The Prognostic Value of the Advanced Lung Cancer Inflammation Index for Major Cardiovascular and Cerebrovascular Events in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Baseline Characteristics
4.2. Parameters Associated with MACCEs
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Wang, J.; Cao, D.; Han, L. Correlation of neutrophil-to-lymphocyte ratio with the prognosis of non-ST-segment elevation in patients with acute coronary syndrome undergoing selective percutaneous coronary intervention. J. Int. Med. Res. 2020, 48, 300060520959510. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Cannon, C.P. Acute coronary syndromes: Diagnosis and management, part I. Mayo Clin. Proc. 2009, 84, 917–938. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.N.; Pocock, S.; Kaul, P.; Owen, R.; Goodman, S.G.; Granger, C.B.; Nicolau, J.C.; Simon, T.; Westermann, D.; Yasuda, S.; et al. Comparing the long-term outcomes in chronic coronary syndrome patients with prior ST-segment and non-ST-segment elevation myocardial infarction: Findings from the TIGRIS registry. BMJ Open 2023, 13, e070237. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Melichova, D.; Aabel, E.W.; Lie, Ø.H.; Klæboe, L.G.; Grenne, B.; Sjøli, B.; Brunvand, H.; Haugaa, K.; Edvardsen, T. Mortality in Patients with Acute Coronary Syndrome-A Prospective 5-Year Follow-Up Study. J. Clin. Med. 2023, 12, 6598. [Google Scholar] [CrossRef] [PubMed]
- Szummer, K.; Jernberg, T.; Wallentin, L. From Early Pharmacology to Recent Pharmacology Interventions in Acute Coronary Syndromes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1618–1636. [Google Scholar] [CrossRef]
- Garcia-Osuna, A.; Sans-Rosello, J.; Ferrero-Gregori, A.; Alquezar-Arbe, A.; Sionis, A.; Ordóñez-Llanos, J. Risk Assessment after ST-Segment Elevation Myocardial Infarction: Can Biomarkers Improve the Performance of Clinical Variables? J. Clin. Med. 2022, 11, 1266. [Google Scholar] [CrossRef]
