Measures of Serum Markers HbA1c, HOMA-IR, HOMA-β, QUICKI and G/I Ratio as Predictors of Abnormal Glucose Tolerance Among Thai Women with Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Kandarakis, H.; Legro, R.S. The role of genes and environment in the etiology of PCOS. Endocrine 2006, 30, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESRHE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Ehrmann, D.A. Polycystic ovary syndrome. N. Engl. J. Med. 2005, 352, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Lillioja, S.; Mott, D.M.; Spraul, M.; Ferraro, R.; Foley, J.E.; Ravussin, E.; Knowler, W.C.; Bennett, P.H.; Bogardus, C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N. Engl. J. Med. 1993, 329, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Glintborg, D.; Henriksen, J.E.; Andersen, M.; Hagen, C.; Hangaard, J.; Rasmussen, P.E.; Schousboe, K.; Hermann, A.P. Prevalence of endocrine diseases and abnormal glucose tolerance tests in 340 Caucasian premenopausal women with hirsutism as the referral diagnosis. Fertil. Steril. 2004, 82, 1570–1579. [Google Scholar] [CrossRef]
- Harris, M.I.; Hadden, W.C.; Knowler, W.C.; Bennett, P.H. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20–74 yr. Diabetes 1987, 36, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Salley, K.E.; Wickham, E.P.; Cheang, K.I.; Essah, P.A.; Karjane, N.W.; Nestler, J.E. Glucose intolerance in polycystic ovary syndrome—A position statement of the Androgen Excess Society. J. Clin. Endocrinol. Metab. 2007, 92, 4546–4556. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.A.; Carmina, E.; Diamanti-Kandarakis, E.; Dokras, A.; Escobar-Morreale, H.F.; Futterweit, W.; Lobo, R.; Norman, R.J.; Talbott, E.; Dumesic, D.A. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J. Clin. Endocrinol. Metab. 2010, 95, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care 2011, 34, S11–S61. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36, S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, D.; Li, L.; Feng, S.; Wang, L. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum. Reprod. 2006, 21, 2027–2032. [Google Scholar] [CrossRef]
- Tosi, F.; Bonora, E.; Moghetti, P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: A comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum. Reprod. 2017, 32, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Nakao, K.; Kimura, M.; Takenaka, H.; Horikawa, M. Insulin resistance in nonobese Japanese women with polycystic ovary syndrome is associated with poorer glucose tolerance, delayed insulin secretion, and enhanced insulin response. Reprod. Med. Biol. 2015, 14, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Finegood, D.; Dunaif, A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1998, 83, 2694–2698. [Google Scholar] [CrossRef]
- World Health Organisation. Regional Office for the Western Pacific. In The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; WHO: Sydney, Australia, 2000. [Google Scholar]
- Free & Bioavailable Testosterone Calculator. Available online: https://www.issam.ch/freetesto.htm (accessed on 11 April 2017).
- ISO 15189; Medical Laboratories-Requirements for Quality and Competence. International Organization for Standardization: Geneva, Switzerland, 2012.
