An Inadequate Blood Supply Is a Risk Factor of Anastomotic Biliary Strictures After Liver Transplantation—A Single-Center Study
Abstract
1. Introduction
2. Material and Methods
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Gurakar, A.; Jabbour, N. Biliary strictures following liver transplantation: Past, present and preventive strategies. Liver Transpl. 2008, 14, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Pascher, A.; Neuhaus, P. Biliary complications after deceased-donor liver transplantation. J. Hepatobiliary Pancreat. Surg. 2006, 13, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, G.; Parungao, J.M.; Hanouneh, I.A.; Parsi, M. Biliary complications following liver transplantation. World J. Gastroenterol. 2013, 19, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, N.; Sugawara, Y.; Hashimoto, D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: A systemic review of the incidence, risk factors and outcome. Transpl. Int. 2011, 24, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Kienlein, S.; Schoening, W.; Andert, A.; Kroy, D.; Neumann, U.P.; Schmeding, M. Biliary complications after liver transplantation: Impact of anastomotic technique and ischemic time on short- and long-term outcome. World J. Transpl. 2015, 5, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Scatton, O.; Meunier, B.; Cherqui, D.; Boillot, O.; Sauvanet, A.; Boudjema, K.; Launois, B.; Fagniez, P.-L.; Belghiti, J.; Wolff, P.; et al. Randomized trial of choledochocholedochostomy with and without T-tube in orthotopic liver transplantation. Ann. Surg. 2001, 233, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Schmidt, S.C.; Ulrich, F.; Andreas, P.; Guido, S.; Martin, S.; Gero, P.; Olaf, G.; Ulf, N.; Johann, P.; et al. Biliary reconstruction using a side-to-side choledochocholedochostomy with or without T-tube in deceased-donor liver transplantation: A prospective randomized trial. Ann. Surg. 2009, 250, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Zeair, S.; Stasiuk, R.; Zair, L.; Wawrzynowicz-Syczewska, M.; Rybicka, A.; Grochans, E.; Kazimierczak, A. Incidents and risk factors of biliary complications after orthotopic liver transplantation. Medicine 2021, 100, 34. