Soluble Fibrin Monomer Complex and D-Dimer Concentrations Between Patients at Low and High Risk of Venous Thromboembolism Before Delivery According to RCOG Score Assessment: An Observational Study Among 100 Third-Trimester Vietnamese Pregnancies
Abstract
1. Introduction
2. Materials and Methods
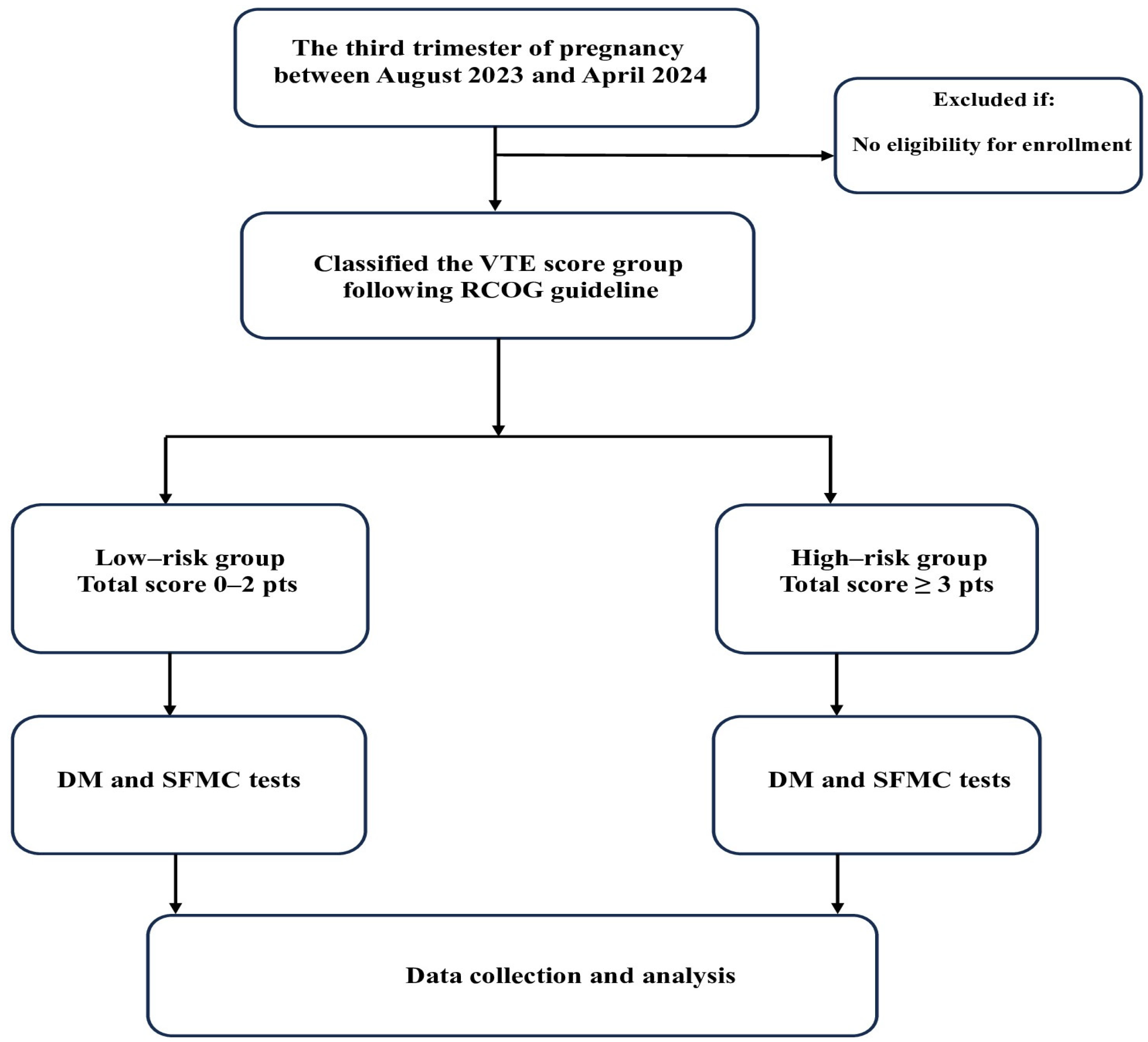
2.1. Study Population
2.2. Study Tool
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zheng, J.; Chen, Q.; Fu, J.; Lu, Y.; Han, T.; He, P. Critical appraisal of international guidelines for the prevention and treatment of pregnancy-associated venous thromboembolism: A systematic review. BMC Cardiovasc. Disord. 2019, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Vuong, A.D.B.; Pham, T.H.; Bui, V.H.; Nguyen, X.T.; Trinh, N.B.; Nguyen, Y.O.N.; Le, D.K.T.; Nguyen, P.N. Successfully conservative management of the uterus in acute pulmonary embolism during cesarean section for placenta previa: A case report from Tu Du Hospital, Vietnam and literature review. Int. J. Emerg. Med. 2024, 17, 14. [Google Scholar] [CrossRef]
- Varrias, D.; Spanos, M.; Kokkinidis, D.G.; Zoumpourlis, P.; Kalaitzopoulos, D.R. Venous Thromboembolism in Pregnancy: Challenges and Solutions. Vasc. Health Risk Manag. 2023, 19, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Gendron, N.; Philippe, A.; Diehl, J.L.; Ochat, N.; Bory, O.; Beauvais, A.; Mareau, A.; Jannot, A.S.; Chocron, R. Fibrin monomers evaluation during hospitalization for COVID-19 is a predictive marker of in-hospital mortality. Front. Cardiovasc. Med. 2023, 10, 1001530. [Google Scholar] [CrossRef]
- Mohd Ariff, N.S.; Abdul Halim Zaki, I.; Mohd Noordin, Z.; Md Hussin, N.S.; Goh, K.W.; Ming, L.C.; Zulkifly, H.H. A Review of the Prevalence of Thromboembolic Complications among Pregnant Women Infected with COVID-19. J. Clin. Med. 2022, 11, 5934. [Google Scholar] [CrossRef] [PubMed]
- Leal, D.; Ferreira, J.; Mansilha, A. Thromboembolic risk in pregnant women with SARS-CoV-2 infection—A systematic review. Taiwan. J. Obstet. Gynecol. 2022, 61, 941–950. [Google Scholar] [CrossRef]