- Onishi, Y.; Hayashi, T.; Sato, K.K.; Ogihara, T.; Kuzuya, N.; Anai, M.; Tsukuda, K.; Boyko, E.J.; Fujimoto, W.Y.; Kikuchi, M. Fasting tests of insulin secretion and sensitivity predict future prediabetes in Japanese with normal glucose tolerance. J. Diabetes Investig. 2010, 1, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian. J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef]
- Celik, C.; Abali, R.; Bastu, E.; Tasdemir, N.; Tasdemir, U.G.; Gul, A. Assessment of impaired glucose tolerance prevalence with hemoglobin A1c and oral glucose tolerance test in 252 Turkish women with polycystic ovary syndrome: A prospective, controlled study. Hum. Reprod. 2013, 28, 1062–1068. [Google Scholar] [CrossRef]
- Wongwananuruk, T.; Rattanachaiyanont, M.; Leerasiri, P.; Indhavivadhana, S.; Techatraisak, K.; Angsuwathana, S. The Usefulness of Homeostatic Measurement Assessment-Insulin Resistance (HOMA-IR) for Detection of Glucose Intolerance in Thai Women of Reproductive Age with Polycystic Ovary Syndrome. Int. J. Endocrinol. 2012, 2012, 571035. [Google Scholar] [CrossRef] [PubMed]
- Charnvises, K.; Weerakiet, S.; Tingthanatikul, Y.; Wansumrith, S.; Chanprasertyothin, S.; Rojanasakul, A. Acanthosis nigricans: Clinical predictor of abnormal glucose tolerance in Asian women with polycystic ovary syndrome. Gynecol. Endocrinol. 2005, 21, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Schwetz, V.; Giuliani, A.; Obermayer-Pietsch, B. Assessment of glucose metabolism in polycystic ovary syndrome: HbA1c or fasting glucose compared with the oral glucose tolerance test as a screening method. Hum. Reprod. 2013, 28, 2537–2544. [Google Scholar] [CrossRef]
- Gooding, H.C.; Milliren, C.; St Paul, M.; Mansfield, M.J.; DiVasta, A. Diagnosing dysglycemia in adolescents with polycystic ovary syndrome. J. Adolesc. Health 2014, 55, 79–84. [Google Scholar] [CrossRef]
- Ehrmann, D.A.; Barnes, R.B.; Rosenfield, R.L.; Cavaghan, M.K.; Imperial, J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 1999, 22, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Hurd, W.W.; Abdel-Rahman, M.Y.; Ismail, S.A.; Abdellah, M.A.; Schmotzer, C.L.; Sood, A. Comparison of diabetes mellitus and insulin resistance screening methods for women with polycystic ovary syndrome. Fertil. Steril. 2011, 96, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Rawal, G.; Yadav, S.; Kumar, R.; Singh, A. Glycosylated hemoglobin (HbA1C): A brief overview for clinicians. Indian J. Immunol. Respir. Med. 2016, 1, 33–36. [Google Scholar]
