Right Ventricular Diastolic Dysfunction Before Coronary Artery Bypass Grafting: Impact on 5-Year Follow-Up Outcomes
Abstract
1. Introduction
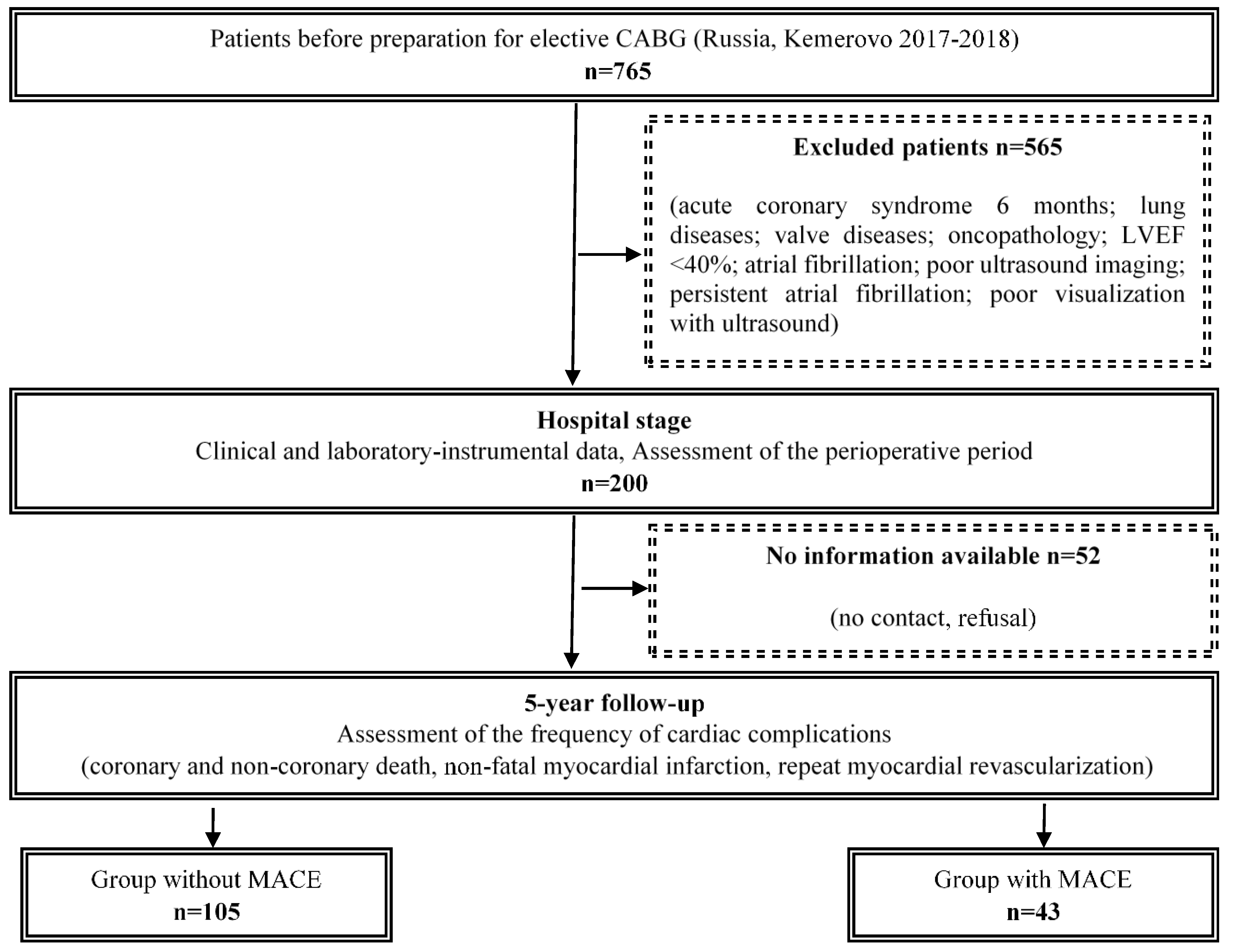
2. Material and Methods
2.1. Study Group Characteristics
2.2. Data Collection
2.3. Echocardiographic Examination
2.4. Follow-Up
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, G.; Frieling, N.; Schneck, E.; Habicher, M.; Koch, C.; Aßmus, B.; Sander, M. Comparison of preoperative NT-proBNP and simple cardiac risk scores for predicting postoperative morbidity after non-cardiac surgery with intermediate or high surgical risk. Perioper. Med. 2024, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.; Fux, T.; Kaakinen, T.; Rutanen, J.; Toivonen, J.M.; Nyström, F.; Wahba, A.; Hammas, B.; Parviainen, M.; Cunha-Goncalves, D.; et al. In Nordic countries 30-day mortality rate is half that estimated with EuroSCORE II in high-risk adult patients given aprotinin and undergoing mainly complex cardiac procedures. Scand. Cardiovasc. J. 2024, 58, 2330347. [Google Scholar] [CrossRef] [PubMed]
- Metkus, T.S.; Suarez-Pierre, A.; Crawford, T.C.; Lawton, J.S.; Goeddel, L.; Dodd-O, J.; Mukherjee, M.; Abraham, T.P.; Whitman, G.J. Diastolic dysfunction is common and predicts outcome after cardiac surgery. J. Cardiothorac. Surg. 2018, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Vizzardi, E.; Gavazzoni, M.; Sciatti, E.; Dallapellegrina, L.; Bernardi, N.; Raddino, R.; Fiorina, C.; Adamo, M.; Metra, M. Right ventricular deformation and right ventricular-arterial coupling in patients with heart failure due to severe aortic stenosis undergoing TAVI: Long-term results. Am. J. Cardiovasc. Dis. 2020, 10, 150–163. [Google Scholar] [PubMed]
- Braksator, M.; Jachymek, M.; Witkiewicz, K.; Witkiewicz, W.; Peregud-Pogorzelska, M.; Kotfis, K.; Kaźmierczak, J.; Brykczyński, M. The Impact of Left Ventricular Diastolic Dysfunction on Respiratory Adverse Events in Cardiac Surgery Patients—An Observational Prospective Single-Center Study. J. Clin. Med. 2023, 12, 4960. [Google Scholar] [CrossRef]
- Efremov, S.; Zagatina, A.; Filippov, A.; Ryadinskiy, M.; Novikov, M.; Shmatov, D. Left Ventricular Diastolic Dysfunction in Cardiac Surgery: A Narrative Review. J. Cardiothorac. Vasc. Anesth. 2024, 38, 2459–2470. [Google Scholar] [CrossRef]
- Kuwajima, K.; Ogawa, M.; Ruiz, I.; Hasegawa, H.; Yagi, N.; Rader, F.; Siegel, R.J.; Shiota, T. Echocardiographic Characteristics of Left and Right Ventricular Longitudinal Function in Patients With a History of Cardiac Surgery. Am. J. Cardiol. 2024, 211, 72–78. [Google Scholar] [CrossRef]