- Li, Q.; Ma, X.; Shao, Q.; Yang, Z.; Wang, Y.; Gao, F.; Zhou, Y.; Yang, L.; Wang, Z. Prognostic Impact of Multiple Lymphocyte-Based Inflammatory Indices in Acute Coronary Syndrome Patients. Front. Cardiovasc. Med. 2022, 9, 811790. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Pizzino, F.; Lilli, A.; De Caterina, A.R.; Esposito, A.; Dalmiani, S.; Mazzone, A.; Di Bella, G.; Berti, S.; Paradossi, U. Advanced Lung Cancer Inflammation Index as Predictor of All-Cause Mortality in ST-Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 6059. [Google Scholar] [CrossRef]
- Kalyoncuoglu, M.; Durmus, G. Relationship between C-reactive protein-to-albumin ratio and the extent of coronary artery disease in patients with non-ST-elevated myocardial infarction. Coron. Artery Dis. 2020, 31, 130–136. [Google Scholar] [CrossRef]
- Menekşe, T.S.; Kaçer, İ.; Hacımustafaoğlu, M.; Gül, M.; Ateş, C. C-reactive protein to albumin ratio may predict in-hospital mortality in non-ST elevation myocardial infarction. Biomark. Med. 2024, 18, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, E.Ö.; Bayam, E.; Çelik, M.; Kahyaoğlu, M.; Eren, K.; Imanov, E.; Karagöz, A.; İzgi, İ.A. Uric Acid-to-Albumin Ratio: A Novel Marker for the Extent of Coronary Artery Disease in Patients with Non-ST-Elevated Myocardial Infarction. Pulse 2021, 8, 99–107. [Google Scholar] [CrossRef]
- Kalyoncuoğlu, M.; Katkat, F.; Biter, H.I.; Cakal, S.; Tosu, A.R.; Can, M.M. Predicting One-Year Deaths and Major Adverse Vascular Events with the Controlling Nutritional Status Score in Elderly Patients with Non-ST-Elevated Myocardial Infarction Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2021, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Raposeiras Roubín, S.; Abu Assi, E.; Cespón Fernandez, M.; Barreiro Pardal, C.; Lizancos Castro, A.; Parada, J.A.; Pérez, D.D.; Blanco Prieto, S.; Rossello, X.; Ibanez, B.; et al. Prevalence and Prognostic Significance of Malnutrition in Patients With Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 76, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.H.; Shi, R.; Mills, G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC Cancer 2013, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, Y.; Peng, Y.; Wang, M.; Zhou, Y.; Gu, W.; Li, Y.; Zou, J.; Zhu, N.; Chen, L. Advanced lung cancer inflammation index combined with geriatric nutritional risk index predict all-cause mortality in heart failure patients. BMC Cardiovasc. Disord. 2023, 23, 565. [Google Scholar] [CrossRef]