- Charuruk, N.; Milintagas, A.; Watanaboonyoungchareon, P.; Ariyaboonsiri, C. Determination of reference intervals of HbA1c (DCCT/NGSP) and HbA1c (IFCC) in adults. J. Med. Assoc. Thai 2005, 88, 810–816. [Google Scholar]
- Paisooksantivatana, K.; Kongsomgan, A.; Leohirun, L.; Atamasirikul, K.; Kunakorn, M. HemoglobinA1c level in healthy Thai adults: Reference interval and fasting plasma glucose. Diabetes Res. Clin. Pract. 2009, 83, e43–e46. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Mean ± SD, Median (P25, P75) or n (%) |
|---|---|
| Age (yr) | 26.2 ± 5.3 |
| Parity | |
| Yes | 9 (24.8) |
| No | 112 (75.2) |
| BMI | 25.5 ± 6.9 |
| Underweight | 12 (9.9) |
| Normal | 39 (32.2) |
| Overweight | 17 (14.0) |
| Obese | 53 (43.8) |
| Waist circumference (cm) | 83.3 ± 17.9 |
| Presence of acanthosis nigricans | 23 (19.0) |
| MFGs | 4 (3, 5) |
| Haemoglobin (g/dL) | 13.1 ± 1.2 |
| Total testosterone (ng/mL) | 0.4 ± 0.3 |
| Free testosterone (ng/dL) | 0.7 ± 0.5 |
| Fasting glucose (mg/dL) | 82.5 ± 24.2 |
| Fasting insulin (µU/mL) | 10.91 (10.93, 19.20) |
| 2-hour glucose (mg/dL) | 124.6 ± 54 |
| HbA1c (%) | 5.5 ± 0.9 |
| QUICKI | 0.3 ± 0.1 |
| HOMA-IR | 2.29 (1.34, 4.01) |
| HOMA-β | 197.33 (128.46, 321.50) |
| G/I ratio | 7.76 (4.40, 11.94) |
| Parameters | Normal Glucose Tolerance Test (n = 91) | Abnormal Glucose Tolerance Test (n = 30) | p |
|---|---|---|---|
| Age (yr) | 25.6 ± 5.3 | 27.8 ± 5.2 | 0.05 |
| Parity | |||
| Yes | 6 (6.6) | 3 (10.0) | 0.681 |
| No | 85 (93.4) | 27 (90.0) | |
| BMI | 23.8 ± 5.7 | 30.6 ± 7.9 | <0.01 |
| Underweight | 10 (11.0) | 2 (6.7) | |
| Normal | 37 (40.7) | 2 (6.7) | |
| Overweight | 14 (15.4) | 3 (10.0) | |
| Obese | 30 (33.0) | 23 (76.7) | |
| Waist circumference (cm) | 79.7 ± 12.2 | 94.0 ± 17.3 | <0.01 |
| Acanthosis nigricans | 10 (11.0) | 13 (43.3) | <0.01 |
| MFGs | 4 (2, 5) | 4 (3, 5) | 0.700 |
| Haemoglobin (g/dL) | 12.9 ± 1.2 | 13.5 ± 1.4 | 0.013 |
| Total testosterone (ng/mL) | 0.4 ± 0.2 | 0.6 ± 0.6 | 0.148 |
| Free testosterone (ng/dL) | 0.6 ± 0.4 | 1.0 ± 0.8 | 0.007 |
| Fasting glucose (mg/dL) | 82.3 ± 6.9 | 103.4 ± 43.8 | 0.014 |
| Insulin (µU/mL) | 9.81 (6.06, 14.29) | 27.39 (12.86, 39.37) | <0.01 |
| 2-h glucose (mg/dL) | 102.8 ± 19.1 | 190.5 ± 70.4 | <0.01 |
