Intracranial Hemorrhage During Pregnancy: An Interdisciplinary Literature Review and a Rare Case Report of Early-Onset Eclampsia with Intracranial Hemorrhage and HELLP Syndrome
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. Hemodynamic Changes in Pregnancy and Labor
3.2. ICH in Pregnancy—Etiology
3.3. Arteriovenous Malformations Bleeding in Pregnancies
3.4. Preeclampsia, Eclampsia and Cerebral Autoregulation
3.5. ICH Management During Pregnancy
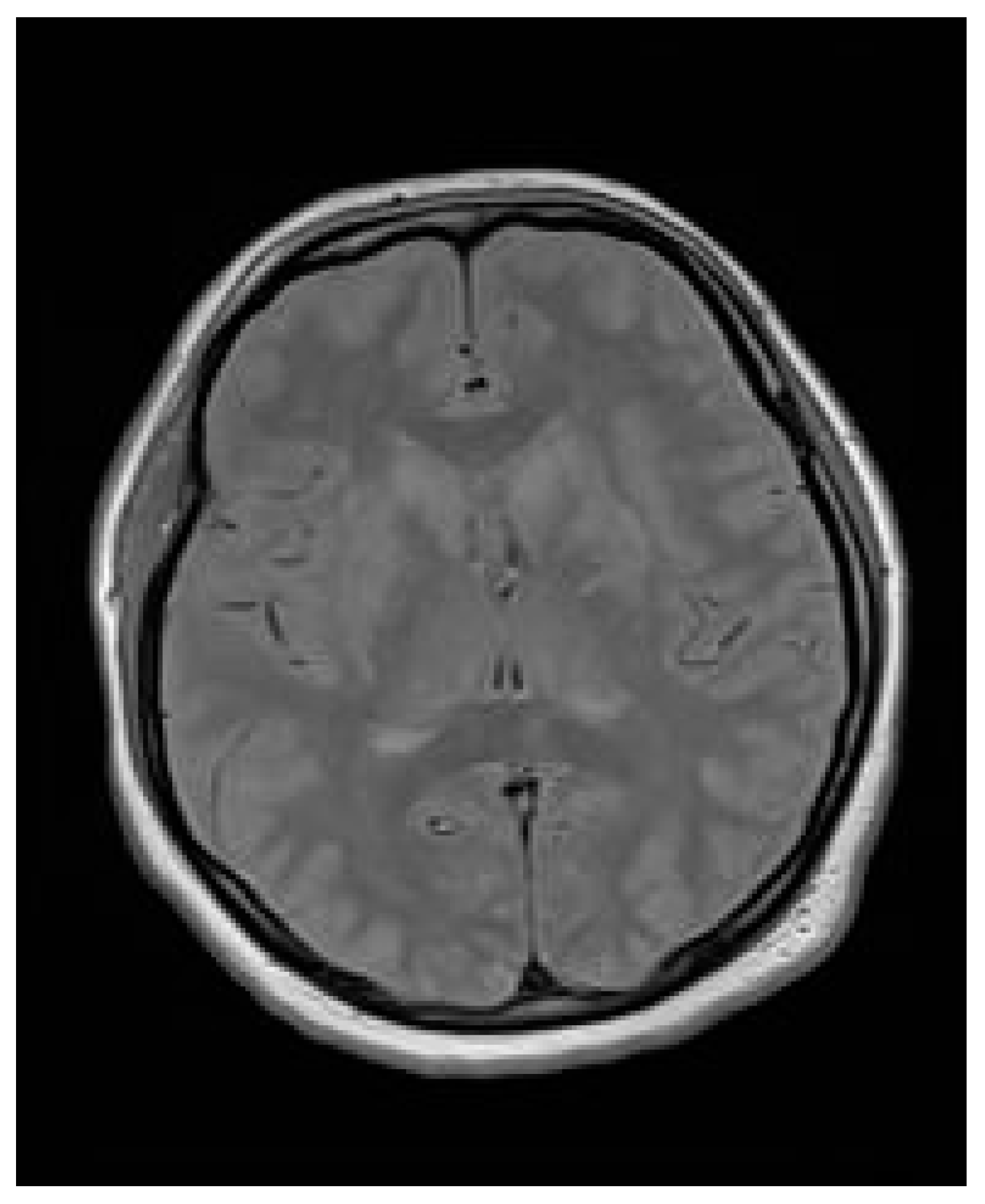
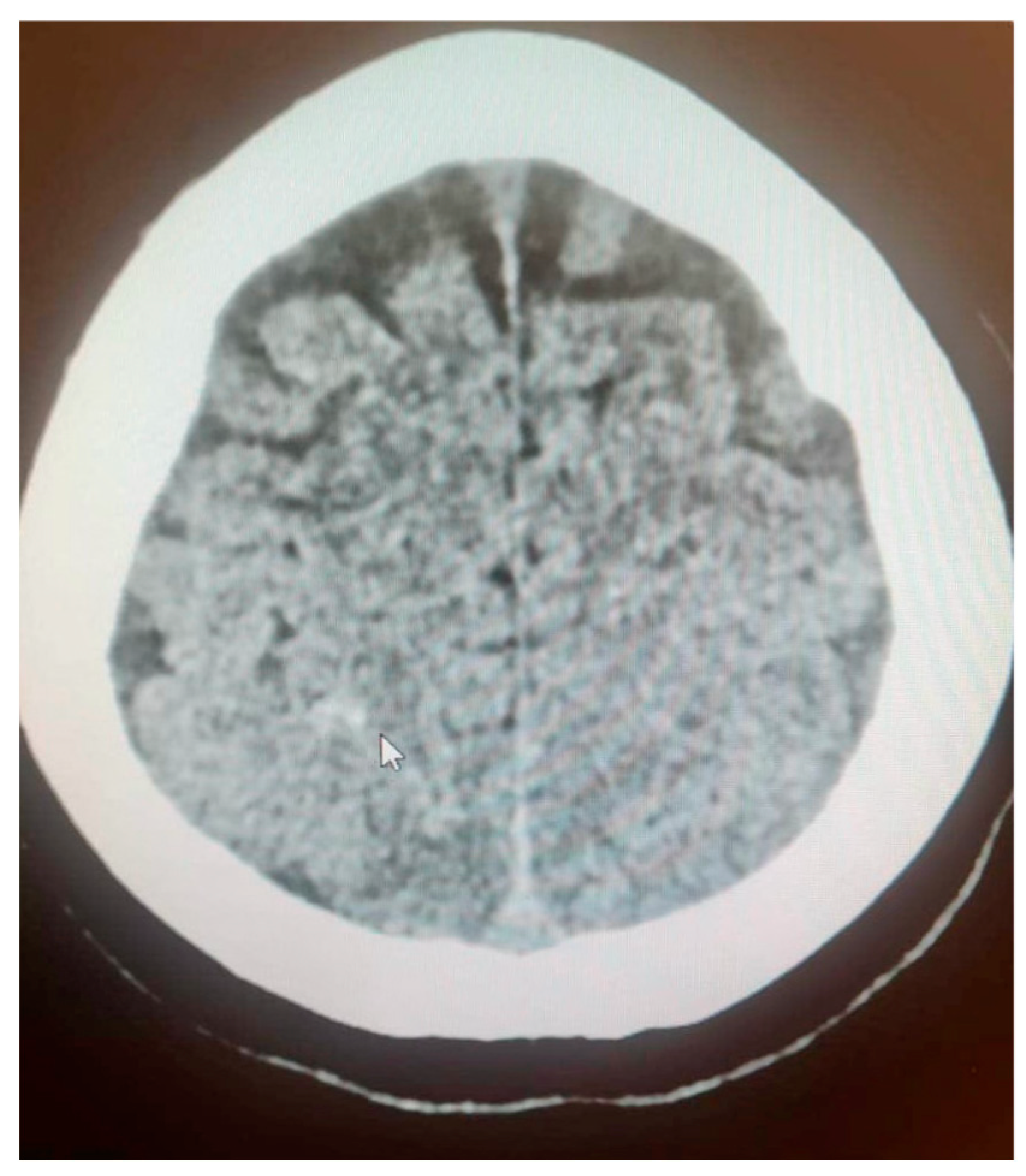
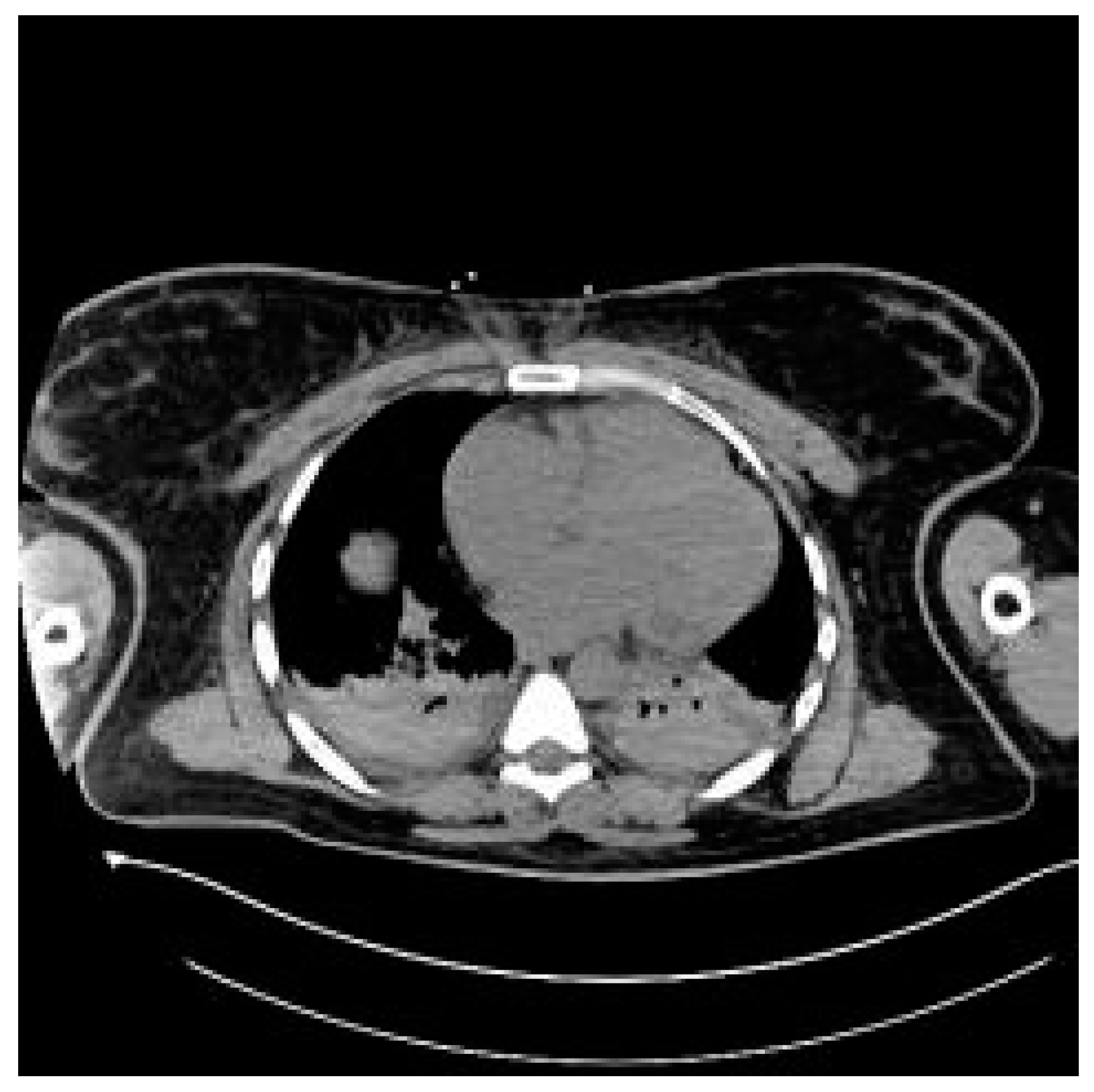
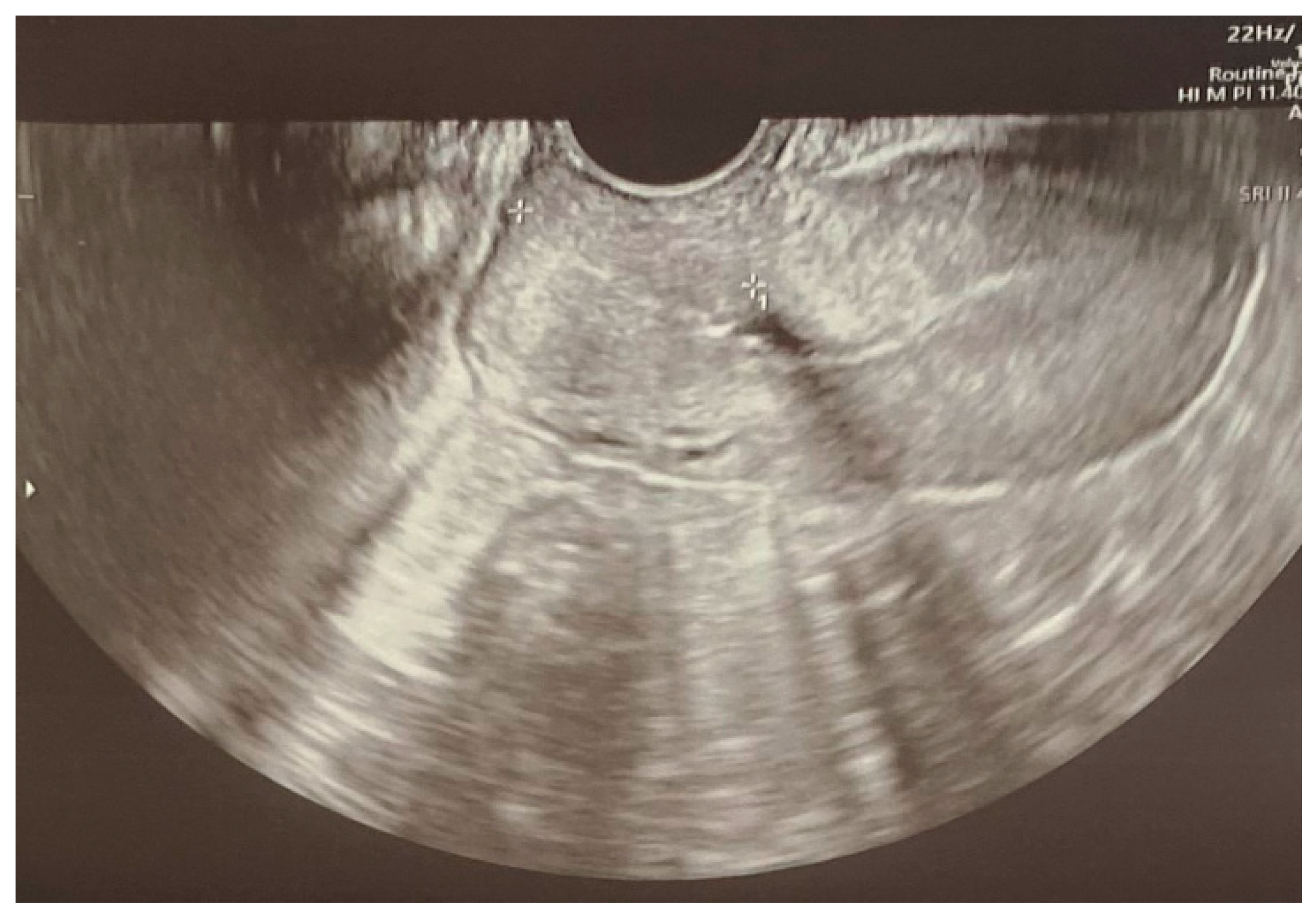
3.6. Case Report
3.7. Case Report—Differential Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| ICH | Intracranial hemorrhage |
| WFNS | World Federation of Neurological Surgeons |
| aSAH | Aneurysmal subarachnoid hemorrhage |
| OB/GYN | Obstetrics and gynecology |
| PE | Preeclampsia |
| HELLP | Hemolysis, elevated liver enzymes and low platelets syndrome |
| DIC | Disseminated intravascular coagulation |
| CVST | Cerebral venous sinus thrombosis |
| AVM | Arteriovenous malformations |
| BBB | Blood–brain barrier |
| CBF | Cerebral blood flow |
| CPP | Cerebral perfusion pressure |
| MR/MRI | Magnetic resonance imaging |
| CT | Computer tomography |
| CNS | Central nervous system |
| SAH | Subarachnoid hemorrhage |
| PRES | Posterior reversible encephalopathy syndrome |
| CS | Cesarean section |
References