- Schwegel, N.; Zach, D.; Peikert, A.; Santner, V.; Höller, V.; Gollmer, J.; Späth, J.; Riepl, H.; Rainer, P.P.; Wallner, M.; et al. The Prognostic Value of Right Ventricular Function in Patients with Chronic Heart Failure—A Prospective Study. J. Clin. Med. 2024, 13, 1930. [Google Scholar] [CrossRef]
- Haruki, K.; Suzuki, A.; Yoshida, A.; Ashihara, K.; Yamaguchi, J.; Shiga, T. Persistently low tricuspid annular plane systolic excursion and its prognosis in Japanese hospitalized patients with heart failure with reduced ejection fraction. Heart Vessels 2024. [Google Scholar] [CrossRef]
- Chou, J.; Ma, M.; Gylys, M.; Salvatierra, N.; Kim, R.; Ailin, B.; Rinehart, J. Preexisting right ventricular systolic dysfunction in high-risk patients undergoing non-emergent open abdominal surgery: A retrospective cohort study. Ann. Card. Anaesth. 2021, 24, 62–71. [Google Scholar] [CrossRef]
- Rong, L.Q.; Yum, B.; Abouzeid, C.; Palumbo, M.C.; Brouwer, L.R.; Devereux, R.B.; Girardi, L.N.; Weinsaft, J.W.; Gaudino, M.; Kim, J. Echocardiographic predictors of intraoperative right ventricular dysfunction: A 2D and speckle tracking echocardiography study. Cardiovasc. Ultrasound 2019, 17, 11. [Google Scholar] [CrossRef]
- Jabagi, H.; Nantsios, A.; Ruel, M.; Mielniczuk, L.M.; Denault, A.Y.; Sun, L.Y. A standardized definition for right ventricular failure in cardiac surgery patients. ESC Heart Fail. 2022, 9, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.; Strumia, A.; Benedetto, M.; Nenna, A.; Schiavoni, L.; Barbato, R.; Mastroianni, C.; Giacinto, O.; Lusini, M.; Chello, M.; et al. Perioperative Right Ventricular Dysfunction and Abnormalities of the Tricuspid Valve Apparatus in Patients Undergoing Cardiac Surgery. J. Clin. Med. 2023, 12, 7152. [Google Scholar] [CrossRef] [PubMed]
- Labus, J.; Winata, J.; Schmidt, T.; Nicolai, J.; Zwaag, S.V.; Sveric, K.; Wilbring, M.; Scholz, M.; Fassl, J. Normal range of intraoperative three-dimensionally derived right ventricular free-wall strain in coronary artery bypass surgery patients. Echocardiography 2023, 40, 615–622. [Google Scholar] [CrossRef]
- Merlo, A.; Cirelli, C.; Vizzardi, E.; Fiorendi, L.; Roncali, F.; Marino, M.; Merlo, M.; Senni, M.; Sciatti, E. Right Ventricular Dysfunction before and after Cardiac Surgery: Prognostic Implications. J. Clin. Med. 2024, 13, 1609. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Cook, J.M.; Moukarbel, G.V.; Ashtiani, S.; Schwann, T.A.; Bonnell, M.R.; Cooper, C.J.; Khouri, S.J. Pre-operative right ventricular echocardiographic parameters associated with short-term outcomes and long-term mortality after CABG. Echo Res. Pract. 2018, 5, 155–166. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, G.; Lv, M.; Wang, H.; Xu, H.; Sun, Y.; Song, X.; Dong, L.; Feng, H.; Wang, Y. The relationship between tricuspid annular plane systolic excursion on transesophageal echocardiography and the incidence of postoperative acute kidney injury in patients undergoing coronary artery bypass grafting surgery: A multicenter prospective cohort study. BMC Anesthesiol. 2024, 24, 328. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H.; Wang, Z.; Jiang, H.; Tao, D.; Wu, H. The evaluation of preoperative right ventricular diastolic dysfunction on coronary artery disease patients with left ventricular dysfunction. Echocardiography 2014, 31, 1259–1264. [Google Scholar] [CrossRef]
- Zand, M.; Sattarzadeh, R.; Larti, F.; Mansouri, P.; Tavoosi, A. Right ventricular diastolic function predicts clinical atrial fibrillation after coronary artery bypass graft. J. Res. Med. Sci. 2022, 27, 35. [Google Scholar] [CrossRef]
- Sumin, A.N.; Shcheglova, A.V.; Korok, E.V.; Sergeeva, T.J. The Outcomes of Coronary Artery Bypass Surgery after 18 Months—Is There an Influence of the Initial Right Ventricle Diastolic Dysfunction? J. Cardiovasc. Dev. Dis. 2023, 10, 18. [Google Scholar] [CrossRef]
- Richter, M.J.; Fortuni, F.; Wiegand, M.A.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Seeger, W.; Sommer, N.; et al. Association of right atrial conduit phase with right ventricular lusitropic function in pulmonary hypertension. Int. J. Cardiovasc. Imaging 2020, 36, 633–642. [Google Scholar] [CrossRef]
- Meng, H.; Song, W.; Liu, S.; Hsi, D.; Wan, L.Y.; Li, H.; Zheng, S.S.; Wang, Z.W.; Ren, R.; Yang, W.X. Right Ventricular Diastolic Performance in Patients With Chronic Thromboembolic Pulmonary Hypertension Assessed by Echocardiography. Front. Cardiovasc. Med. 2021, 8, 755251. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Ren, X.; Suffredini, G.; Dodd-O, J.M.; Gao, W.D. Right ventricular diastolic dysfunction and failure: A review. Heart Fail. Rev. 2022, 27, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, J.S.; Andersen, S.; Ringgaard, S.; Smal, R.; Lluciá-Valldeperas, A.; Nielsen-Kudsk, J.E.; de Man, F.S.; Andersen, A. Right ventricular diastolic adaptation to pressure overload in different rat strains. Physiol. Rep. 2024, 12, e16132. [Google Scholar] [CrossRef] [PubMed]
- Sumin, A.N.; Korok, E.V.; Sergeeva, T.Y. Impaired right ventricular filling in patients with a chronic coronary syndrome. Med. Ultrason. 2021, 23, 311–318. [Google Scholar] [CrossRef]
- Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; Di Mario, C.; et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Sumin, A.N.; Korok, E.V.; Sergeeva, T.J. Preexisting Right Ventricular Diastolic Dysfunction and Postoperative Cardiac Complications in Patients Undergoing Nonemergency Coronary Artery Bypass Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 799–806. [Google Scholar] [CrossRef]