- Gong, M.; Sasmita, B.R.; Zhu, Y.; Chen, S.; Wang, Y.; Xiang, Z.; Jiang, Y.; Luo, S.; Huang, B. Prognostic Value of the Advanced Lung Cancer Inflammation Index Ratio in Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Cohort Study. Rev. Cardiovasc. Med. 2024, 25, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Tang, W.; Yang, C.; Liu, X.; Huang, J. The Prognostic Value of Advanced Lung Cancer Inflammation Index in Elderly Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Int. Heart J. 2024, 65, 621–629. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Amstardam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, 139–228. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Global Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Benson, B.; Belle, A.; Lee, S.; Bassin, B.S.; Medlin, R.P.; Sjoding, M.W.; Ward, K.R. Prediction of episode of hemodynamic instability using an electrocardiogram based analytic: A retrospective cohort study. BMC Anesthesiol. 2023, 23, 324. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.C.; Cruz-Fernández, J.M.; Fernández-Bergés, D.; García-Moll, X.; Martín Jadraque, L.; Mostaza, J.; López García-Aranda, V.; González Juanatey, J.R.; Castro Beiras, A.; Martín Luengo, C.; et al. Inflammation markers and risk stratification in patients with acute coronary syndromes: Design of the SIESTA Study (Systemic Inflammation Evaluation in Patients with non-ST segment elevation Acute coronary syndromes). Rev. Esp. Cardiol. 2003, 56, 389–395. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Banahene, N.O.; Sinha, T.; Shaikh, S.; Zin, A.K.; Khreis, K.; Chaudhari, S.S.; Wei, C.R.; Palleti, S.K. Effect of Elevated Neutrophil-to-Lymphocyte Ratio on Adverse Outcomes in Patients With Myocardial Infarction: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e61647. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Ye, Z.; You, H.; Wu, Y.; Chen, W.; Jiang, T. Elevated uric acid/albumin ratio as a predictor of poor coronary collateral circulation development in patients with non-ST segment elevation myocardial infarction. Clin. Cardiol. 2024, 47, e24215. [Google Scholar] [CrossRef]