- Meeks, J.R.; Bambhroliya, A.B.; Alex, K.M.; Sheth, S.A.; Savitz, S.I.; Miller, E.C.; McCullough, L.D.; Vahidy, F.S. Association of Primary Intracerebral Hemorrhage with Pregnancy and the Postpartum Period. JAMA Netw. Open 2020, 3, e202769. [Google Scholar] [CrossRef]
- Fairhall, J.M.; Stoodley, M.A. Intracranial haemorrhage in pregnancy. Obstet. Med. 2009, 2, 142–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klein, H.H.; Pich, S. Physiologische Anderungen des Herz-Kreislauf-Systems in der Schwangerschaft [Cardiovascular changes during pregnancy]. Herz 2003, 28, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Ascanio, L.C.; Maragkos, G.A.; Young, B.C.; Boone, M.D.; Kasper, E.M. Spontaneous Intracranial Hemorrhage in Pregnancy: A Systematic Review of the Literature. Neurocritical Care 2019, 30, 5–15. [Google Scholar] [CrossRef]
- Takahashi, J.C.; Iihara, K.; Ishii, A.; Watanabe, E.; Ikeda, T.; Miyamoto, S. Pregnancy-associated intracranial hemorrhage: Results of a survey of neurosurgical institutes across Japan. J. Stroke Cerebrovasc. Dis. 2014, 23, e65–e71. [Google Scholar] [CrossRef] [PubMed]
- Pelayo-Salazar, M.E.; Montenegro-Rosales, H.A.; Balderrama-Bañares, J.L.; Martínez-Arellano, P.; Campos-Flota, O.A.; Mestre-Orozco, L.; López-Valdés, J.C. Clinical and anatomic description of patients with arteriovenous malformation treated with endovascular therapy in a Mexican population. J. Cerebrovasc. Endovasc. Neurosurg. 