- Pouleur, A.C.; Rousseau, M.F.; Ahn, S.A.; Amzulescu, M.; Demeure, F.; de Meester, C.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.L.; Gerber, B.L. Right Ventricular Systolic Dysfunction Assessed by Cardiac Magnetic Resonance Is a Strong Predictor of Cardiovascular Death After Coronary Bypass Grafting. Ann. Thorac. Surg. 2016, 101, 2176–2184. [Google Scholar] [CrossRef][Green Version]
- Sumin, A.N.; Shcheglova, A.V.; Korok, E.V.; Sergeeva, T.J. Indicators of the Right Ventricle Systolic and Diastolic Function 18 Months after Coronary Bypass Surgery. J. Clin. Med. 2022, 11, 3994. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, R.; Leng, S.; Chen, L.; Ma, Y.; Hu, L.; Sun, A.; Wang, Q.; Zhao, X.; Tan, R.S.; Guo, C.; et al. Assessment of right ventricular diastolic function in pediatric patients with repaired tetralogy of Fallot by cardiovascular magnetic resonance and echocardiography. Eur. Radiol. 2024, 34, 5487–5500. [Google Scholar] [CrossRef]
- Hameed, I.; Sulague, R.M.; Li, E.S.; Yalcintepe, D.; Candelario, K.; Amabile, A.; Effiom, V.B.; Larson, H.; Geirsson, A.; Williams, M.L. Association between preoperative right heart catheterization parameters and outcomes in patients undergoing isolated coronary artery bypass grafting. Interdiscip. Cardiovasc. Thorac. Surg. 2024, 39, ivae158. [Google Scholar] [CrossRef] [PubMed]
- Canton, L.; Suma, N.; Amicone, S.; Impellizzeri, A.; Bodega, F.; Marinelli, V.; Ciarlantini, M.; Casuso, M.; Bavuso, L.; Belà, R.; et al. Clinical impact of multimodality assessment of myocardial viability. Echocardiography 2024, 41, e15854. [Google Scholar] [CrossRef] [PubMed]
- Arjomandi Rad, A.; Tserioti, E.; Magouliotis, D.E.; Vardanyan, R.; Samiotis, I.V.; Skoularigis, J.; Ariff, B.; Xanthopoulos, A.; Triposkiadis, F.; Casula, R.; et al. Assessment of Myocardial Viability in Ischemic Cardiomyopathy with Reduced Left Ventricular Function Undergoing Coronary Artery Bypass Grafting. Clin. Cardiol. 2024, 47, e24307. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.Y.; Yeom, S.Y.; Choi, J.W.; Oh, S.J.; Park, E.A.; Lee, W.; Kim, K.B. Cardiac Magnetic Resonance Predictor of Ventricular Function after Surgical Coronary Revascularization. J. Korean Med. Sci. 2017, 32, 2009–2015. [Google Scholar] [CrossRef]
- Koizumi, S.; Keiichi, I.; Sakai, T.; Kubota, Y.; Yokota, H.; Takaoka, H.; Kohno, H.; Matsumiya, G. Cardiac Magnetic Resonance Feature Tracking Analysis for Change in Right Ventricular Function After Cardioplegic Arrest. Heart Lung Circ. 2024, 33, 1457–1464. [Google Scholar] [CrossRef]