- Daru, J.; White, K.; Hunt, B.J. COVID-19, thrombosis and pregnancy. Thromb. Update 2021, 5, 100077. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy. Obstet. Gynecol. 2018, 132, e1–e17. [Google Scholar] [CrossRef]
- Borsi, S.H.; Shoushtari, M.H.; MalAmir, M.D.; Angali, K.A.; Mavalizadeh, M.S. Comparison of the D-dimer concentration in pregnant women with or without pulmonary thromboembolism. J. Fam. Med. Prim. Care 2020, 9, 4343–4347. [Google Scholar]
- Xu, Q.; Dai, L.; Chen, H.-Q.; Xia, W.; Wang, Q.-L.; Zhu, C.-R.; Zhou, R. Specific changes and clinical significance of plasma D-dimer during pregnancy and puerperium: A prospective study. BMC Pregnancy Childbirth 2023, 23, 248. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Z.; Miao, C.; Wang, X.; Liu, W.; Chen, S.; Gao, H.; Li, W.; Wu, Z.; Cao, H.; et al. Trajectories of maternal D-dimer are associated with the risk of developing adverse maternal and perinatal outcomes: A prospective birth cohort study. Clin. Chim. Acta 2023, 543, 117324. [Google Scholar] [CrossRef]
- Refaai, M.A.; Riley, P.; Mardovina, T.; Bell, P.D. The Clinical Significance of Fibrin Monomers. Thromb. Haemost. 2018, 118, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.; Michaux, I.; Bulpa, P.; Schonau, B.; Nicolay, B.; de Maistre, E.; Godon, A.; Lecompte, T.; Mullier, F. Serial fibrin monomer and D-dimer plasma levels measurements can capture thrombotic complications in critically ill COVID-19 patients: A prospective observational study. Thromb. Res. 2023, 221, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersen, A.H.; Petersen, P.H.; Bjørge, L.; Røraas, T.; Sandberg, S. Concentration of fibrin monomer in pregnancy and during the postpartum period. Ann. Clin. Biochem. 2019, 56, 692–700. [Google Scholar] [CrossRef]
- Onishi, H.; Kaniyu, K.; Iwashita, M.; Tanaka, A.; Watanabe, T. Fibrin monomer complex in normal pregnant women: A potential thrombotic marker in pregnancy. Ann. Clin. Biochem. 2007, 44, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.Z.; Zhang, C.; Cai, Y.Q.; Huang, H.F. Application of the RCOG Risk Assessment Model for Evaluating Postpartum Venous Thromboembolism in Chinese Women: A Case-Control Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e929904. [Google Scholar] [CrossRef] [PubMed]