2023, 25, 36–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, X.; Liu, P.; Li, Y. The clinical characteristics and treatment of cerebral AVM in pregnancy. Neuroradiol. J. 2015, 28, 234–237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, K.; Chen, Y.; Yan, D.; Li, R.; Li, Z.; Zhang, H.; Wang, K.; Han, H.; Zhao, Y.; Ma, L.; et al. Re-rupture in ruptured brain arteriovenous malformations: A retrospective cohort study based on a nationwide multicenter prospective registry. J. NeuroInterv. Surg. 2024, 16, 1145–1151. [Google Scholar] [CrossRef]
- Miller, E.C. Preeclampsia and Cerebrovascular Disease. Hypertension 2019, 74, 5–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bergman, L.; Cluver, C.; Carlberg, N.; Belfort, M.; Tolcher, M.C.; Panerai, R.B.; van Veen, T. Cerebral perfusion pressure and autoregulation in eclampsia-a case control study. Am. J. Obstet. Gynecol. 2021, 225, 185.e1–185.e9. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.H. Computed Tomography in Pregnant Patients. J. Med. Imaging Radiat. Sci. 2009, 40, 100–104. [Google Scholar] [CrossRef]
- Bourgioti, C.; Konidari, M.; Gourtsoyianni, S.; Moulopoulos, L.A. Imaging during pregnancy: What the radiologist needs to know. Diagn. Interv. Imaging 2021, 102, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Maetani, Y.; Nakamori, M.; Watanabe, T.; Matsushima, H.; Imamura, E.; Wakabayashi, S. Subarachnoid hemorrhage after transient global amnesia caused by cerebral venous congestion: Case report. BMC Neurol. 2018, 18, 36. [Google Scholar] [CrossRef]