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, R.C.; Buis, C.I.; Porte, R.J.; van der Jagt, E.J.; Limburg, A.J.; Berg, A.P.v.D.; Slooff, H.M.J.; Peeters, P.M.; de Jong, K.P.; Kleibeuker, J.H.; et al. Anastomotic biliary strictures after liver transplantation: Causes and consequences. Liver Transpl. 2006, 12, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Huang, Z.Y.; Chen, X.P. Biliary complications after living donor liver transplantation. Liver Transpl. 2011, 17, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Lee, I.S.; Choi, J.Y.; Yoon, S.K.; Kim, D.G.; You, Y.K.; Chun, H.J.; Lee, D.K.; Choi, M.-G.; Chong, I.-S. Biliary stricture after adult right- lobe living-donor liver transplantation with duct-to-duct anastomosis: Long-term outcome and its related factors after endoscopic treatment. Gut Liver 2010, 4, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Wojcicki, M.; Lubikowski, J.; Klek, R.; Post, M.; Jarosz, K.; Białek, A.; Wunch, M.; Czuprynska, M. Reduction of biliary complications rate using continuous suture and no biliary drainage for duct-to-duct anastomosis in whole-organ liver transplantation. Transpl. Proc. 2009, 41, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Magro, B.; Tacelli, M.; Mazzola, A.; Conti, F.; Celsa, C. Biliary complications after liver transplantation: Current perspectives and future strategies. Hepatobiliary Surg. Nutr. 2021, 10, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Kalloo, A.N. Biliary complications after liver transplantation. Curr. Treat. Options Gastroenterol. 2002, 5, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Koneru, B.; Sterling, M.J.; Bahramipour, P.F. Bile duct strictures after liver transplantation: A changing landscape of the Achilles’ heel. Liver Transpl. 2006, 12, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.P.; Fernandez, L.A.; Leverson, G.; Anderson, M.; Mezrich, J.; Sollinger, H.W.; D’Alessandro, A. Biliary complications after liver transplantation from donation after cardiac death donors: An analysis of risk factors and long-term outcomes from a single center. Ann. Surg. 2011, 253, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Guichelaar, M.M.J.; Benson, J.T.; Malinchoc, M.; Krom, R.A.F.; Wiesner, R.H.; Charlton, M.R. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am. J. Transpl. 2003, 3, 885–890. [Google Scholar] [CrossRef] [PubMed]
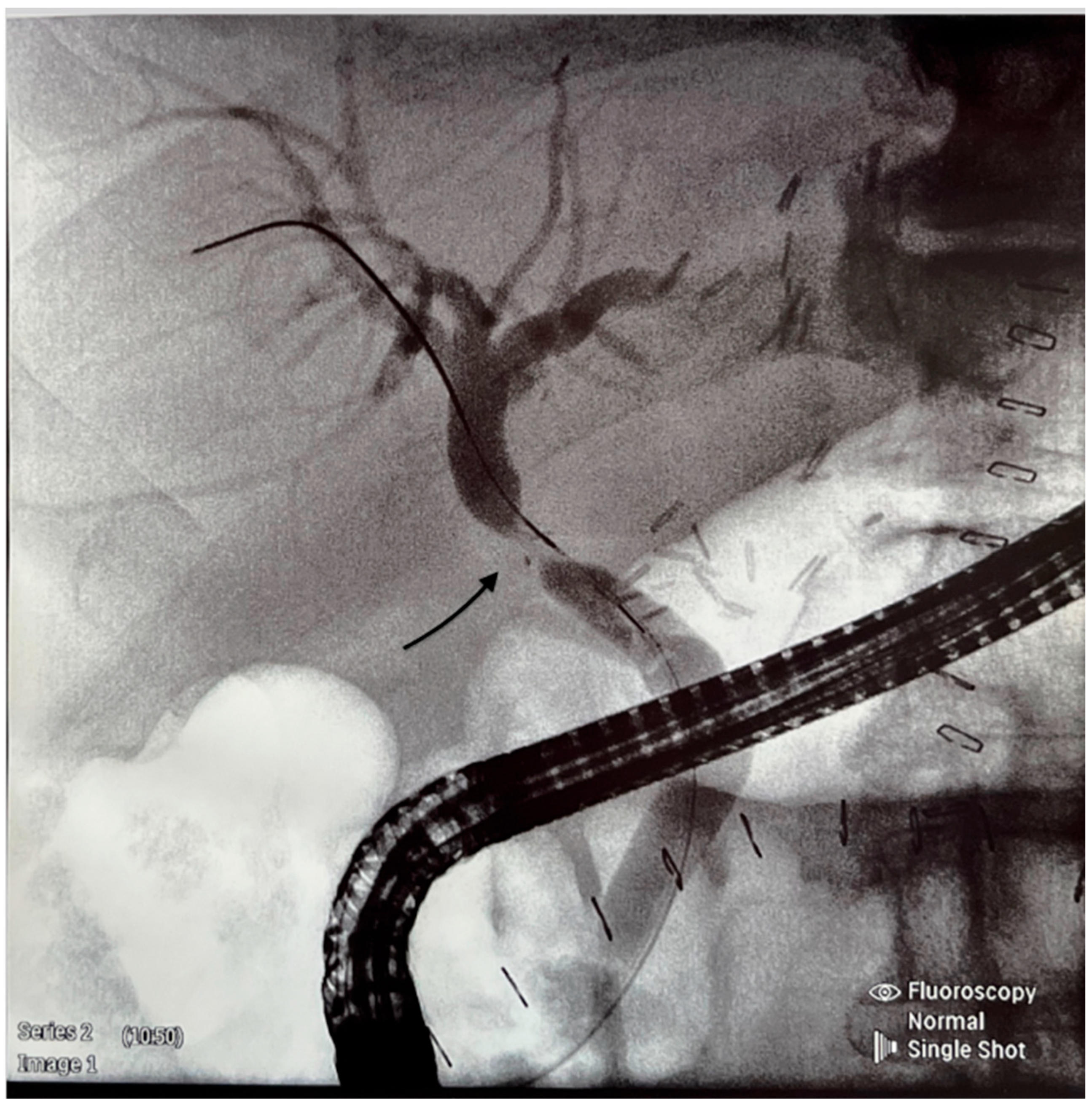
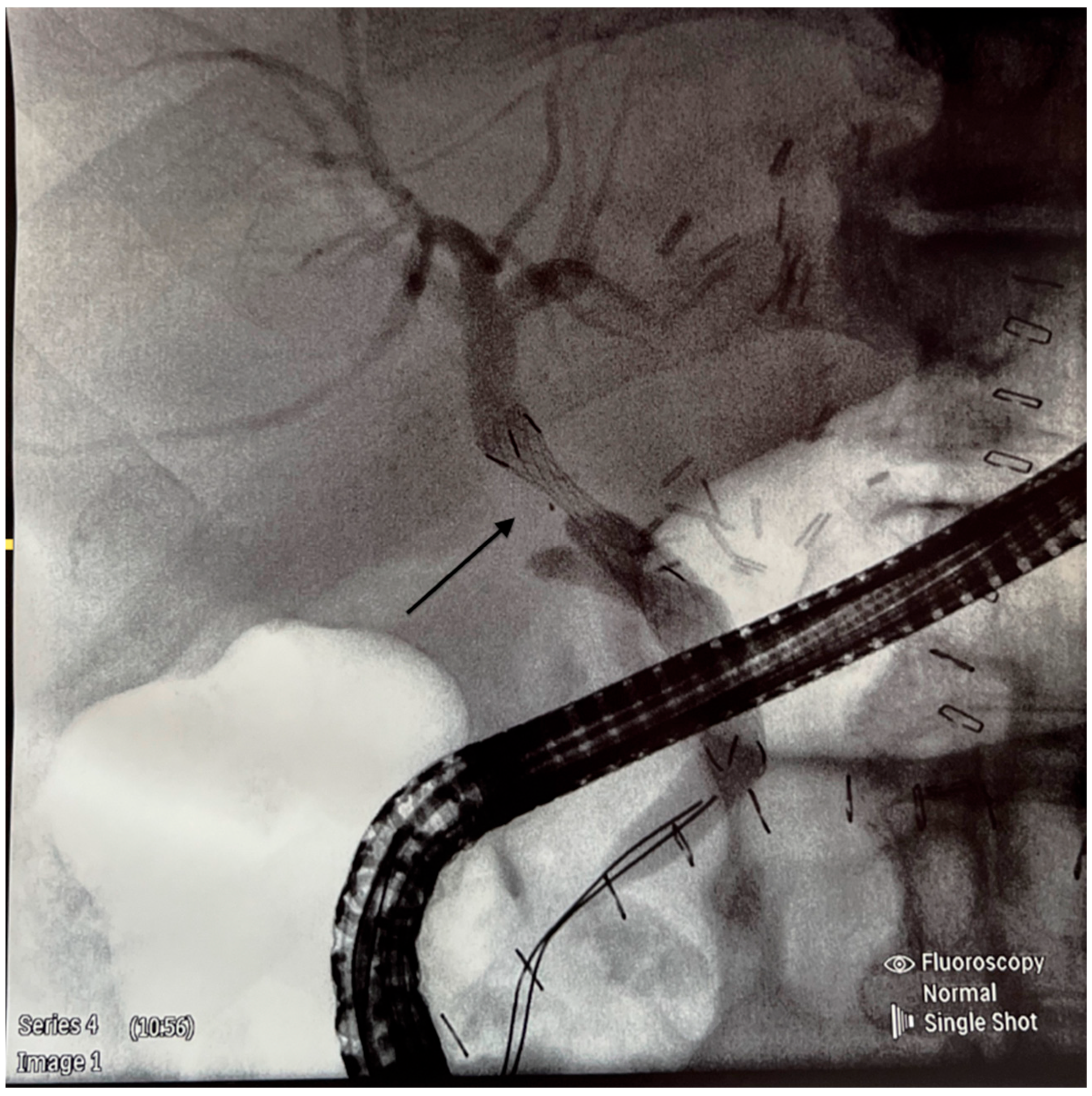

| Median or Numbers | %, 95% CI or IQR | |
|---|---|---|
| Age [median, IQR] | 58 | 46–66 |
| Gender [male, %] | 135 | 58.7 |
| MELD [mean, 95% CI] | 14.9 | 6.0–23.4 |
| Etiology of liver disease: | ||
| Cholestatic [n, %] | 32 | 13.9 |
| Non-cholestatic [n, %] | 198 | 86.1 |
| Patients Requiring ERCP, n = 51 | Controls, n = 179 | Statistical Significance | |
|---|---|---|---|
| Age in years [median, IQR) | 56 (48.75–67.25) | 58 (45–66) | NS |
| Male sex [n, %] | 32 (63) | 103 (58) | NS |
| MELD [mean, 95% CI] | 13.9 (6.4–24.1) | 14.5 (6.1–23.8) | NS |
| Age of donors in years [median, IQR] | 49.5 (37.5–60.75 | 47 (36.5–60) | NS |
| Male sex of donors [n, %] | 32 (63) | 115 (64) | NS |
| Donor ICU stay in days (mean, 95% CI) | 4.96 (3.68–6.24) | 5.41 (4.66–6.16) | NS |
| CIT in min [mean, 95% CI] | 373 (340.37–404.47) | 344.64 (323.94–365.35) | NS |
| WIT in min (mean, 95% CI) | 30.5 (29.14–31.86) | 31.93 (30.96–32.9) | NS |
| Splintage of bile ducts [n, %] | 11 (21,56) | 48 (26.81) | NS |
| MAP before reperfusion in mmHg [mean, 95% CI] | 70.58 (66.19–74.97) | 70.99 (68.72–73.26) | NS |
| Additional use of vaso-pressors [n, %] | 45 (86.53) | 138 (77.09) | p = 0.0001 |
| Reperfusion syndrome [n, %] | 8 (15.68) | 11 (6.11) | p = 0.00001 |
| Acidosis correction [n, %] | 35 (68.63) | 133 (74.3) | NS |
| Classical hepatic artery anastomosis [n, %] | 30 (63) | 107 (61) | NS |
| Variant hepatic artery anastomosis [n, %] | 19 (37) | 63 (39) | NS |
| Patients requiring blood transfusion [n, %] | 34 (69) | 113 (66) | NS |
| Median number of RBC units | 2 | 1.91 | NS |
| Study Parameter | Pearson’s Correlation Coefficient | p-Value |
|---|---|---|
| CIT in min | 0.02 | 0.87 |