- Skeith, L. Prevention and management of venous thromboembolism in pregnancy: Cutting through the practice variation. Hematology 2021, 2021, 559–569. [Google Scholar] [CrossRef]
- Lamont, M.C.; McDermott, C.; Thomson, A.J.; Greer, I.A. United Kingdom recommendations for obstetric venous thromboembolism prophylaxis: Evidence and rationale. Semin. Perinatol. 2019, 43, 222–228. [Google Scholar] [CrossRef]
- Tuan, T.A.; Xoay, T.D.; My, T.T.K.; Duyen, N.T.; Trang, N.T.; Kien, N.T.; Son, C.T.; Thang, N.V.; Quan, T.Q.; Hung, D.V. Reference Value Fibrin Monomer in Healthy Children: A Cross-Sectional Study. Clin. Appl. Thromb. Hemost. 2024, 30, 10760296241247560. [Google Scholar] [CrossRef]
- Li, H.; Wan, S.; Pei, J.; Zhang, L.; Peng, J.; Che, R. Use of the RCOG risk assessment model and biomarkers to evaluate the risk of postpartum venous thromboembolism. Thromb. J. 2023, 21, 66. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hatayama, Y.; Namba, H.; Kojima, N.; Horie, T.; Yamashita, N.; Ichikawa, H.; Fukuda, T.; Motokura, T. Fibrin monomer complex as a potential thrombosis marker related to venous thromboembolism risk in pregnant women. Ann. Clin. Biochem. 2023, 60, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.R.; Emmett, S.; Wong, A. Venous thromboembolism prophylaxis in pregnancy: Are we adequately identifying and managing risks? Aust. N. Z. J. Obstet. Gynaecol. 2022, 62, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Siennicka, A.; Kłysz, M.; Chełstowski, K.; Tabaczniuk, A.; Marcinowska, Z.; Tarnowska, P.; Kulesza, J.; Torbe, A.; Jastrzębska, M. Reference Values of D-Dimers and Fibrinogen in the Course of Physiological Pregnancy: The Potential Impact of Selected Risk Factors-A Pilot Study. BioMed Res. Int. 2020, 2020, 3192350. [Google Scholar] [CrossRef] [PubMed]