- Boushra, M.; Natesan, S.M.; Koyfman, A.; Long, B. High risk and low prevalence diseases: Eclampsia. Am. J. Emerg. Med. 2022, 58, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Fishel Bartal, M.; Sibai, B.M. Eclampsia in the 21st century. Am. J. Obstet. Gynecol. 2022, 226, S1237–S1253. [Google Scholar] [CrossRef] [PubMed]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Magee, L.A.; Nicolaides, K.H.; von Dadelszen, P. Preeclampsia. N. Engl. J. Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef]
- Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef]
- Mateos Rodriguez, A.A.; Benito Vellisca, M.A. Management of eclampsia in the prehospital setting. Emerg. Med. J. 2007, 24, 504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walsh, S.W.; Strauss, J.F., 3rd. The Road to Low-Dose Aspirin Therapy for the Prevention of Preeclampsia Began with the Placenta. Int. J. Mol. Sci. 2021, 22, 6985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefanovic, V. International Academy of Perinatal Medicine (IAPM) guidelines for screening, prediction, prevention and management of pre-eclampsia to reduce maternal mortality in developing countries. J. Perinat. Med. 2021, 51, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Nicolaides, K.H.; Demers, S.; Villa, P.; Bujold, E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: A meta-analysis. Ultrasound Obstet. Gynecol. 