- Keast, T.; McErlane, J.; Kearns, R.; McKinlay, S.; Raju, I.; Watson, M.; Robertson, K.E.; Berry, C.; Greenlaw, N.; Ackland, G.; et al. Study protocol for IMPRoVE: A multicentre prospective observational cohort study of the incidence, impact and mechanisms of perioperative right ventricular dysfunction in non-cardiac surgery. BMJ Open 2023, 13, e074687. [Google Scholar] [CrossRef]
- Gozdzik, A.; Letachowicz, K.; Grajek, B.B.; Plonek, T.; Obremska, M.; Jasinski, M.; Gozdzik, W. Application of strain and other echocardiographic parameters in the evaluation of early and long-term clinical outcomes after cardiac surgery revascularization. BMC Cardiovasc. Disord. 2019, 19, 189. [Google Scholar] [CrossRef]
- Olsen, F.J.; Lindberg, S.; Pedersen, S.; Iversen, A.; Davidovski, F.S.; Galatius, S.; Fritz-Hansen, T.; Gislason, G.H.; Søgaard, P.; Møgelvang, R.; et al. Global longitudinal strain predicts cardiovascular events after coronary artery bypass grafting. Heart 2021, 107, 814–821. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; Gensini, G.F.; Ambrosio, G. Does chest shape influence exercise stress echocardiographic results in patients with suspected coronary artery disease? Intern. Emerg. Med. 2022, 17, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Heller, T.; Lang, T.; Patzelt, J.; Schreieck, J.; Schlensak, C.; Rosenberger, P.; Magunia, H. Acute changes of global and longitudinal right ventricular function: An exploratory analysis in patients undergoing open-chest mitral valve surgery, percutaneous mitral valve repair and off-pump coronary artery bypass grafting. Cardiovasc. Ultrasound. 2020, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Donauer, M.; Schneider, J.; Jander, N.; Beyersdorf, F.; Keyl, C. Perioperative Changes of Right Ventricular Function in Cardiac Surgical Patients Assessed by Myocardial Deformation Analysis and 3-Dimensional Echocardiography. J. Cardiothorac. Vasc. Anesth. 2020, 34, 708–718. [Google Scholar] [CrossRef]
- Schneider, M.; Aschauer, S.; Mascherbauer, J.; Ran, H.; Binder, C.; Lang, I.; Goliasch, G.; Binder, T. Echocardiographic assessment of right ventricular function: Current clinical practice. Int. J. Cardiovasc. Imaging 2019, 35, 49–56. [Google Scholar] [CrossRef] [PubMed]

| Variable n (%), |Me (LQ;UQ) | Total (n = 148) | Group 1 Without MACE (n = 105) | Group 2 with MACE (n = 43) | p |
|---|---|---|---|---|
| Demographic | ||||
| Sex, male | 115 (77.7) | 80 (76.2) | 35 (81.4) | 0.489 |
| Age, years | 64.0 [60.0;69.0] | 64.0 [62.0;69.0] | 64.0 [60.0;69.0] | 0526 |
| Body mass index, kgm–2 | 28.7 [25.8;31.2] | 28.7 [26.2;31.1] | 28.7 [24.6;31.2] | 0.511 |
| Smoking history active | 48 (32.4) | 34 (33.0) | 14 (33.3) | 0.971 |
