Injective Therapies for Managing Sacroiliac Joint Pain in Spondyloarthropathy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Assessment of Risk of Bias and Quality of Evidence
2.3. Statistical Analysis
3. Results
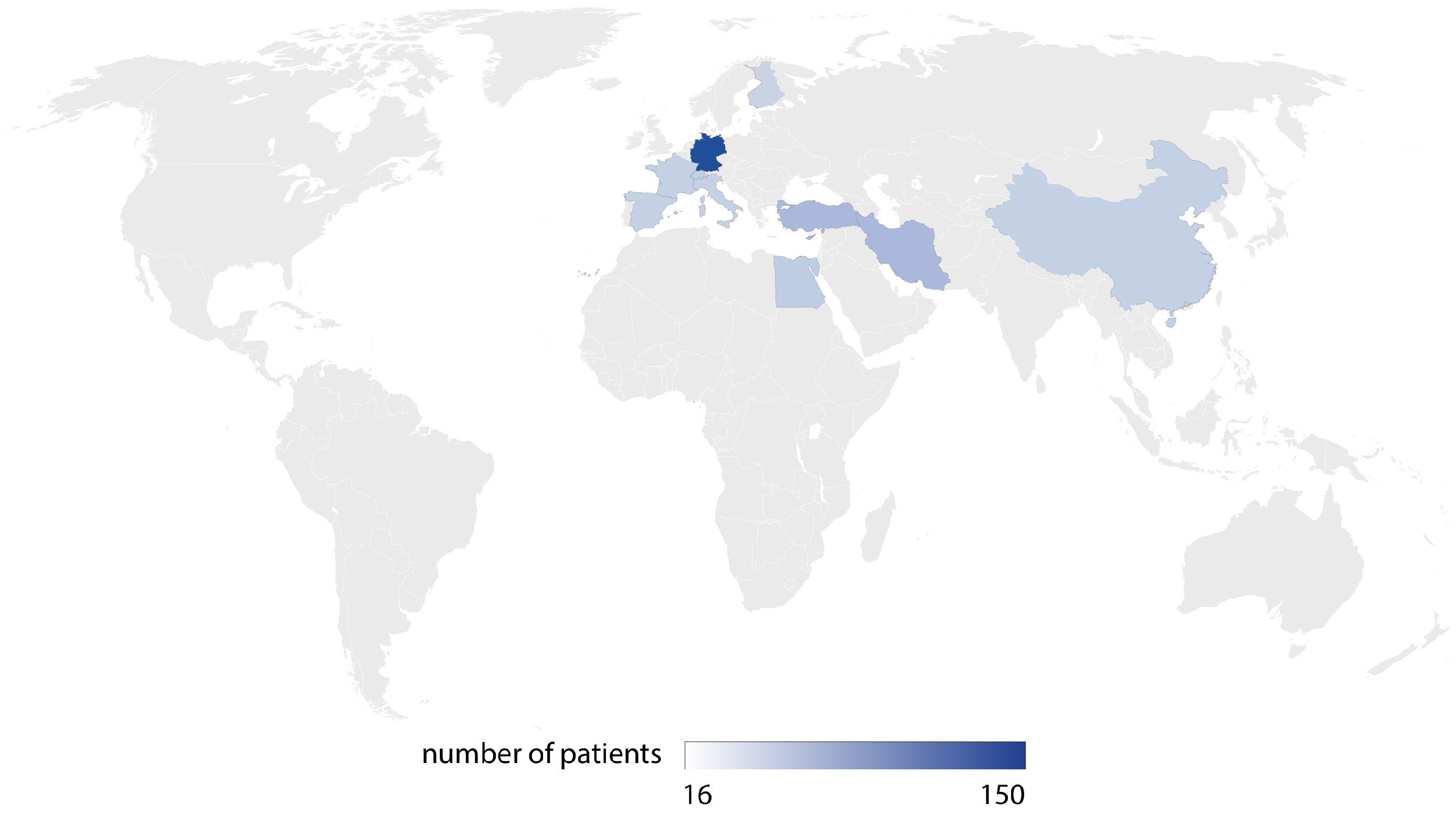