- Grossman, K.B.; Arya, R.; Peixoto, A.B.; Akolekar, R.; Staboulidou, I.; Nicolaides, K.H. Maternal and pregnancy characteristics affect plasma fibrin monomer complexes and D-dimer reference ranges for venous thromboembolism in pregnancy. Am. J. Obstet. Gynecol. 2016, 215, 466.e1–466.e8. [Google Scholar] [CrossRef]
- Grouzi, E.; Pouliakis, A.; Aktypi, A.; Christoforidou, A.; Kotsi, P.; Anagnostou, G.; Foifa, A.; Papadakis, E. Pregnancy and thrombosis risk for women without a history of thrombotic events: A retrospective study of the real risks. Thromb. J. 2022, 20, 60. [Google Scholar] [CrossRef]
| Characteristics | Group of VTE Risk | Total (N = 100) | p-Value | ||
|---|---|---|---|---|---|
| Low-Risk (N = 50) | High-Risk (N = 50) | ||||
| Maternal age (years) | 30.92 ± 0.80 (16–42) | 36.42 ± 0.77 (22–56) | 33.67 ± 0.62 (16–56) | <0.0001 * | |
| Body mass index (kg/m2) | 26.04 ± 0.48 (18.29–34.24) | 34.45 ± 5.67 (20.02–36.98) | 26.83 ± 0.50 (18.29–36.98) | 0.025 * | |
| Occupation | Houseworker | 20 (48.8) | 21 (51.2) | 41 (100.0) | NA |
| Worker | 7 (36.8) | 12 (63.2) | 19 (100.0) | ||
| Farmer | 0 (0.0) | 2 (100.0) | 2 (100.0) | ||
| Teacher/staff | 17 (54.8) | 14 (45.2) | 31 (100.0) | ||
| Others | 6 (85.7) | 1 (14.3) | 7 (100.0) | ||
| Demographic region | HCM City | 9 (60.0) | 6 (40.0) | 15 (100.0) | 0.401 † |
| Others | 41 (48.2) | 44 (51.8) | 85 (100.0) | ||
| Gravida (times) | 2.32 ± 1.38 (1–6) | 2.80 ± 1.64 (1–7) | 2.56 ± 1.53 (1–7) | 0.116 * | |
| Parity (times) | <3 | 46 (51.1) | 44 (48.9) | 90 (100.0) | 0.505 † |
| ≥3 | 4 (40.0) | 6 (60.0) | 10 (100.0) | ||
| Miscarriage (times) | <3 | 49 (51.0) | 47 (49.0) | 96 (100.0) | 0.617 †† |
| ≥3 | 1 (25.0) | 3 (75.0) | 4 (100.0) | ||
| Cesarean section scar (times) | 0 | 40 (51.9) | 37 (48.1) | 77 (100.0) | 0.476 † |
| ≥1 | 10 (43.5) | 13 (56.5) | 23 (100.0) | ||
| Gestational age (weeks) | 38.38 ± 0.26 (31.6–40.4) | 34.88 ± 0.40 (28.2–40.1) | 36.63 ± 0.30 (28.2–40.4) | <0.0001 * | |
| History of disease | Hypertension | 1 (16.7) | 5 (83.3) | 6 (100.0) | NA |
| GDM | 1 (33.3) | 2 (66.7) | 3 (100.0) | ||
| Systematic erythematous lupus | 0 (0.0) | 6 (100.0) | 6 (100.0) | ||
| Nephrotic syndrome | 0 (0.0) | 2 (100.0) | 2 (100.0) | ||