| Hypertension | 140 (94.6) | 102 (97.4) | 38 (88.4) | 0.032 |
| Diabetes | 37 (25.0) | 27 (25.7) | 10 (23.3) | 0.753 |
| Hyperlipidemia | 95 (64.2) | 70 (66.7) | 25 (58.1) | 0.325 |
| Cardiovascular history | ||||
| Angina | 129 (87.2) | 92 (87.6) | 37 (86.1) | 0.795 |
| Myocardial infarction | 89 (60.1) | 56 (53.3) | 33 (76.7) | 0.008 |
| Rhythm disturbances | 29 (19.6) | 19 (18.1) | 10 (23.3) | 0.472 |
| Stroke | 13 (8.8) | 9 (8.6) | 4 (9.3) | 0.886 |
| Previous PCI | 25 (16.9) | 19 (18.1) | 6 (14.0) | 0.541 |
| CHF NYHA III FC | 38 (25.7) | 20 (19.1) | 18 (41.9) | 0.013 |
| Carotid artery bilateral ≥50% | 26 (17.6) | 20 (19.1) | 6 (14.0) | 0.459 |
| Medical treatment | ||||
| β-blockers | 138 (93.2) | 98 (93.3) | 40 (93.0) | 0.946 |
| Statins | 143 (96.6) | 103 (98.1) | 40 (93.0) | 0.121 |
| CCB | 109 (73.6) | 78 (74.3) | 31 (72.1) | 0.783 |
| ACEI | 113 (76.4) | 78 (74.3) | 35 (81.4) | 0.355 |
| Aspirin | 135 (91.2) | 95 (90.5) | 40 (93.0) | 0.619 |
| Laboratory parameters | ||||
| Total cholesterol, mmol/L | 4.4 [3.7;5.3] | 4.6 [3.7;5.4] | 4.2 [3.7;5.0] | 0.296 |
| LDL cholesterol, mmol/L | 2.7 [2.1;3.5] | 2.7 [2.1;3.6] | 2.6 [1.9;3.3] | 0.442 |
| HDL cholesterol, mmol/L | 1.13 [0.9;1.3] | 1.1 [0.92;1.3] | 1.16 [0.9;1.4] | 0.583 |
| Triglycerides, mmol/L | 1.42 [1.1;2.2] | 1.5 [1.2;2.2] | 1.3 [1.0;1.8] | 0.009 |
| Glucose, mmol/L | 5.7 [5.3;6.5] | 5.7 [5.3;6.6] | 5.7 [5.2;6.3] | 0.471 |
| Creatinine, mmol/L | 85.5 [76.5;97.5] | 85.0 [75.0;101.0] | 86.0 [80.0;92.0] | 0.958 |
| NT-proBNP, pg/ml | 67.4 [32.3;122.0] | 62.0 [32.4;103.0] | 78.5 [32.1;171.0] | 0.465 |
| Coronary angiography | ||||
| 1—coronary artery disease | 12 (8.1) | 10 (9.5) | 2 (4.7) | 0.324 |
| 2—coronary artery disease | 61 (41.2) | 42 (40.0) | 19 (44.2) | 0.639 |
| 3—coronary artery disease | 72 (48.6) | 50 (47.6) | 22 (51.2) | 0.695 |
| Variable n (%)|Me (LQ;UQ) | Total (n = 148) | Group 1 Without MACE (n = 105) | Group 2 with MACE (n = 43) | p |
|---|---|---|---|---|
| Intraoperative characteristics | ||||
| Number of shunts | 3.0 [2.0;3.0] | 3.0 [2.0;3.0] | 2.0 [2.0;3.0] | 0.324 |
| Cardiopulmonary bypass duration, minutes | 77.0 [67.0;94.0] | 77.0 [68.0;93.0] | 75.5 [65.0;96.0] | 0.711 |
| Aortic cross-clamp time, min | 51.0 [41.5;60.0] | 50.5 [42.0;59.0] | 52.0 [41.0;62.0] | 0.636 |
| Ventriculoplasty | 11 (7.4) | 6 (5.7) | 5 (11.6) | 0.213 |
| Thrombectomy | 7 (4.7) | 4 (3.8) | 3 (7.0) | 0.409 |
| Radiofrequency ablation | 4 (2.7) | 3 (2.9) | 1 (2.3) | 0.856 |
| Carotid endarterectomy | 18 (12.2) | 13 (12.4) | 5 (11.6) | 0.898 |
| Mitral valve replacement | 1 (0.7) | 0 (0) | 1 (2.3) | 0.116 |
| Prosthetics of the aortic valve | 1 (0.7) | 1 (0.95) | 0 (0) | 0.521 |