- Kalkan, S.; Cagan Efe, S.; Karagöz, A.; Zeren, G.; Yılmaz, M.F.; Şimşek, B.; Batgerel, U.; Özkalaycı, F.; Tanboğa, İ.H.; Oduncu, V.; et al. A New Predictor of Mortality in ST-Elevation Myocardial Infarction: The Uric Acid Albumin Ratio. Angiology 2022, 73, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Camprubi, M.; Cabrera, S.; Sans, J.; Vidal, G.; Salvadó, T.; Bardají, A. Body mass index and hospital mortality in patients with acute coronary syndrome receiving care in a university hospital. J. Obes. 2012, 2012, 287939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadakia, M.B.; Fox, C.S.; Scirica, B.M.; Murphy, S.A.; Bonaca, M.P.; Morrow, D.A. Central obesity and cardiovascular outcomes in patients with acute coronary syndrome: Observations from the MERLIN-TIMI 36 trial. Heart 2011, 97, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Das, S.R.; Alexander, K.P.; Chen, A.Y.; Powell-Wiley, T.M.; Diercks, D.B.; Peterson, E.D.; Roe, M.T.; de Lemos, J.A. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J. Am. Coll. Cardiol. 2011, 58, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; Arangalage, D.; Maurizi, N.; Pizzi, C.; Valgimigli, M.; Iglesias, J.F.; Landi, A.; Leo, L.A.; Eeckhout, E.; Schwitter, J.; et al. Hepatic T1 Mapping as a Novel Cardio-Hepatic Axis Imaging Biomarker Early after STEMI. Eur. Heart J. Cardiovasc. Imaging. 2025, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.L.; Kennedy, K.F.; Cohen, D.J.; Diercks, D.B.; Moscucci, M.; Ramee, S.; Wang, T.Y.; Connolly, T.; Spertus, J.A. Predicting In-Hospital Mortality in Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 68, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Gaba, P.; Gersh, B.J.; Ali, Z.A.; Moses, J.W.; Stone, G.W. Complete versus incomplete coronary revascularization: Definitions, assessment and outcomes. Nat. Rev. Cardiol. 2021, 18, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, H.; Li, L.; Zhao, X.; Zhang, C.; Wang, W.E. The prognostic utility of GRACE risk score in predictive adverse cardiovascular outcomes in patients with NSTEMI and multivessel disease. BMC Cardiovasc. Disord. 