2013, 41, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Saccone, G.; Morelli, M.; Interlandi, F.; Votino, C.; Zuccalà, V.; Di Carlo, C.; Zullo, F.; Venturella, R. Stillbirth, potentially preventable cases: An Italian retrospective study. Ital. J. Gynaecol. Obstet. 2022, 34, 89–102. [Google Scholar] [CrossRef]
- Iweka, R.O.; Okonofua, O.J.; Usiholo, E.A.; Okonofua, F.E. Eclampsia complicated by acute subdural haematoma: A case report. Int. J. Surg. Case Rep. 2024, 119, 109666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Parameter | Value | Unit | Reference Value |
|---|---|---|---|
| Platelets | 224 | K/μL | 140–400 |
| Urinary total protein | 3073.4 | mg/dL | - |
| Alanine transaminase | 47 | IU/L | 14–59 |
| Aspartate aminotransferase | 55 | IU/L | 55 |
| Parameter | Value | Unit | Reference Value |
|---|---|---|---|
| Platelets | 148 | ×109/L | 150–410 |
| Urinary total protein | 13.3 | g/L | - |
| Alanine transaminase | 122 | U/L | <34 |
| Aspartate aminotransferase | 252 | U/L | 11–34 |
| Lactate dehydrogenase | 1233 | U/L | 125–220 |
| Parameter | Value | Unit | Reference Value |
|---|---|---|---|
| Platelets | 488 | ×109/L | 150–410 |
| Urinary total protein | 0.51 | g/L | - |
| Alanine transaminase | 34 | U/L | <34 |
| Aspartate aminotransferase | 17 | U/L | 11–34 |