| HbA1c (%) | 5.3 ± 0.3 | 6.0 ± 16 | 0.022 |
| QUICKI | 0.35 ± 0.04 | 0.31 ± 0.05 | <0.01 |
| HOMA-IR | 2 (1.21, 2.95) | 6.52 (2.64, 9.59) | <0.01 |
| HOMA-β | 180.57 (123.87, 255.29) | 351.31 (209.26, 493.78) | <0.01 |
| GI ratio | 8.28 (6, 13.37) | 3.83 (2.43, 7.91) | <0.01 |
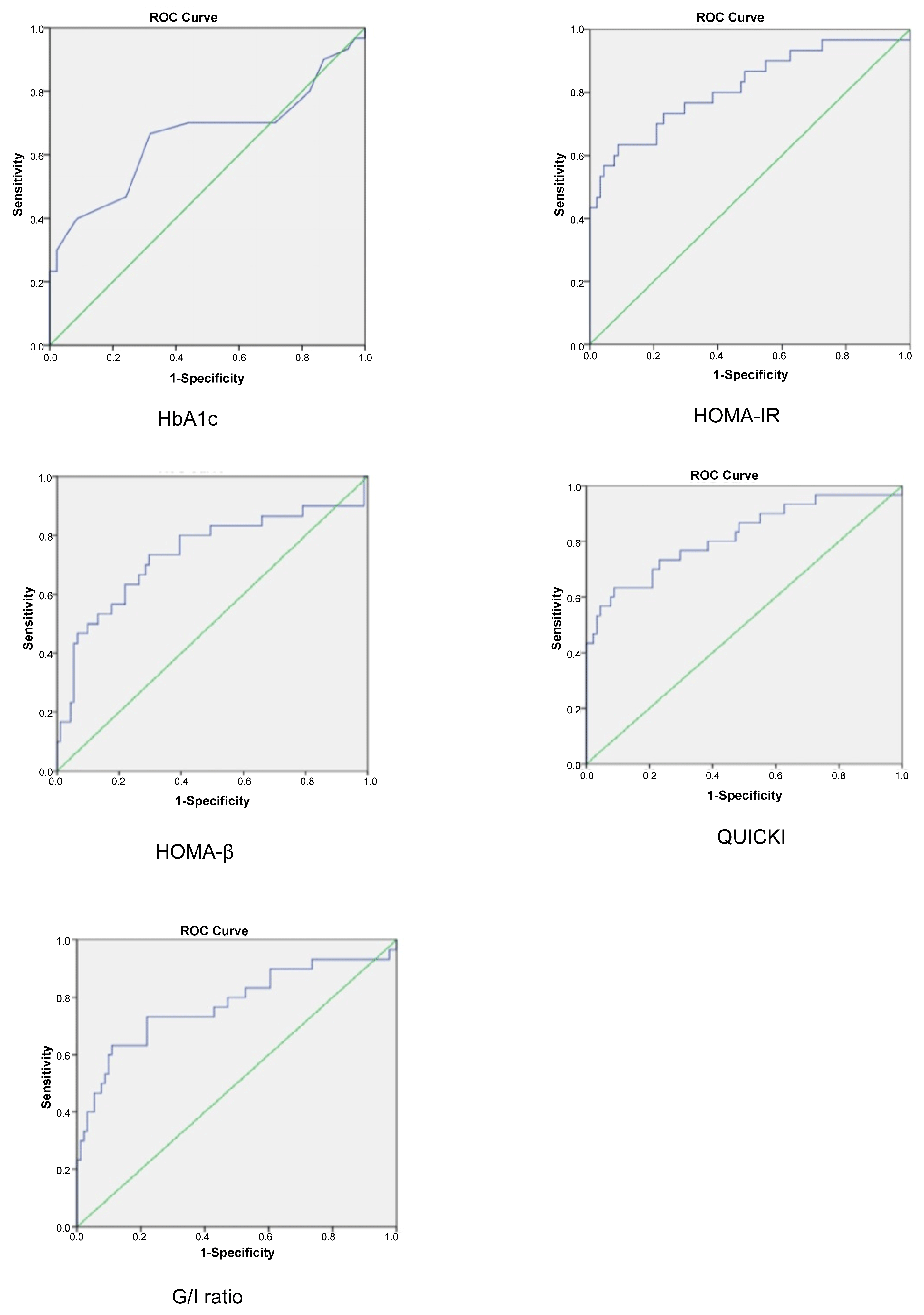
| Test | AUC | Cut-Off Point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| HbA1c | 0.656 | 5.4 | 70.0 | 56.0 | 34.4 | 85.0 | 59.5 |
| HOMA-IR | 0.817 | 2.3 | 80.0 | 60.4 | 40.0 | 90.2 | 65.0 |
| HOMA-β | 0.737 | 200.0 | 80.0 | 60.4 | 40.0 | 90.2 | 65.3 |
| QUICKI | 0.817 | 0.336 | 80.0 | 61.5 | 40.7 | 90.3 | 66.1 |
| G/I ratio | 0.777 | 6.0 | 73.3 | 74.7 | 48.9 | 89.5 | 74.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongwananuruk, T.; Prasongvej, P.; Chantrapanichkul, P.; Indhavivadhana, S.; Tanmahasamut, P.; Rattanachaiyanont, M.; Techatraisak, K.; Angsuwathana, S. Measures of Serum Markers HbA1c, HOMA-IR, HOMA-β, QUICKI and G/I Ratio as Predictors of Abnormal Glucose Tolerance Among Thai Women with Polycystic Ovary Syndrome. J. Clin. Med. 2025, 14, 1452. https://doi.org/10.3390/jcm14051452
Wongwananuruk T, Prasongvej P, Chantrapanichkul P, Indhavivadhana S, Tanmahasamut P, Rattanachaiyanont M, Techatraisak K, Angsuwathana S. Measures of Serum Markers HbA1c, HOMA-IR, HOMA-β, QUICKI and G/I Ratio as Predictors of Abnormal Glucose Tolerance Among Thai Women with Polycystic Ovary Syndrome. Journal of Clinical Medicine. 2025; 14(5):1452. https://doi.org/10.3390/jcm14051452
Chicago/Turabian StyleWongwananuruk, Thanyarat, Pichita Prasongvej, Panicha Chantrapanichkul, Suchada Indhavivadhana, Prasong Tanmahasamut, Manee Rattanachaiyanont, Kitirat Techatraisak, and Surasak Angsuwathana. 2025. "Measures of Serum Markers HbA1c, HOMA-IR, HOMA-β, QUICKI and G/I Ratio as Predictors of Abnormal Glucose Tolerance Among Thai Women with Polycystic Ovary Syndrome" Journal of Clinical Medicine 14, no. 5: 1452. https://doi.org/10.3390/jcm14051452
APA StyleWongwananuruk, T., Prasongvej, P., Chantrapanichkul, P., Indhavivadhana, S., Tanmahasamut, P., Rattanachaiyanont, M., Techatraisak, K., & Angsuwathana, S. (2025). Measures of Serum Markers HbA1c, HOMA-IR, HOMA-β, QUICKI and G/I Ratio as Predictors of Abnormal Glucose Tolerance Among Thai Women with Polycystic Ovary Syndrome. Journal of Clinical Medicine, 14(5), 1452. https://doi.org/10.3390/jcm14051452