2022, 22, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wan, Z.; Zhang, Y.; Fan, Y.; Gu, W.; Li, F.; Meng, L.; Zeng, X.; Han, D.; Li, X. Neutrophil count improves the GRACE risk score prediction of clinical outcomes in patients with ST-elevation myocardial infarction. Atherosclerosis 2015, 241, 723–728. [Google Scholar] [CrossRef]

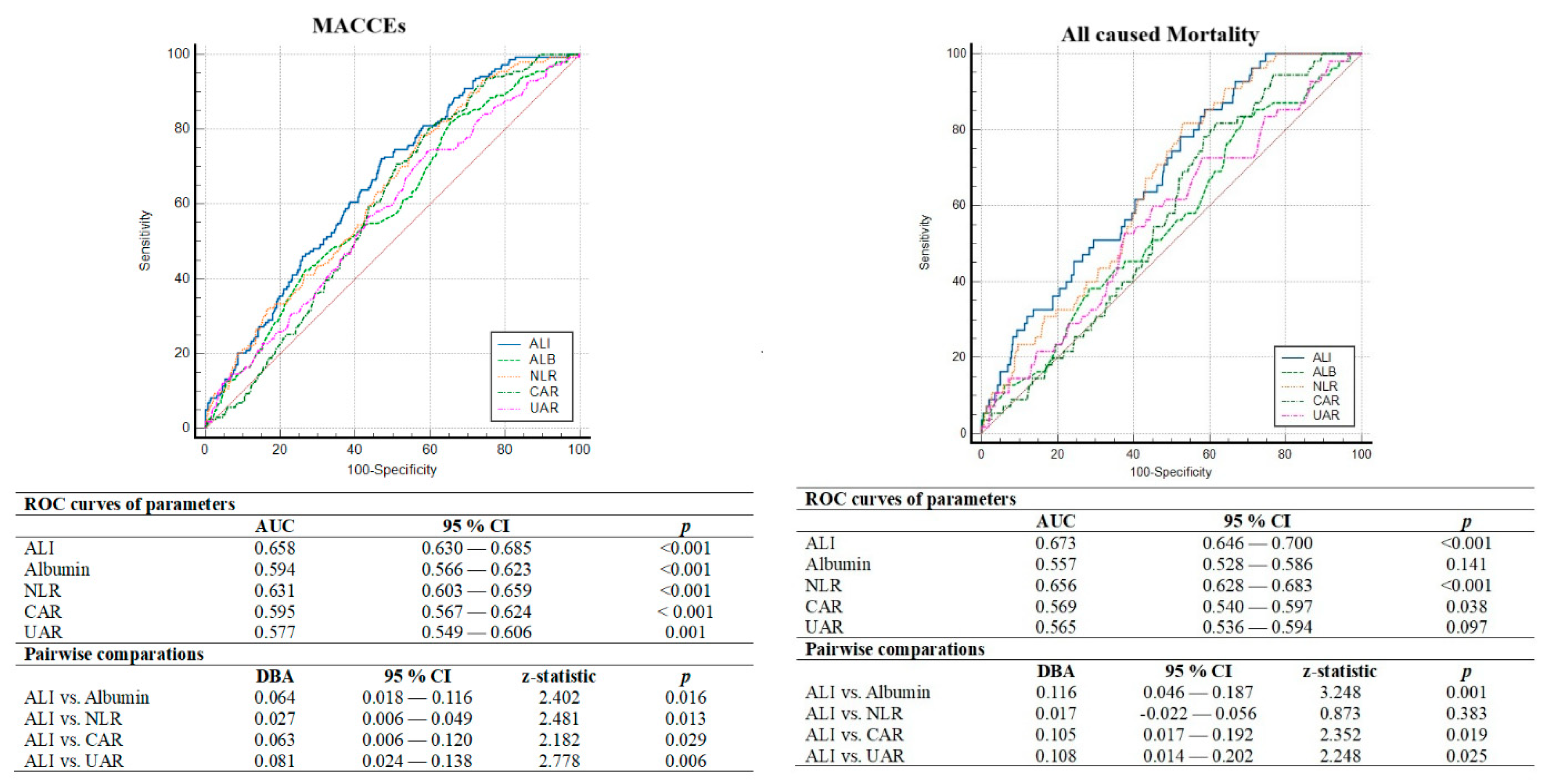

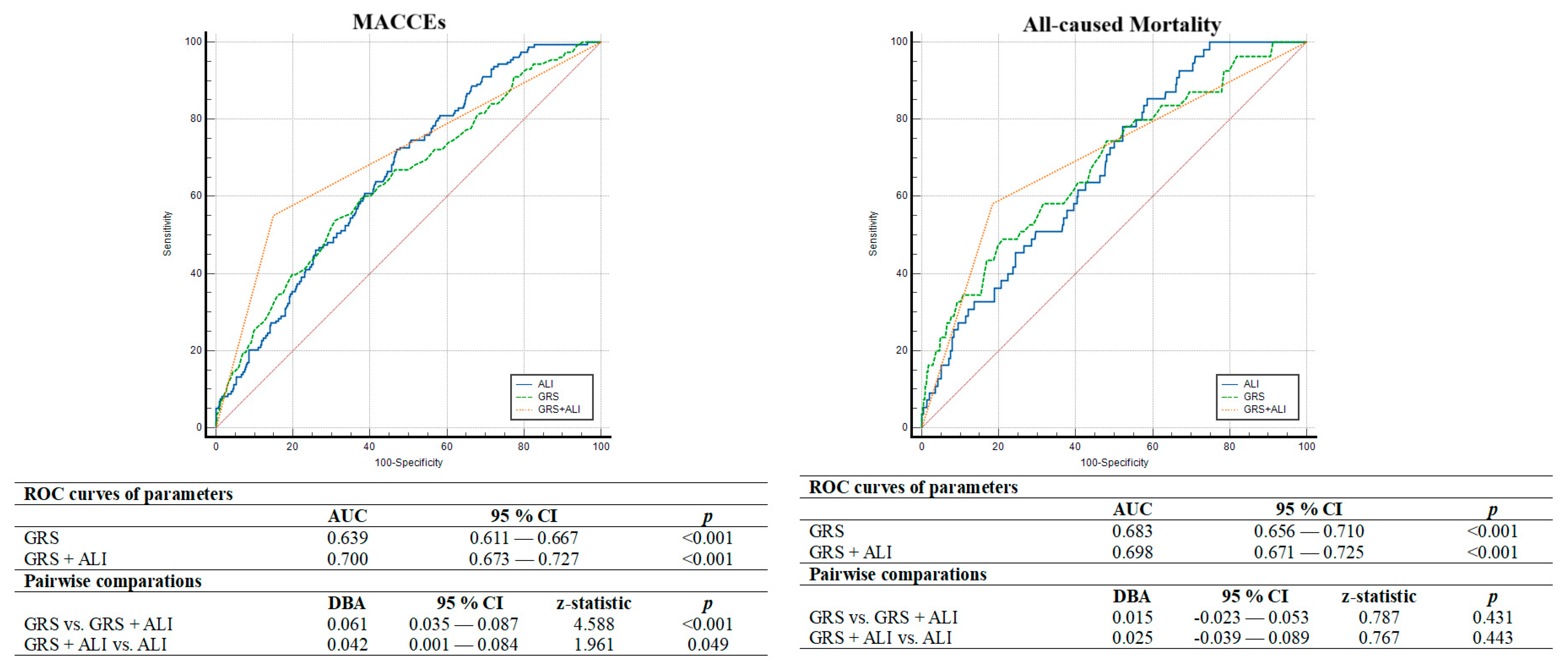
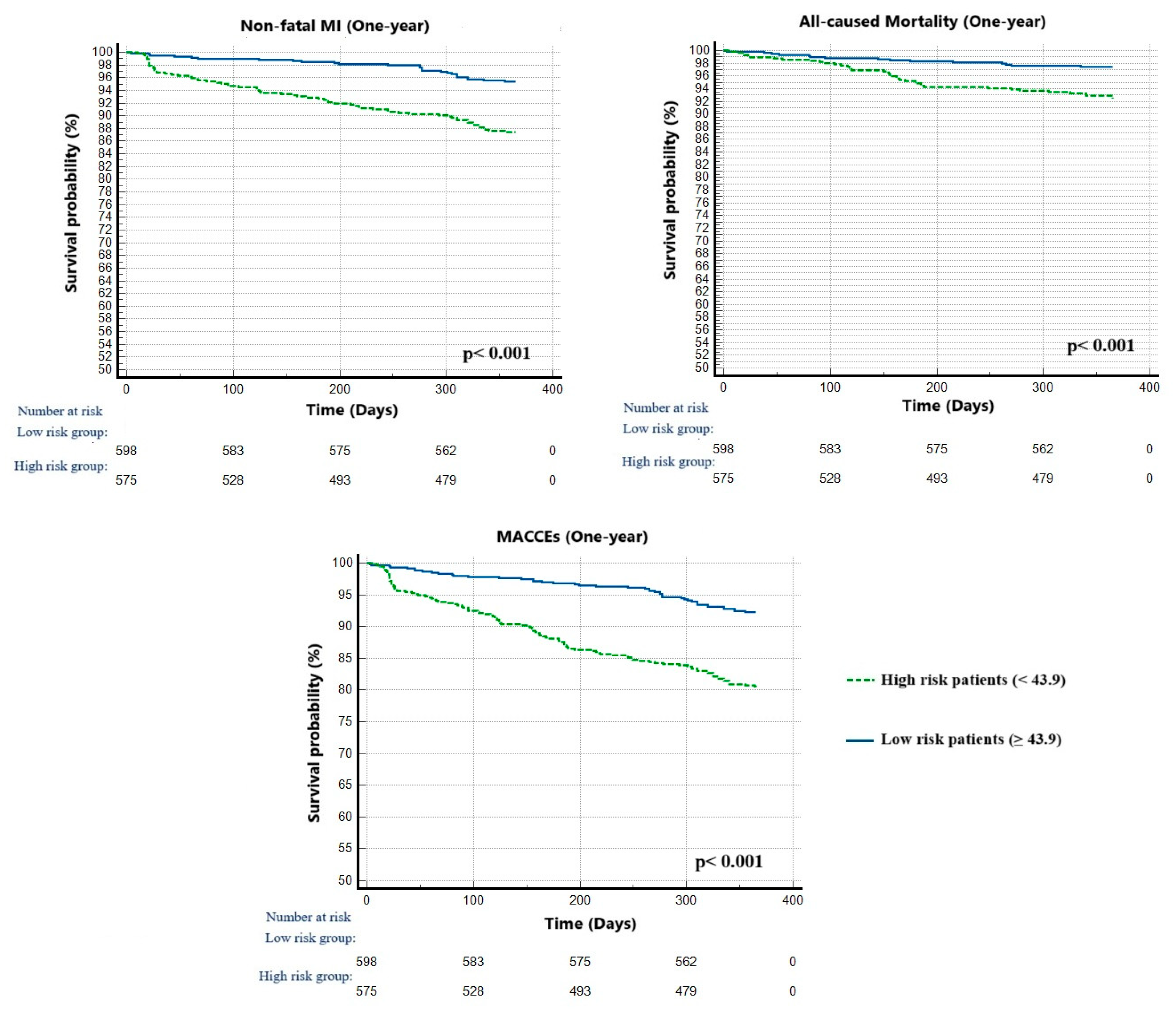
| Variables | All Population (n = 1173) | Low ALI (<43.9) (n = 575; 49.0%) | High ALI (≥43.9) (n = 598; 51.0%) | p |
|---|---|---|---|---|
| Female gender, n % | 296 (25.2) | 149 (25.9) | 147 (24.6) | 0.600 |
| Age | 61.9 ± 12.5 | 62.1 ± 12.7 | 61.6 ± 12.4 | 0.491 |
| BMI (kg/m2) | 27.7 ± 3.4 | 26.9 ± 3.4 | 28.4 ± 3.3 | <0.001 |
| Hypertension, n (%) | 674 (57.5) | 324 (56.3) | 350 (58.5) | 0.450 |
| Diabetes, n (%) | 417 (35.5) | 207 (36.0) | 210 (35.1) | 0.752 |
| Hyperlipidemia, n (%) | 548 (46.7) | 268 (46.6) | 280 (46.8) | 0.941 |
| Family history, n (%) | 411 (35.0) | 214 (37.2) | 197 (32.9) | 0.125 |
| Smoking, n (%) | 522 (44.5) | 269 (46.8) | 253 (42.3) | 0.123 |
| CAD history, n (%) | 500 (42.6) | 254 (44.2) | 246 (41.1) | 0.293 |
| MI history, n (%) | 362 (30.9) | 189 (32.9) | 173 (28.9) | 0.144 |
| PCI history, n (%) | 376 (32.1) | 192 (33.4) | 184 (30.8) | 0.336 |
| PAD history, n (%) | 33 (2.8) | 19 (3.3) | 14 (2.3) | 0.319 |
| CRF, n (%) | 132 (11.3) | 70 (12.2) | 62 (10.4) | 0.328 |
| CRF, dialysis, n (%) | 11 (0.9) | 5 (0.9) | 6 (1.0) | 0.812 |
| Killip II-IV, n (%) | 105 (9.0) | 57 (9.9) | 48 (8.0) | 0.258 |
| GRACE score | 100.0 ± 27.0 | 101.9 ± 28.2 | 98.0 ± 25.7 | 0.014 |
| LVEF (%) | 51.3 ± 9.9 | 50.3 ± 10.4 | 52.2 ± 9.3 | 0.002 |
| Hemodynamic instability, n (%) | 35 (3.0) | 25 (4.3) | 10 (1.7) | 0.007 |
| Medications, n (%) | ||||
| Acetylsalicyclic acid | 424 (36.1) | 210 (36.5) | 214 (35.8) | 0.793 |
| Clopidogrel | 137 (11.7) | 76 (13.2) | 61 (10.2) | 0.108 |
| Oral anticoagulants | 57 (4.9) | 31 (5.4) | 26 (4.4) | 0.410 |
