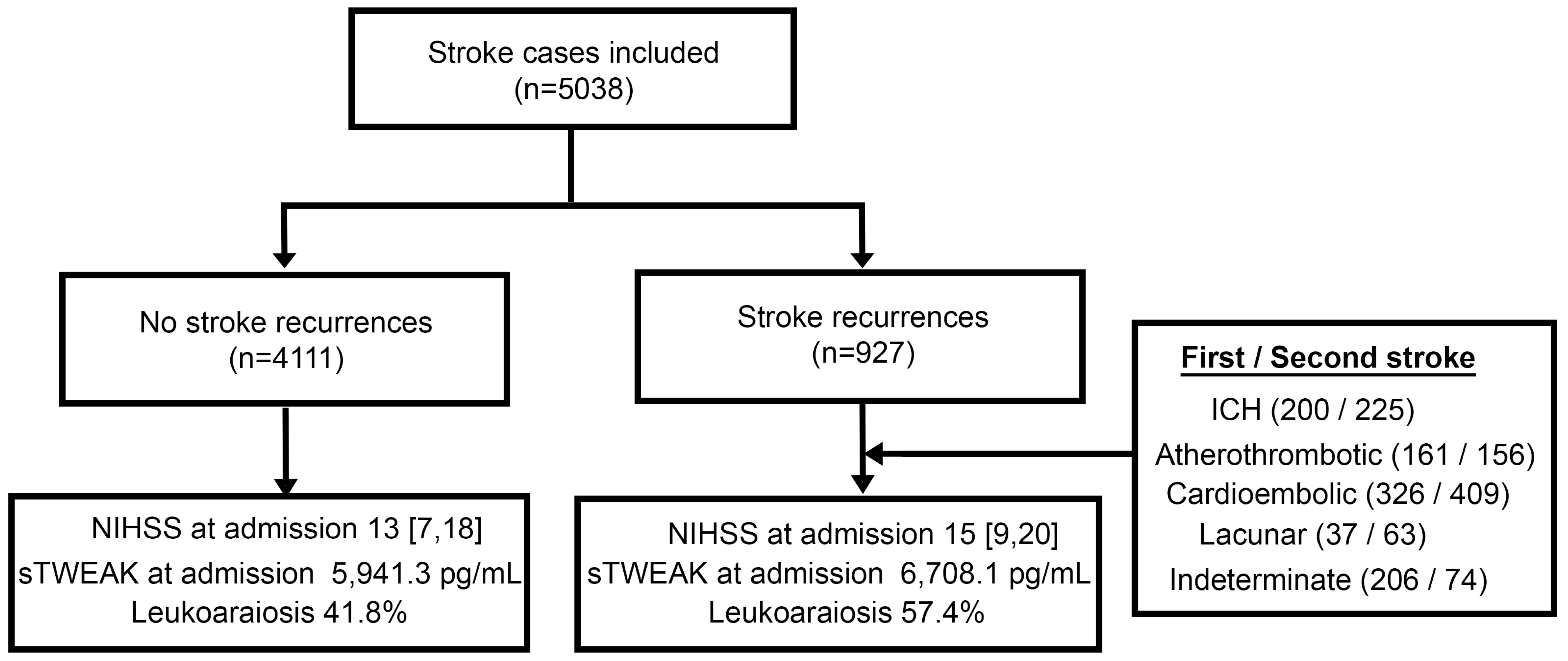
| No Stroke Recurrences n = 4111 | Stroke Recurrences n = 927 | p | |
|---|---|---|---|
| Age, years | 71.4 ± 14 | 76.1 ± 11 | <0.001 |
| Women, % | 44.6 | 44.4 | 0.943 |
| Hypertension, % | 61.6 | 71.3 | <0.001 |
| Dyslipidemia, % | 33.6 | 45.0 | <0.001 |
| Diabetes, % | 22.4 | 29.3 | <0.001 |
| Smokers, % | 16.0 | 12.2 | 0.002 |
| Atrial fibrillation, % | 19.1 | 30.0 | <0.001 |
| Ischemic heart disease, % | 10.3 | 13.9 | 0.002 |
| Heart failure, % | 4.0 | 5.8 | 0.013 |
| Antiplatelet (chronic), % | 17.5 | 52.7 | <0.001 |
| Anticoagulant (chronic), % | 7.7 | 19.4 | <0.001 |
| Glycemia *, mg/dL | 136.9 ± 55 | 140.8 ± 63 | 0.077 |
| Leukocytes *, ×103/mL | 9.0 ± 3.2 | 9.7 ± 3.3 | <0.001 |
| C-reactive protein *, mg/dL | 3.9 ± 4.4 | 4.6 ± 4.5 | <0.001 |
| Glycosylated hemoglobin, % | 6.1 ± 2.2 | 6.1 ± 1.6 | 0.354 |
| LDL cholesterol, mg/dL | 114.4 ± 43.5 | 98.2 ± 36.6 | <0.001 |
| HDL cholesterol, mg/dL | 41.5 ± 19.1 | 39.7 ± 14.6 | 0.035 |
| Triglycerides, mg/dL | 120.2 ± 64.2 | 107.6 ± 55.2 | <0.001 |
| IMT, mm | 0.9 ± 0.7 | 1.1 ± 1.1 | 0.062 |
| Leukoaraiosis, % | 41.8 | 57.4 | <0.001 |
| Degree of leukoaraiosis, % | <0.001 | ||
| No | 58.6 | 42.6 | |
| Fazecas I | 40.6 | 50.8 | |
| Fazecas II | 0.6 | 4.8 | |
| Fazecas III | 0.1 | 1.7 | |
| NIHSS at admission | 13 [7, 18] | 15 [9, 20] | <0.001 |
| Etiology of stroke | <0.001 | ||
| PIC, % | 17.7 | 21.6 | |
| Cardioembolic, % | 28.6 | 35.2 | |
| Atherothrombotic, % | 18.9 | 17.4 | |
| Lacunar, % | 7.7 | 4.0 | |
| Indeterminate, % | 26.0 | 21.1 | |
| Others, % | 1.1 | 0.8 | |
| Any reperfusion therapy, % | 0.067 | ||