| Lactate dehydrogenase | 255 | U/L | 125–220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, N.K.; Fercho, J.M.; Kałas, M.; Szaruta-Raflesz, K.; Grzybowska, M.E.; Siemiński, M.; Wydra, D.G. Intracranial Hemorrhage During Pregnancy: An Interdisciplinary Literature Review and a Rare Case Report of Early-Onset Eclampsia with Intracranial Hemorrhage and HELLP Syndrome. J. Clin. Med. 2025, 14, 1361. https://doi.org/10.3390/jcm14041361
Mazur NK, Fercho JM, Kałas M, Szaruta-Raflesz K, Grzybowska ME, Siemiński M, Wydra DG. Intracranial Hemorrhage During Pregnancy: An Interdisciplinary Literature Review and a Rare Case Report of Early-Onset Eclampsia with Intracranial Hemorrhage and HELLP Syndrome. Journal of Clinical Medicine. 2025; 14(4):1361. https://doi.org/10.3390/jcm14041361
Chicago/Turabian StyleMazur, Natalia Katarzyna, Justyna Małgorzata Fercho, Maria Kałas, Karolina Szaruta-Raflesz, Magdalena Emilia Grzybowska, Mariusz Siemiński, and Dariusz Grzegorz Wydra. 2025. "Intracranial Hemorrhage During Pregnancy: An Interdisciplinary Literature Review and a Rare Case Report of Early-Onset Eclampsia with Intracranial Hemorrhage and HELLP Syndrome" Journal of Clinical Medicine 14, no. 4: 1361. https://doi.org/10.3390/jcm14041361
APA StyleMazur, N. K., Fercho, J. M., Kałas, M., Szaruta-Raflesz, K., Grzybowska, M. E., Siemiński, M., & Wydra, D. G. (2025). Intracranial Hemorrhage During Pregnancy: An Interdisciplinary Literature Review and a Rare Case Report of Early-Onset Eclampsia with Intracranial Hemorrhage and HELLP Syndrome. Journal of Clinical Medicine, 14(4), 1361. https://doi.org/10.3390/jcm14041361