| Beta-Blockers | 348 (29.7) | 177 (30.8) | 171 (28.6) | 0.412 |
| RAS Blockers | 549 (46.8) | 255 (44.3) | 294 (49.2) | 0.098 |
| Calcium-channel blockers | 424 (36.1) | 201 (35.0) | 223 (37.3) | 0.405 |
| Statin | 264 (22.5) | 129 (22.4) | 135 (22.6) | 0.954 |
| Antianginals | 115 (9.8) | 59 (10.3) | 56 (9.4) | 0.606 |
| Syntax Score I | 20.1 ± 6.0 | 20.7 ± 6.3 | 19.6 ± 5.7 | 0.001 |
| ‡ Complete revascularization | 564 (48.1) | 271 (47.1) | 293 (49.0) | 0.522 |
| * TIMI < 3 | 102 (8.7) | 47 (8.2) | 55 (9.2) | 0.534 |
| * Stent thrombosis | 14 (1.2) | 9(1.6) | 5 (0.8) | 0.250 |
| * Require TVR, n (%) | 39 (3.3) | 24(4.2) | 15 (2.5) | 0.112 |
| Nonfatal MI, n (%) | 96 (8.2) | 69 (12.0) | 27 (4.5) | <0.001 |
| Nonfatal stroke, n (%) | 7 (0.6) | 3 (0.5) | 4 (0.7) | 0.744 |
| Death, all-cause, n (%) | 55 (4.7) | 40 (7.0) | 15 (2.5) | <0.001 |
| MACCEs, n (%) | 158 (13.5) | 112 (19.5) | 46 (7.7) | <0.001 |
| Variables | All Population (n = 1173) | Low ALI (<43.9) (n = 575; 49.0%) | High ALI (≥43.9) (n = 598; 51.0%) | p |
|---|---|---|---|---|
| Glucose, mg/dL | 114.8 ± 32.5 | 115.5 ± 33.2 | 114.1 ± 31.8 | 0.457 |
| eGFR, mL/min/1.73 m2 | 80.8 ± 23.9 | 80.0 ± 24.2 | 81.6 ± 23.5 | 0.240 |
| Serum uric acid, mg/dL | 5.52 ± 1.8 | 5.62 ± 1.8 | 5.42 ± 1.7 | 0.053 |
| Albumin, g/dL | 3.88 ± 0.48 | 3.75 ±0.47 | 4.0 ± 0.44 | <0.001 |
| CRP, mg/dL, IQR | 5.36 [3.45–8.23] | 5.45 [3.50–8.60] | 5.23 [3.30–8.00] | 0.131 |
| Baseline Troponin I, ng/mL | 0.06 [0.02–0.21] | 0.060 [0.021–0.23] | 0.058 [0.020–0.19] | 0.011 |
| TC, mg/dL | 193.2 ± 43.5 | 193.2 ± 41.2 | 193.3 ± 45.7 | 0.968 |
| LDL-C, mg/dL | 117.1 ± 37.6 | 117.0 ± 35.6 | 117.3 ± 39.5 | 0.893 |
| HDL-C, mg/dL | 39.2 ± 10.3 | 39.5 ± 10.9 | 38.9 ± 9.6 | 0.348 |
| Triglyceride, mg/dL | 122.0 [99.0–167.0] | 121.0 [100.0–167.0] | 123.0 [99.0–169.0] | 0.867 |
| Hemoglobin, g/dL | 13.7 ± 1.9 | 13.6 ± 1.9 | 13.8 ± 1.8 | 0.065 |
| WBC, 109/L | 8.56 ± 2.2 | 8.96 ± 2.6 | 8.18 ± 1.8 | <0.001 |
| Neutrophil, 109/L | 5.29 ± 1.9 | 6.07 ± 2.1 | 4.54 ± 1.2 | <0.001 |
| Lymphocyte, 109/L | 2.06 [1.58–2.58] | 1.69 [1.28–2.15] | 2.43 [1.98–2.95] | <0.001 |