| Systemic fibrinolysis, % | 22.6 | 26.3 | |
| Thrombectomy, % | 5.5 | 3.7 | |
| Combination therapy, % | 0.5 | 1.2 | |
| NT-pro-BNP *, pg/mL | 1426.8 ± 2353.3 | 1871.3 ± 2097.1 | <0.001 |
| TNF-a *, pg/mL | 17.71 ± 7.5 | 18.98 ± 7.7 | 0.002 |
| IL-6 *, pg/mL | 14.55 ± 11.3 | 15.87 ± 11.8 | 0.022 |
| IL-10 *, pg/mL | 6.79 ± 4.7 | 7.07 ± 4.5 | 0.277 |
| sTWEAK *, pg/mL | 5941.3 ± 3859.5 | 6708.1 ± 3939.1 | <0.001 |
| Microalbuminuria *, mg/g | 7.3 ± 26.7 | 8.1 ± 26.8 | 0.555 |
| Binary | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p | aOR * | CI 95% | p | |
| Age | 1.03 | 1.02–1.03 | <0.001 | 0.98 | 0.91–1.07 | 0.752 |
| Hypertension | 1.54 | 1.33–1.80 | <0.001 | 23.25 | 1.71–315-34 | 0.018 |
| Diabetes | 1.44 | 1.23–1.68 | <0.001 | 1.19 | 0.25–5.72 | 0.829 |
| Smoker | 0.71 | 0.57–0.88 | 0.002 | 0.26 | 0.05–1.49 | 0.132 |
| Dyslipidemia | 1.61 | 1.40–1.86 | <0.001 | 2.14 | 0.52–8.85 | 0.295 |
| IHD | 1.41 | 1.15–1.73 | 0.001 | 13.58 | 1.23–150.01 | 0.033 |
| Previous AF | 1.81 | 1.55–2.11 | <0.001 | 3.87 | 0.34–44.59 | 0.278 |
| Heart failure | 1.49 | 1.09–2.03 | 0.012 | 1.49 | 0.14–16.23 | 0.739 |
| Antiplatelets | 5.25 | 4.53–6.09 | <0.001 | 46.39 | 6.30–341.38 | <0.001 |
| Anticoagulants | 2.89 | 2.39–3.51 | <0.001 | 26.11 | 1.71–397.90 | 0.019 |
| NIHSS † | 1.03 | 1.03–1.04 | <0.001 | 1.04 | 0.94–1.15 | 0.442 |
| LDL cholesterol | 0.99 | 0.98–0.099 | <0.001 | 1.01 | 0.99–1.03 | 0.176 |
| HDL cholesterol | 0.99 | 0.98–0.99 | 0.032 | 1.00 | 0.96–1.05 | 0.840 |
| Triglycerides | 0.99 | 0.99–0.99 | <0.001 | 1.00 | 0.99–1.02 | 0.337 |
| Leucocytes † | 1.06 | 1.04–1.08 | <0.001 | 1.19 | 0.98–1.46 | 0.076 |