| Variable n (%), Me (LQ;UQ) | Total (n = 148) | Group 1 Without MACE (n = 105) | Group 2 MACE (n = 43) | p |
|---|---|---|---|---|
| Structural indicators and systolic function | ||||
| Aorta, mm | 3.55 [3.3;3.8] | 3.6 [3.3;3.8] | 3.5 [3.2;3.8] | 0.864 |
| LA, mm | 4.5 [4.1;4.9] | 4.5 [4.1;4.8] | 4.6 [4.2;5.0] | 0.256 |
| EDD, mm | 5.5 [5.3;6.1] | 5.5 [5.2;6.1] | 5.6 [5.4;6.1] | 0.228 |
| ESD, mm | 3.6 [3.3;4.0] | 3.6 [3.3;4.0] | 3.7 [3.5;4.4] | 0.02 |
| ESDi, mm/m2 | 1.88 [1.72;2.1] | 1.82 [1.7;2.0] | 2.0 [1.8;2.3] | 0.001 |
| EDDi, mm/m2 | 2.91 [2.8;3.1] | 2.9 [2.8;3.1] | 3.1 [2.8;3.2] | 0.06 |
| EDV, mL | 147.0 [132.5;187.0] | 147.0 [130.0;187.0] | 154.0 [141.0;187.0] | 0.231 |
| ESV, mL | 51.0 [44.0;70.0] | 51.0 [43.0;70.0] | 51.0 [47.0;83.0] | 0.151 |
| ESVi, mL/m2 | 27.5 [21.8;35.1] | 26.7 [21.6;34.3] | 29.2 [25.3;39.8] | 0.07 |
| EDVi, mL/m2 | 79.3 [70.3;93.4] | 77.3 [68.5;92.1] | 83.6 [74.5;96.9] | 0.08 |
| LVEF, % | 61.0 [56.0;65.0] | 62.0 [56.0;66.0] | 60.0 [52.0;63.0] | 0.056 |
| SV, mL | 147.0 [132.5;187.0] | 147.0 [130.0;187.0] | 160.5 [135.0;180.0] | 0.652 |
| LVM, g | 312.3 [259.0;374.6] | 313.0 [242.1;374.0] | 302.6 [284.0;375.0] | 0.428 |
| LVMi | 149.7 [131.9;184.1] | 146.6 [128.2;182.3] | 163.0 [144.5;203.6] | 0.112 |
| IVST, cm | 1.1 [1.0;1.2] | 1.1 [1.0;1.3] | 1.1 [1.0;1.2] | 0.857 |
| PW LV, cm | 1.1 [1.0;1.2] | 1.1 [1.0;1.2] | 1.1 [1.0;1.2] | 0.109 |
| Diastolic function | ||||
| IVRT, m/s | 92.0 [90.0;98.0] | 92.0 [90.0;98.0] | 92.0 [90.0;98.0] | 0.307 |
| DT | 236.0 [209.0;263.0] | 229.0 [209.0;263.0] | 236.0 [202.0;270.0] | 0.489 |
| E, cm/s | 59.0 [46.0;66.0] | 59.0 [46.0;67.5] | 57.0 [44.0;65.0] | 0.298 |
| A, cm/s | 67.5 [59.0;80.5] | 67.0 [57.0;80.0] | 70.0 [60.0;81.0] | 0.475 |
| E/A | 0.77 [0.66;1.06] | 0.79 [0.68;1.06] | 0.75 [0.69;1.05] | 0.237 |
| e’, cm/s | 9.1 [7.5;11.0] | 9.4 [7.7;11.6] | 8.5 [7.3;11.0] | 0.093 |
| a′, cm/s | 9.9 [8.6;11.6] | 9.8 [8.6;11.4] | 10.5 [8.9;12.0] | 0.323 |
| e′/a′ | 0.87 [0.71;1.27] | 0.91 [0.7;1.3] | 0.76 [0.64;1.1] | 0.041 |
| s′, cm/s | 9.0 [8.0;10.5] | 9.0 [8.0;10.6] | 9.0 [8.0;9.9] | 0.714 |
| E/e′, | 6.2 [4.8;7.6] | 6.2 [4.8;7.5] | 6.5 [4.9;7.7] | 0.652 |
| Tei LV | 0.32 [0.25;0.4] | 0.33 [0.25;0.41] | 0.28 [0.25;0.39] | 0.317 |
| Variable n (%), Me (LQ;UQ) | Total (n = 148) | Group 1 Without MACE (n = 105) | Group 2 MACE (n = 43) | p |
|---|---|---|---|---|
| Structural indicators and systolic function | ||||
| RV, mm | 2.0 [1.9;2.3] | 2.0 [1.8;2.3] | 2.0 [2.0;2.2] | 0.902 |
| RVth,MM [LQ, UQ] | 0.4 [0.3;0.4] | 0.4 [0.3;0.4] | 0.4 [0.3;0.4] | 0.962 |
| TAPSE, mm | 23.0 [21.0;26.0] | 23.0 [21.0;26.0] | 22.0 [21.0;26.0] | 0.608 |
| RVEF, % | 55.0 [53.0;57.0] | 55.0 [53.0;57.0] | 55.0 [53.0;57.0] | 0.713 |