| Platelet, 109/L | 256.7 ± 76.4 | 255.6 ± 77.9 | 253.1 ± 72.0 | 0.565 |
| NLR | 2.40 [1.85–3.30] | 3.30 [2.74–4.34] | 1.87 [1.54–2.23] | <0.001 |
| ALI, kg × g/m2 × dL | 43.7 [31.1–58.5] | 30.8 [23.0–37.2] | 57.9 [50.3–73.5] | <0.001 |
| CAR, mg/g | 1.42 [0.88–2.17] | 1.51 [0.92–2.35] | 1.30 [0.84–2.0] | <0.001 |
| UAR, mg/g | 1.45 ± 0.5 | 1.54 ± 0.6 | 1.37 ± 0.5 | <0.001 |
| Clinical Variables | Laboratory Variables | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Age | 1.030 (1.017–1.044) | <0.001 | eGFR | 0.978 (0.972–0.983) | <0.001 |
| Gender | 1.689 (1.219–2.341) | 0.002 | Albumin | 0.531 (0.396–0.711) | <0.001 |
| Diabetes | 2.125 (1.555–2.904) | 0.001 | Uric acid | 1.104 (1.014–1.202) | 0.022 |
| CAD | 1.599 (1.170–2.185) | 0.003 | Troponin | 1.423 (1.333–1.519) | <0.001 |
| GRACE | 1.020 (1.014–1.026) | <0.001 | Hemoglobin | 0.796 (0.739–0.857) | <0.001 |
| LVEF | 0.951 (0.939–0.964) | <0.001 | NLR | 1.170 (1.109–1.234) | <0.001 |
| SxSI | 1.061 (1.039–1.083) | <0.001 | ALI | 0.971 (0.962–0.979) | <0.001 |
| TIMI < 3 | 1.822 (1.162–2.859) | 0.009 | CAR | 1.238 (1.071–1.430) | 0.004 |
| CR | 0.974 (0.264–0.532) | <0.001 | UAR | 1.644 (1.272–2.125) | <0.001 |
| Model 1 * | Model 2 * | Model 3 * | ||||
|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| Age | 0.983 (0.958–1.008) | 0.174 | 0.986 (0.961–1.012) | 0.278 | 0.986 (0.961–1.012) | 0.291 |
| Gender | 1.274 (0.880–1.845) | 0.200 | 1.282 (0.882–1.864) | 0.192 | 1.325 (0.913–1.924) | 0.139 |
| Diabetes | 1.470 (1.047–2.063) | 0.026 | 1.475 (1.052–2.069) | 0.024 | 1.447 (1.032–2.030) | 0.032 |
| CAD | 1.257 (0.904–1.748) | 0.174 | 1.222 (0.879–1.698) | 0.233 | 1.220 (0.878–1.695) | 0.237 |
| GRACE score | 1.009 (0.997–1.022) | 0.141 | 1.008 (0.995–1.020) | 0.238 | 1.007 (0.995–1.020) | 0.251 |
| LVEF | 0.981 (0.967–0.996) | 0.014 | 0.984 (0.969–0.999) | 0.035 | 0.983 (0.968–0.998) | 0.027 |
| SxSI | 1.050 (1.027–1.072) | <0.001 | 1.058 (1.036–1.080) | <0.001 | 1.056 (1.034–1.079) | <0.001 |
| TIMI < 3 | 1.424 (0.895–2.265) | 0.135 | 1.322 (0.831–2.102) | 0.239 | 1.393 (0.876–2.214) | 0.161 |
| CR | 0.514 (0.356–0.742) | <0.001 | 0.509 (0.352–0.736) | <0.001 | 0.510 (0.353–0.737) | <0.001 |