| CRP † | 1.03 | 1.01–1.05 | <0.001 | 0.90 | 0.76–1.08 | 0.261 |
| IL-6 † | 1.03 | 1.01–1.01 | <0.001 | 1.02 | 0.95–1.09 | 0.572 |
| Multivariable | |||
|---|---|---|---|
| aOR * | CI 95% | p | |
| Lacunar stroke | |||
| Hypertension | 4.98 | 2.04–12.25 | <0.001 |
| Ischemic heart disease | 1.44 | 0.42–4.98 | 0.565 |
| Antiplatelets | 1.19 | 0.53–2.67 | 0.681 |
| Anticoagulation | 0.17 | 0.02–1.36 | 0.095 |
| Leukoaraiosis | 9.50 | 3.12–28.93 | <0.001 |
| Cardioembolic stroke | |||
| Hypertension | 1.06 | 0.75–1.49 | 0.759 |
| IHD | 1.51 | 0.95–2.41 | 0.083 |
| Antiplatelets | 0.25 | 0.17–0.35 | <0.001 |
| Anticoagulation | 0.19 | 0.13–0.29 | <0.001 |
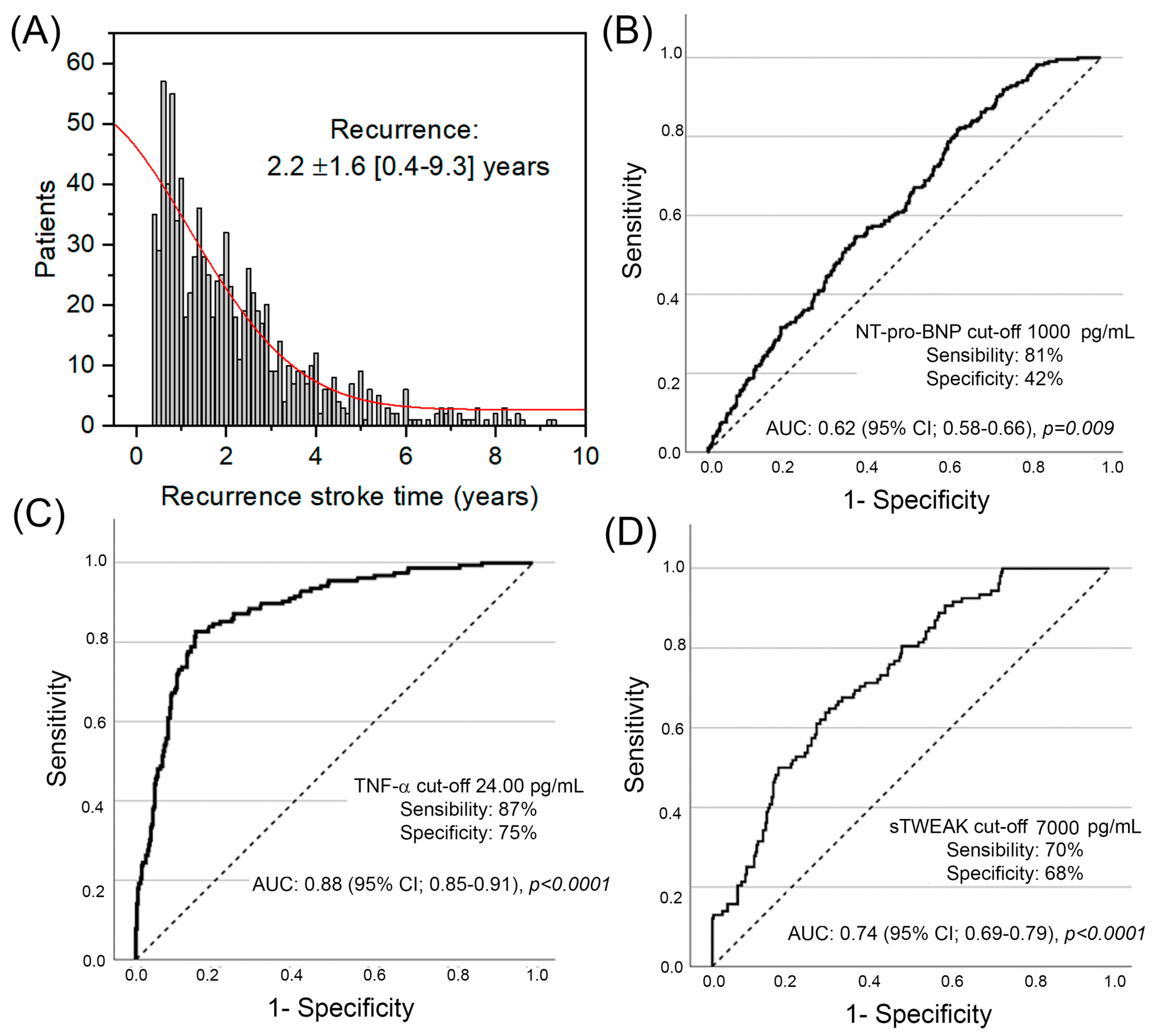
| NT-pro-BNP † >1000 pg/mL | 1.80 | 1.23–2.61 | 0.002 |
| Atherothrombotic stroke | |||
| Hypertension | 1.49 | 0.89–2.49 | 0.129 |
| IHD | 1.29 | 0.72–2.33 | 0.390 |
| Antiplatelets | 1.89 | 1.14–3.15 | 0.014 |
| Anticoagulation | 0.79 | 0.41–1.55 | 0.500 |
| TNF-a † >24 pg/mL | 21.61 | 12.42–37.59 | <0.001 |
| Intracerebral hemorrage | |||
| Hypertension | 1.33 | 0.76–2.32 | 0.316 |
| IHD | 2.16 | 0.85–5.45 | 0.104 |
| Antiplatelets | 2.04 | 1.16–3.57 | 0.013 |
| Anticoagulation | 2.27 | 1.11–4.65 | 0.002 |
| sTWEAK † >7000 pg/mL | 4.81 | 2.86–8.07 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oblitas, C.-M.; Sampedro-Viana, A.; Fernández-Rodicio, S.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Gonzalez-Quintela, A.; Mosqueira, A.J.; Porto-Álvarez, J.; Martínez Fernández, J.; González-Simón, I.; et al. Molecular and Neuroimaging Profile Associated with the Recurrence of Different Types of Strokes: Contribution from Real-World Data. J. Clin. Med. 2025, 14, 1460. https://doi.org/10.3390/jcm14051460