| RA, mm | 40.0 [31.0;50.0] | 40.0 [30.0;49.0] | 39.0 [34.0;51.0] | 0.988 |
| mPAP, mmhg | 12.0 [11.0;14.0] | 11.0 [11.0;13.0] | 13.0 [12.0;15.0] | 0.137 |
| sPAP, mmhg | 27.0 [24.0;30.0] | 27.0 [24.0;30.0] | 25.0 [24.0;28.0] | 0.755 |
| Diastolic function | ||||
| Et, cm/s | 44.0 [37.0;49.0] | 44.0 [38.0;48.0] | 42.0 [35.0;49.0] | 0.632 |
| At, cm/s | 42.0 [34.0;49.0] | 42.0 [34.0;48.0] | 43.0 [35.0;49.0] | 0.376 |
| Et/At | 1.1 [0.8;1.4] | 1.5 [1.3;1.7] | 1.4 [0.99;1.5] | 0.06 |
| e′t, cm/s | 9.4 [8.2;11.3] | 9.4 [8.0;11.3] | 9.4 [8.4;11.3] | 0.933 |
| a′t, cm/s | 14.3 [12.1;16.0] | 14.0 [12.0;15.6] | 14.8 [12.9;17.0] | 0.117 |
| e′t/a′t, cm/s | 0.69 [0.6;0.8] | 0.7 [0.6;0.8] | 0.68 [0.57;0.74] | 0.233 |
| s’t, cm/s | 13.3 [11.9;15.0] | 13.3 [11.9;15.0] | 13.3 [11.9;15.2] | 0.582 |
| Et/e′t | 4.4 [3.5;5.5] | 4.5 [3.7;5.5] | 4.3 [3.4;5.1] | 0.577 |
| RV Tei index | 0.3 [0.23;0.37] | 0.3 [0.24;0.37] | 0.28 [0.22;0.36] | 0.335 |
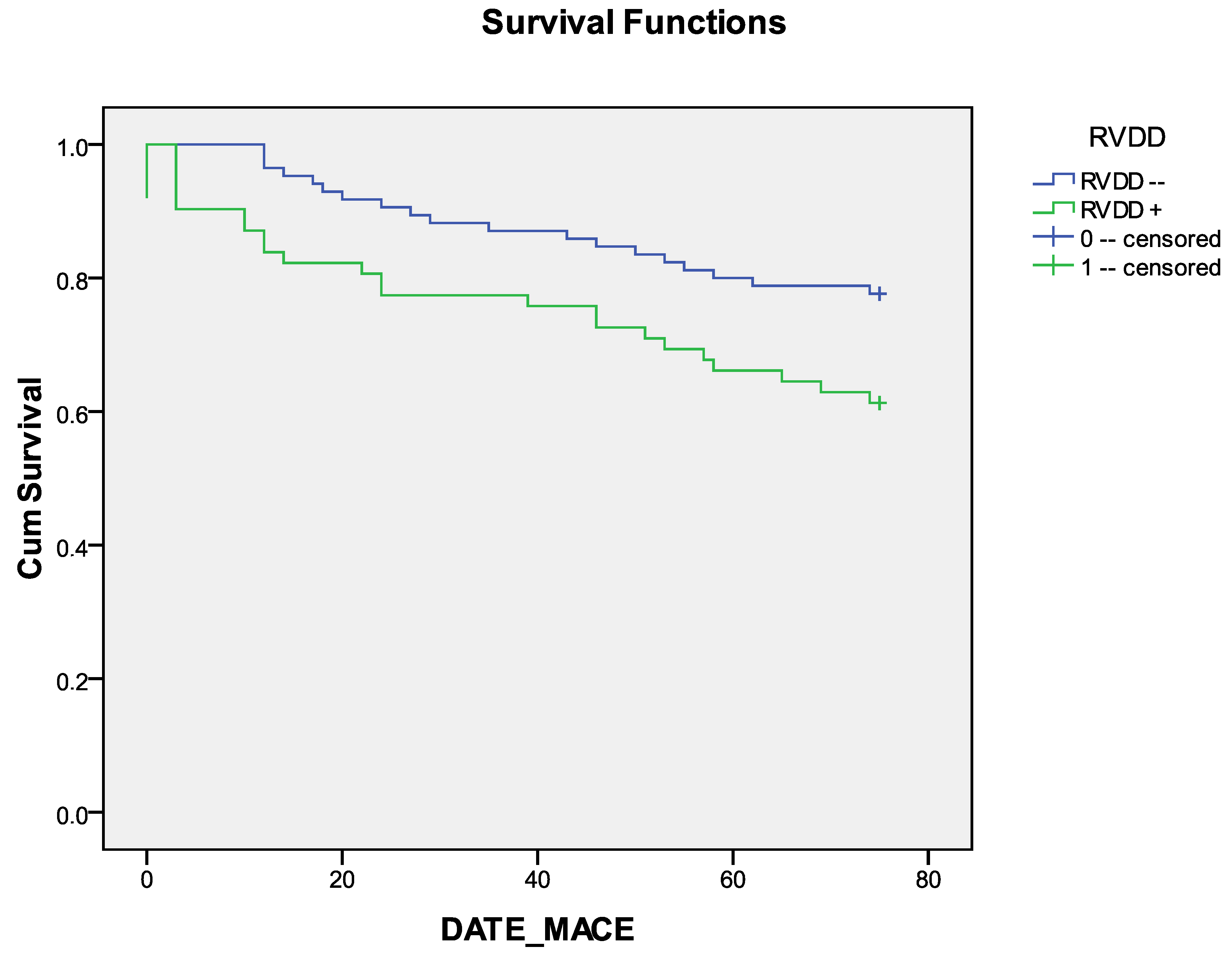
| RVDD, n (%) | 62 (41.9) | 39 (37.1) | 23 (56.1) | 0.037 |
| RVDD | Mean | |||
|---|---|---|---|---|
| 95% Confidence Interval | ||||
| Estimate | Std. Error | Lower Bound | Upper Bound | |
| RVDD− | 65.729 | 2.161 | 61.495 | 69.964 |
| RVDD+ | 57.081 | 3.471 | 50.278 | 63.884 |
| Overall | 62.082 | 1.957 | 58.247 | 65.916 |
| Chi-Square | df | Sig. | |
|---|---|---|---|
| Log Rank (Mantel-Cox) | 4.976 | 1 | 0.026 |
| Breslow (Generalized Wilcoxon) | 5.277 | 1 | 0.022 |
| Tarone-Ware | 5.132 | 1 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumin, A.N.; Shcheglova, A.V.; Oganyan, N.D.; Romanenko, E.Y.; Sergeeva, T.Y. Right Ventricular Diastolic Dysfunction Before Coronary Artery Bypass Grafting: Impact on 5-Year Follow-Up Outcomes. J. Clin. Med. 2025, 14, 1398. https://doi.org/10.3390/jcm14041398
Sumin AN, Shcheglova AV, Oganyan ND, Romanenko EY, Sergeeva TY. Right Ventricular Diastolic Dysfunction Before Coronary Artery Bypass Grafting: Impact on 5-Year Follow-Up Outcomes. Journal of Clinical Medicine. 2025; 14(4):1398. https://doi.org/10.3390/jcm14041398
Chicago/Turabian StyleSumin, Alexey N., Anna V. Shcheglova, Nazeli D. Oganyan, Evgeniya Yu. Romanenko, and Tatjana Yu. Sergeeva. 2025. "Right Ventricular Diastolic Dysfunction Before Coronary Artery Bypass Grafting: Impact on 5-Year Follow-Up Outcomes" Journal of Clinical Medicine 14, no. 4: 1398. https://doi.org/10.3390/jcm14041398
APA StyleSumin, A. N., Shcheglova, A. V., Oganyan, N. D., Romanenko, E. Y., & Sergeeva, T. Y. (2025). Right Ventricular Diastolic Dysfunction Before Coronary Artery Bypass Grafting: Impact on 5-Year Follow-Up Outcomes. Journal of Clinical Medicine, 14(4), 1398. https://doi.org/10.3390/jcm14041398