| eGFR | 0.987 (0.979–0.995) | 0.001 | 0.988 (0.980–0.996) | 0.003 | 0.988 (0.980–0.997) | 0.005 |
| Uric acid | 1.047 (0.962–1.140) | 0.290 | 1.051 (0.967–1.142) | 0.246 | - | - |
| Troponin | 1.358 (1.257–1.468) | <0.001 | 1.373 (1.270–1.486) | <0.001 | 1.370 (1.266–1.481) | <0.001 |
| Hemoglobin | 0.992 (0.902–1.090) | 0.865 | 0.984 (0.895–1.083) | 0.748 | 0.988 (0.897–1.088) | 0.800 |
| NLR | - | - | 1.122 (1.059–1.188) | <0.001 | 1.127 (1.065–1.193) | <0.001 |
| ALI | 0.974 (0.965–0.982) | <0.001 | - | - | - | - |
| CAR | - | - | 1.232 (1.063–1.428) | 0.006 | - | - |
| UAR | - | - | - | - | 1.404 (1.092–1.806) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaca, M.; Kalyoncuoğlu, M.; Zengin, A.; Eren, S.; Keskin, K.; Oflar, E.; Karataş, M.B.; Çalık, A.N. The Prognostic Value of the Advanced Lung Cancer Inflammation Index for Major Cardiovascular and Cerebrovascular Events in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2025, 14, 1403. https://doi.org/10.3390/jcm14051403
Karaca M, Kalyoncuoğlu M, Zengin A, Eren S, Keskin K, Oflar E, Karataş MB, Çalık AN. The Prognostic Value of the Advanced Lung Cancer Inflammation Index for Major Cardiovascular and Cerebrovascular Events in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2025; 14(5):1403. https://doi.org/10.3390/jcm14051403
Chicago/Turabian StyleKaraca, Mehmet, Muhsin Kalyoncuoğlu, Ahmet Zengin, Semih Eren, Kıvanç Keskin, Ersan Oflar, Mehmet Baran Karataş, and Ali Nazmi Çalık. 2025. "The Prognostic Value of the Advanced Lung Cancer Inflammation Index for Major Cardiovascular and Cerebrovascular Events in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention" Journal of Clinical Medicine 14, no. 5: 1403. https://doi.org/10.3390/jcm14051403
APA StyleKaraca, M., Kalyoncuoğlu, M., Zengin, A., Eren, S., Keskin, K., Oflar, E., Karataş, M. B., & Çalık, A. N. (2025). The Prognostic Value of the Advanced Lung Cancer Inflammation Index for Major Cardiovascular and Cerebrovascular Events in Patients with Non-ST Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine, 14(5), 1403. https://doi.org/10.3390/jcm14051403