| WIT in min | 0.3 | 0.67 |
| MAP before reperfusion in mmHg | −0.3 | 0.03 |
| The lowest MAP 5 min after reperfusion in mmHg | −0.11 | 0.45 |
| Early BS, n = 16 | Late BS, n = 35 | Statistical Significance | |
|---|---|---|---|
| Donor ICU stay in days [mean, 95% CI] | 4.33 (2.54–6.16) | 4.97 (3.48–6.47) | NS |
| Median age of recipients | 58.5 | 56 | NS |
| Classical hepatic artery anastomosis [n, %] | 11 (68.75) | 21 (60) | NS |
| Variant hepatic artery anastomosis | 5 (31.25) | 14 (40) | NS |
| Requirement of blood trans-fusion [n, %] | 9 (56.25) | 24 (69) | NS |
| Median number of RBC | 1.88 | 2 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeair, S.; Mamos, M.; Hirchy-Żak, J.; Modelewski, P.; Stasiuk, R.; Post, M.; Uździcki, A.; Witkowski, M.; Łakomiak, A.; Wawrzynowicz-Syczewska, M. An Inadequate Blood Supply Is a Risk Factor of Anastomotic Biliary Strictures After Liver Transplantation—A Single-Center Study. J. Clin. Med. 2025, 14, 1365. https://doi.org/10.3390/jcm14041365
Zeair S, Mamos M, Hirchy-Żak J, Modelewski P, Stasiuk R, Post M, Uździcki A, Witkowski M, Łakomiak A, Wawrzynowicz-Syczewska M. An Inadequate Blood Supply Is a Risk Factor of Anastomotic Biliary Strictures After Liver Transplantation—A Single-Center Study. Journal of Clinical Medicine. 2025; 14(4):1365. https://doi.org/10.3390/jcm14041365
Chicago/Turabian StyleZeair, Samir, Marek Mamos, Julia Hirchy-Żak, Patryk Modelewski, Robert Stasiuk, Mariola Post, Artur Uździcki, Michał Witkowski, Agata Łakomiak, and Marta Wawrzynowicz-Syczewska. 2025. "An Inadequate Blood Supply Is a Risk Factor of Anastomotic Biliary Strictures After Liver Transplantation—A Single-Center Study" Journal of Clinical Medicine 14, no. 4: 1365. https://doi.org/10.3390/jcm14041365
APA StyleZeair, S., Mamos, M., Hirchy-Żak, J., Modelewski, P., Stasiuk, R., Post, M., Uździcki, A., Witkowski, M., Łakomiak, A., & Wawrzynowicz-Syczewska, M. (2025). An Inadequate Blood Supply Is a Risk Factor of Anastomotic Biliary Strictures After Liver Transplantation—A Single-Center Study. Journal of Clinical Medicine, 14(4), 1365. https://doi.org/10.3390/jcm14041365