Oblitas C-M, Sampedro-Viana A, Fernández-Rodicio S, Rodríguez-Yáñez M, López-Dequidt I, Gonzalez-Quintela A, Mosqueira AJ, Porto-Álvarez J, Martínez Fernández J, González-Simón I, et al. Molecular and Neuroimaging Profile Associated with the Recurrence of Different Types of Strokes: Contribution from Real-World Data. Journal of Clinical Medicine. 2025; 14(5):1460. https://doi.org/10.3390/jcm14051460
Chicago/Turabian StyleOblitas, Crhistian-Mario, Ana Sampedro-Viana, Sabela Fernández-Rodicio, Manuel Rodríguez-Yáñez, Iria López-Dequidt, Arturo Gonzalez-Quintela, Antonio J. Mosqueira, Jacobo Porto-Álvarez, Javier Martínez Fernández, Inmaculada González-Simón, and et al. 2025. "Molecular and Neuroimaging Profile Associated with the Recurrence of Different Types of Strokes: Contribution from Real-World Data" Journal of Clinical Medicine 14, no. 5: 1460. https://doi.org/10.3390/jcm14051460
APA StyleOblitas, C.-M., Sampedro-Viana, A., Fernández-Rodicio, S., Rodríguez-Yáñez, M., López-Dequidt, I., Gonzalez-Quintela, A., Mosqueira, A. J., Porto-Álvarez, J., Martínez Fernández, J., González-Simón, I., Bazarra-Barreiros, M., Abengoza-Bello, M. T., Ortega-Espina, S., Ouro, A., Campos, F., Sobrino, T., Castillo, J., Alonso-Alonso, M. L., Hervella, P., & Iglesias-Rey, R. (2025). Molecular and Neuroimaging Profile Associated with the Recurrence of Different Types of Strokes: Contribution from Real-World Data. Journal of Clinical Medicine, 14(5), 1460. https://doi.org/10.3390/jcm14051460