| Renal dysfunction | 0 (0.0) | 3 (100.0) | 3 (100.0) | ||
| Hepatic dysfunction | 0 (0.0) | 1 (100.0) | 1 (100.0) | ||
| Hypothyroidism | 1 (33.3) | 2 (66.7) | 3 (100.0) | ||
| Hyperthyroidism | 1 (25.0) | 3 (75.0) | 4 (100.0) | ||
| Other diseases | 5 (45.45) a | 6 (54.55) b | 11 (100.0) | ||
| Surgical history | Yes | 13 (52.0) | 12 (48.0) | 25 (100.0) | 0.817 † |
| No | 37 (49.3) | 38 (50.7) | 75 (100.0) | ||
| Characteristics | Group of VTE Risk | Total | p-Value | ||
|---|---|---|---|---|---|
| Low-Risk (N = 50) | High-Risk (N = 50) | ||||
| Number of fetuses | Singleton | 49 (57.6) | 36 (42.4) | 85 (100.0) | NA |
| Twin | 0 (0.0) | 13 (100.0) | 13 (100.0) | ||
| Triplet | 1 (50.0) | 1 (50.0) | 2 (100.0) | ||
| Fetal weight (gram) | Mean ± SD (min–max) | 3052.04 ± 435.16 (1470–3760) | 2270.18 ± 614.45 (1150–3600) | 2661.11 ± 65.95 (1150–3760) | <0.0001 * |
| Amniotic fluid volume (mL) | Median IQR [1–3] | 10.50 [7.00–14.00] (3.00–28.00) | 11.00 [8.00–19.00] (3.00–68.00) | 11.00 [7.20–17.00] (3.00–68.00) | 0.483 ** |
| Largest amniotic fluid poche (cm) | Mean ± SD | 4.19 ± 1.52 | 5.06 ± 2.92 | 4.71 ± 0.31 (1.1–20.0) | 0.117 * |
| Intact amniotic membrane rupture | Yes | 45 (47.9) | 49 (52.1) | 94 (100.0) | NA |
| No | 5 (83.3) | 1 (16.7) | 6 (100.0) | ||
| Accompanied disease | Gestational hypertension | 0 (0.0) | 1 (100.0) | 1 (100.0) | NA |
| Non-severe preeclampsia | 2 (22.2) | 7 (77.8) | 9 (100.0) | ||
| Severe preeclampsia | 2 (5.7) | 33 (94.3) | 35 (100.0) | ||
| Non-medical GDM | 6 (54.5) | 5 (45.5) | 11 (100.0) | ||
| Insulin-treated GDM | 0 (0.0) | 4 (100.0) | 4 (100.0) | ||
| Other diseases | 1 (12.5) | 7 (87.5) | 8 (100.0) | ||
| Risk factors for VTE before delivery | Non-surgical previous thrombus | 0 (0.0) | 1 (100.0) | 1 (100.0) | NA |
| Surgical previous thrombus | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| High-risk embolism | 0 (0.0) | 1 (100.0) | 1 (100.0) | ||
| Severe medical disease | 0 (0.0) | 15 (100.0) | 15 (100.0) | ||
| Familial history of thrombus | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Low-risk thromboembolism | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Maternal age greater than 35 years old | 9 (19.6) | 37 (80.4) | 46 (100.0) | ||
| Obesity | 0 (0.0) | 7 (100.0) | 7 (100.0) | ||
| Parity greater than 3 | 8 (25.0) | 24 (75.0) | 32 (100.0) | ||
| Smoking | 0 (0.0) | 1 (100.0) | 1 (100.0) | ||
| Gross varicose of lower limbs | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Preeclampsia in pregnancy | 4 (9.8) | 37 (90.2) | 41 (100.0) | ||
| In vitro fertility | 1 (7.1) | 13 (92.9) | 14 (100.0) | ||
| Multipregnancy | 1 (5.9) | 16 (100.0) | 17 (100.0) | ||
| Other risk factors | 0 (0.0) | 2 (100.0) † | 2 (100.0) | ||
| Total scores | Median (min–max) | 0 [0–1] (0–2) | 4 [3–4] (3–8) | 2.5 [0–4] (0–8) | <0.0001 ** |
| Parameters | Group of VTE Risk | Total | p-Value | ||
|---|---|---|---|---|---|
| Low-Risk (N = 50) | High-Risk (N = 50) | ||||
| Total blood cell count | Platelet (109/L) | 249.72 ± 7.77 (147–456) | 235.64 ± 9.52 (81–392) | 242.68 ± 6.15 (81–456) | 0.255 * |
| Hemoglobin (g/L) | 122.56 ± 1.49 (95–141) | 116.85 ± 4.85 (11–151) | 119.70 ± 2.54 (11–151) | 0.265 * | |
| Red blood count (1012/L) | 4.54 ± 0.22 (3.45–13.42) | 4.43 ± 0.14 (9.70–221.40) | 4.48 ± 0.13 (2.49–13.42) | 0.677 * | |
| Coagulation profile | PT (%) | 104.54 ± 1.28 (88.0–129.4) | 105.32 ± 2.58 (12.7–142.5) | 104.93 ± 1.43 (12.7–142.5) | 0.786 * |
| INR | 0.98 ± 0.01 (0.88–1.09) | 0.95 ± 0.13 (0.54–1.13) | 0.97 ± 0.01 (0.54–1.13) | 0.072 * | |
| APTT (s) | 29.89 ± 0.64 (3.60–34.80) | 30.96 ± 0.47 (22.10 ± 36.50) | 30.43 ±0.40 (3.60–36.50) | 0.179 * | |
| TQ (s) | 12.70 (9.40–14.00) [12.40–13.10] | 12.50 (11.90–13.10) [11.90–13.10] | 12.6 (12.1–13.1) [8.90–14.50] | 0.444 | |
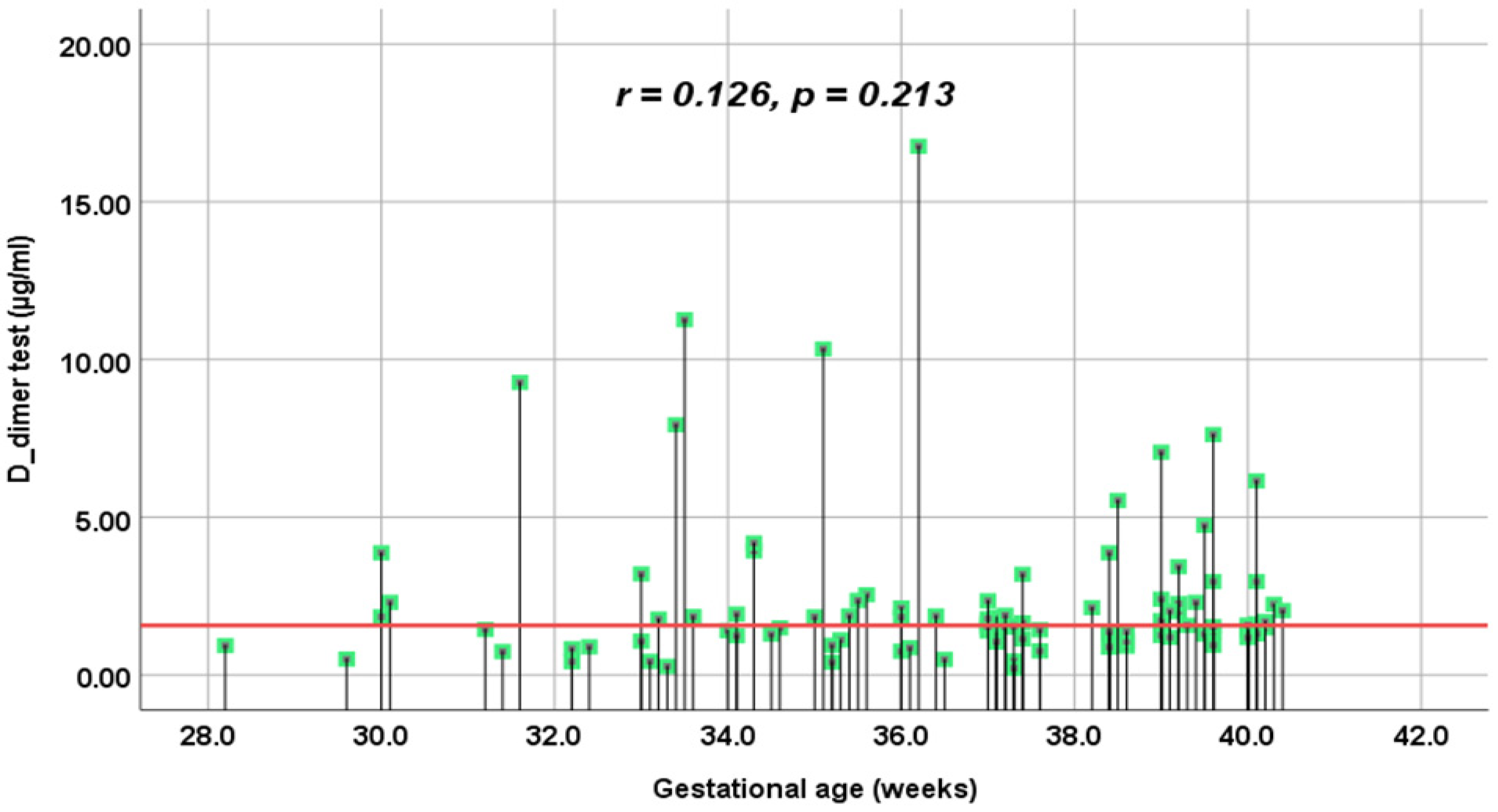
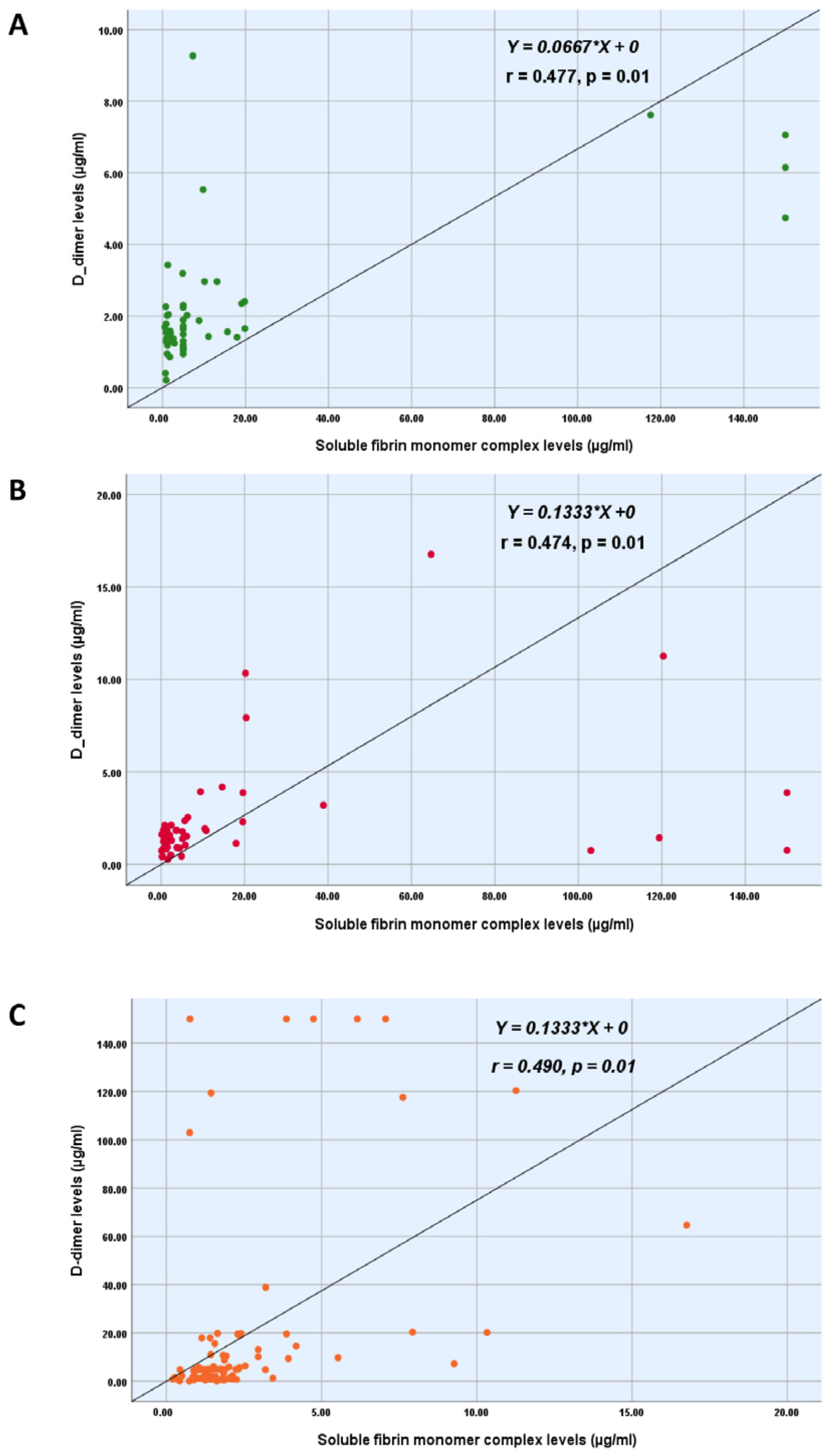
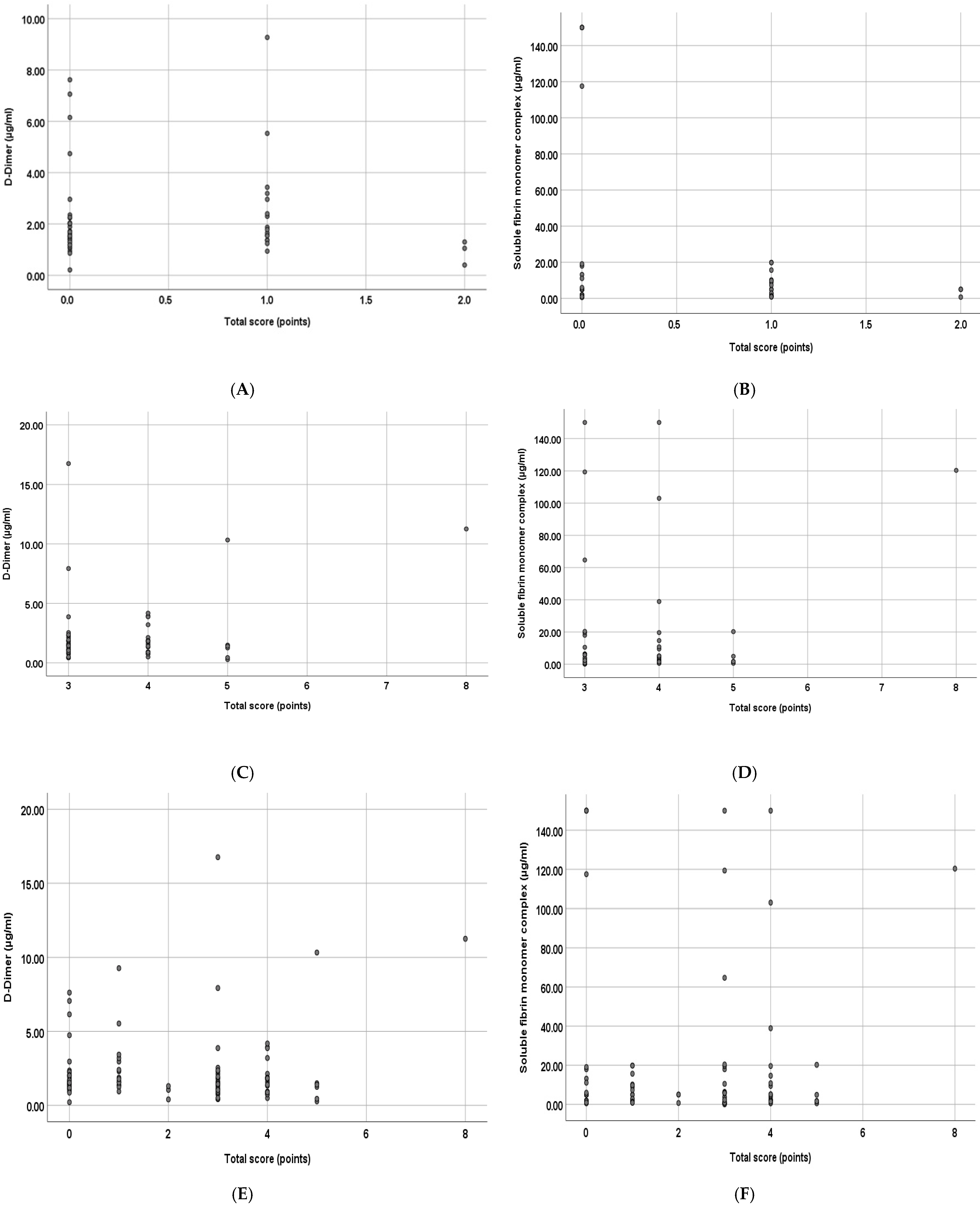
| Study tests | DD (µg/mL) | 1.61 (0.21–9.27) [1.30–2.30] | 1.51 (0.27–16.76) [0.91–2.13] | 1.57 (0.21–16.76) [1.15–2.29] | 0.282 ** |
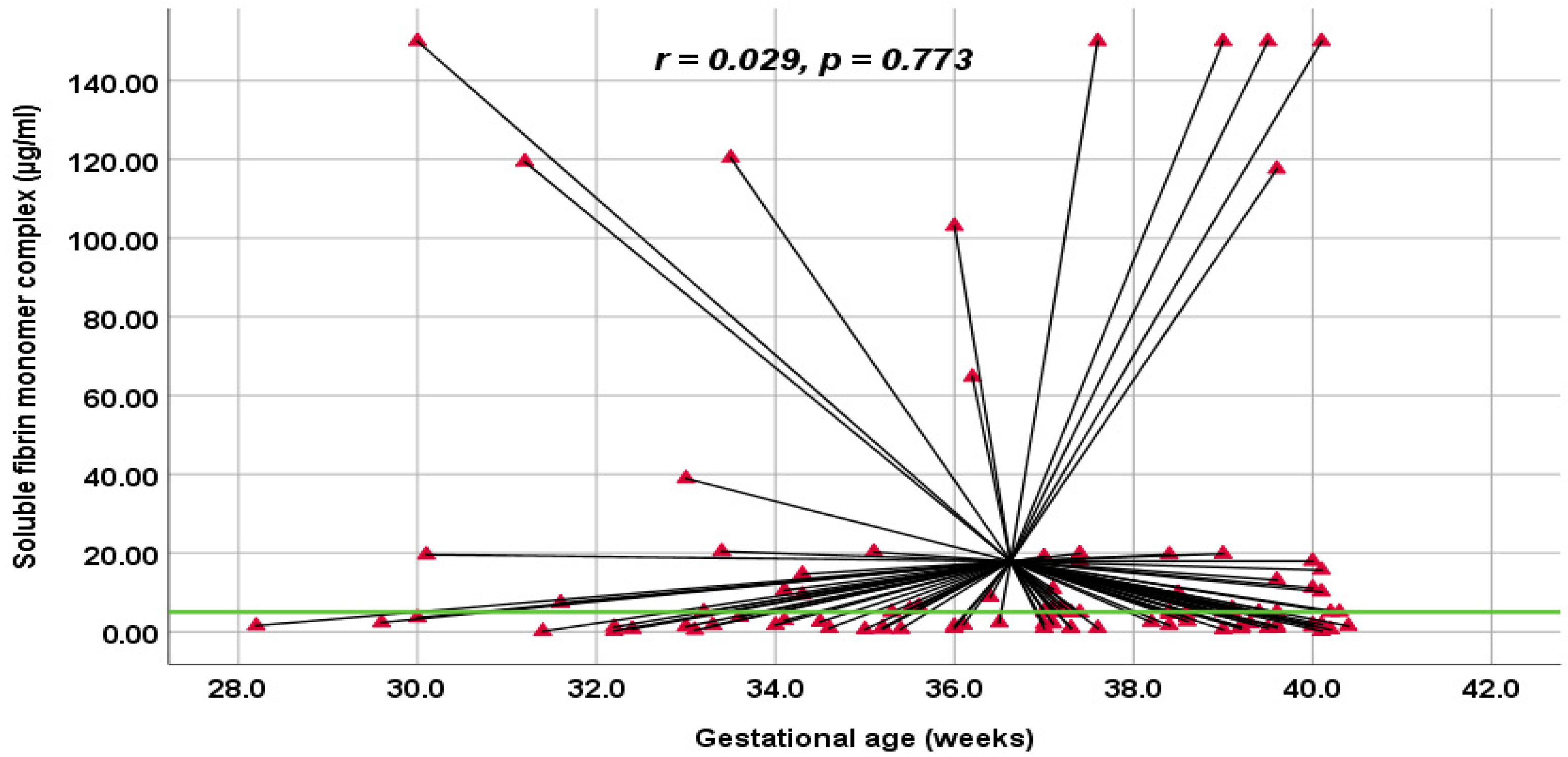
| SFMC (µg/mL) | 5.00 (0.54–150.00) [1.36–9.78] | 3.74 (0.10–150.00) [1.28–14.63] | 4.94 (0.10–150.00) [1.32–10.64] | 0.882 ** | |
| Finding Tests | Group 1 (N = 10) | Group 2 (N = 4) |
|---|---|---|
| DD (µg/mL) | 3.57 [2.06–8.76] (1.77–16.76) | 0.91 [0.69–1.16] (0.49–1.39) |
| SFMC (µg/mL) | 12.03 [5.10–38.9] (1.44–120.37) | 1.92 [1.07–3.72] (0.62–5.12) |
| VTE group | Low-Risk | High-Risk | Overall Study | |||
|---|---|---|---|---|---|---|
| DM | SFMC | DM | SFMC | DM | SFMC | |
| Spearman’s coefficient (r) | −0.095 | −0.007 | 0.004 | 0.057 | −0.002 | −0.031 |
| p-value (2-tailed) | 0.347 | 0.943 | 0.980 | 0.64 | 0.989 | 0.832 |
| Outcomes | Group of VTE Risk | Total (N = 100) | p-Value | ||
|---|---|---|---|---|---|
| Low-Risk (N = 50) | High-Risk (N = 50) | ||||
| Birth method | Cesarean section | 26 (37.7) | 43 (62.3) | 69 (100.0) | 0.0001 † |
| Vaginal birth a | 24 (77.4) | 7 (22.6) | 31 (100.0) | ||
| Induction of labor | No required | 30 (50.0) | 30 (50.0) | 60 (100.0) | NA |
| Foley balloon catheter b | 20 (52.6) | 18 (47.4) | 38 (100.0) | ||
| Dinoprostone b | 0 (0.0) | 2 (100.0) | 2 (100.0) | ||
| Indication of cesarean | Failed IOL | 8 (42.1) | 11 (57.9) | 19 (100.0) | NA |
| Non-reassuring fetal CTG | 5 (45.5) | 6 (54.5) | 11 (100.0) | ||
| Placenta previa | 7 (87.5) | 1 (12.5) | 8 (100.0) | ||
| Twin pregnancy underwent IVF | 0 (0.0) | 11 (100.0) | 11 (100.0) | ||
| CS scar ≥ 2 times | 1 (12.5) | 7 (87.5) | 8 (100.0) | ||
| Other | 3 (30.0) | 7 (100.0) | 10 (100.0) | ||
| Total blood during delivery (mL) | <500 | 44 (50.6) | 43 (49.4) | 87 (100.0) | 0.766 † |
| ≥500 | 6 (46.2) | 7 (53.8) | 13 (100.0) | ||
| Intraoperative EBL (mL) | Median ± SD (min–max) | 300 [200–400] (200–1400) | 300 [200–300] (200–700) | 300 [200–300] (200–1400) | 0.693 ** |
| Hospital length of stay (days) | Median ± SD (min–max) | 6 [5–8] (3–27) | 8 [7–9] (3–30) | 7 [5.5–9] (3–30) | 0.004 ** |
| Postoperative time duration (days) | Median ± SD (min–max) | 5 [3–5] (3–25) | 6 [5–7] (3–17) | 5 [4.5–6] (3–25) | <0.0001 ** |
| Postpartum infection | No | 49 (51.6) | 46 (48.4) | 95 (100.0) | 0.117 †† |
| Yes | 0 (0.0) | 4 (100.0) | 4 (100.0) c | ||
| Apgar score at 1 min (points) | ≤3 | 0 (0.0) | 1 (100.0) | 1 (100.0) | 1.0 †† |
| >3 | 50 (50.5) | 49 (49.5) | 99 (100.0) | ||
| Apgar score at 5 min (points) | <7 | 1 (14.3) | 6 (85.7) | 7 (100.0) | 0.112 †† |
| ≥7 | 49 (52.7) | 44 (47.3) | 93 (100.0) | ||
| Mode of neonatal care | Skin-to-skin | 32 (91.4) | 3 (8.6) | 35 (100.0) | NA |
| Neonatal unit admission <24 h | 17 (30.4) | 39 (69.6) | 55 (100.0) | ||
| NICU admission | 1 (11.1) | 8 (88.9) | 9 (100.0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuong, A.D.B.; Tran, N.H.; Pham, T.H.; Le, H.A.M.; Nguyen, P.N. Soluble Fibrin Monomer Complex and D-Dimer Concentrations Between Patients at Low and High Risk of Venous Thromboembolism Before Delivery According to RCOG Score Assessment: An Observational Study Among 100 Third-Trimester Vietnamese Pregnancies. J. Clin. Med. 2025, 14, 1399. https://doi.org/10.3390/jcm14051399
Vuong ADB, Tran NH, Pham TH, Le HAM, Nguyen PN. Soluble Fibrin Monomer Complex and D-Dimer Concentrations Between Patients at Low and High Risk of Venous Thromboembolism Before Delivery According to RCOG Score Assessment: An Observational Study Among 100 Third-Trimester Vietnamese Pregnancies. Journal of Clinical Medicine. 2025; 14(5):1399. https://doi.org/10.3390/jcm14051399
Chicago/Turabian StyleVuong, Anh Dinh Bao, Ngoc Hai Tran, Thanh Hai Pham, Hoai An Minh Le, and Phuc Nhon Nguyen. 2025. "Soluble Fibrin Monomer Complex and D-Dimer Concentrations Between Patients at Low and High Risk of Venous Thromboembolism Before Delivery According to RCOG Score Assessment: An Observational Study Among 100 Third-Trimester Vietnamese Pregnancies" Journal of Clinical Medicine 14, no. 5: 1399. https://doi.org/10.3390/jcm14051399
APA StyleVuong, A. D. B., Tran, N. H., Pham, T. H., Le, H. A. M., & Nguyen, P. N. (2025). Soluble Fibrin Monomer Complex and D-Dimer Concentrations Between Patients at Low and High Risk of Venous Thromboembolism Before Delivery According to RCOG Score Assessment: An Observational Study Among 100 Third-Trimester Vietnamese Pregnancies. Journal of Clinical Medicine, 14(5), 1399. https://doi.org/10.3390/jcm14051399