3.1. Systematic Review Results
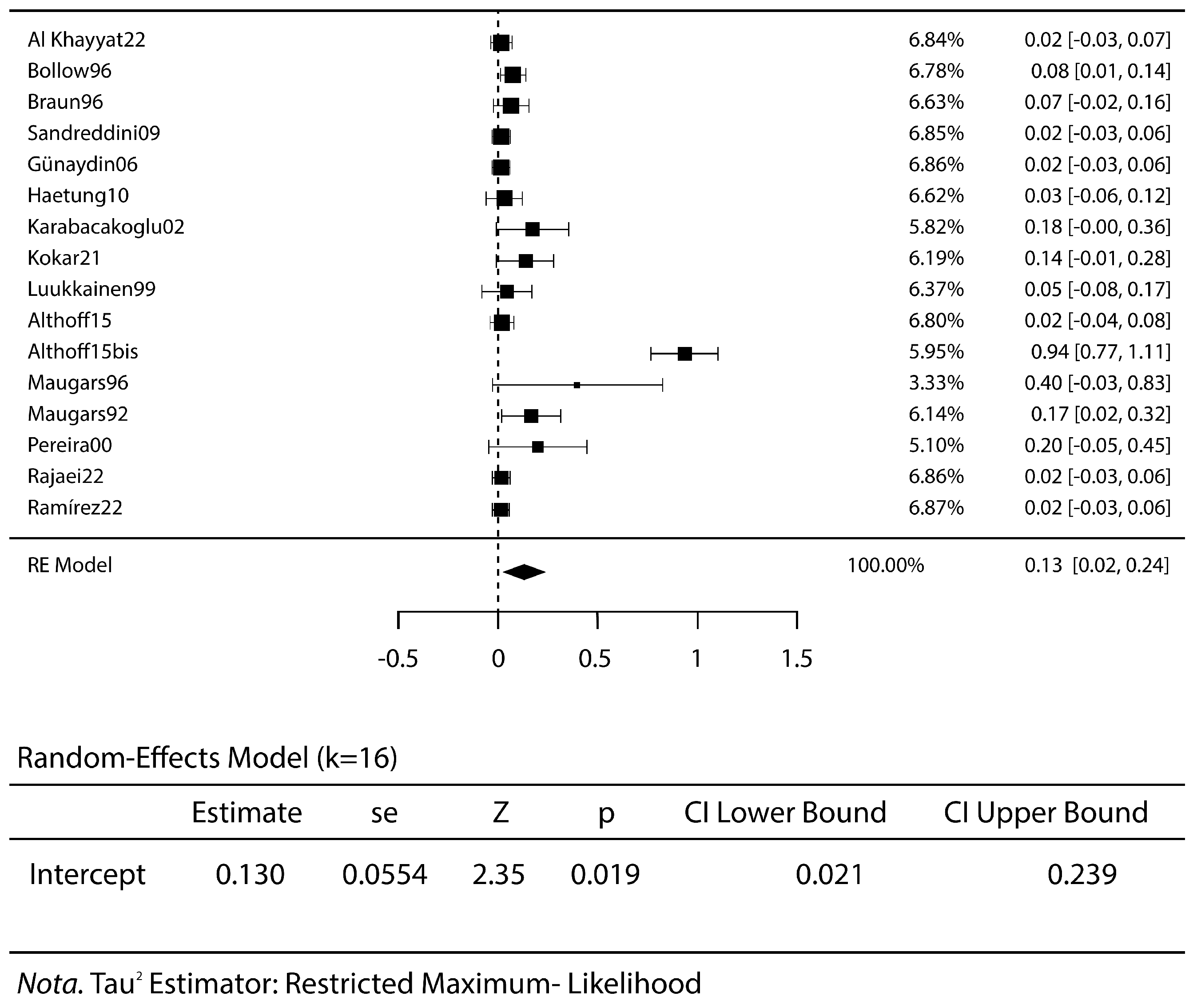
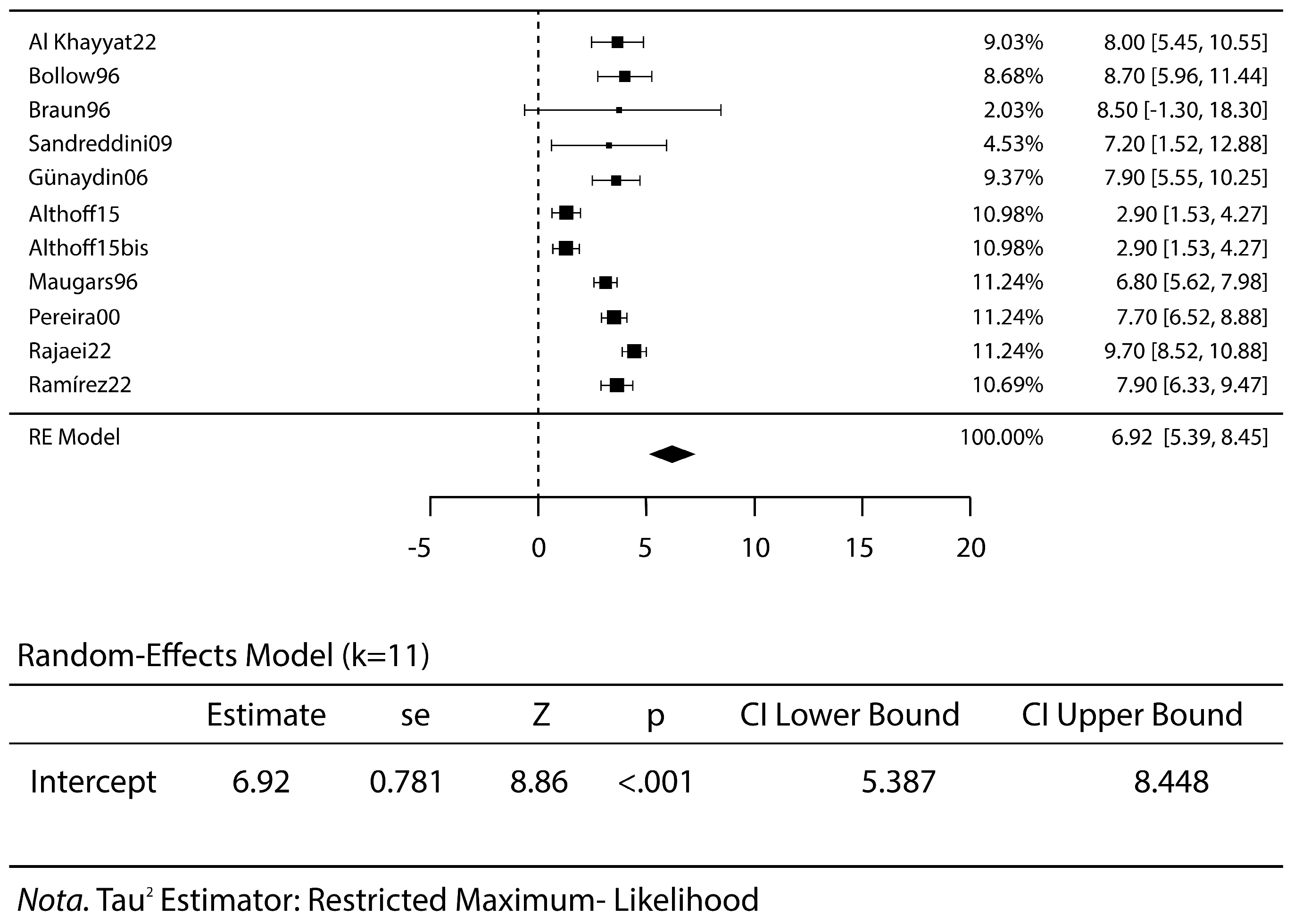
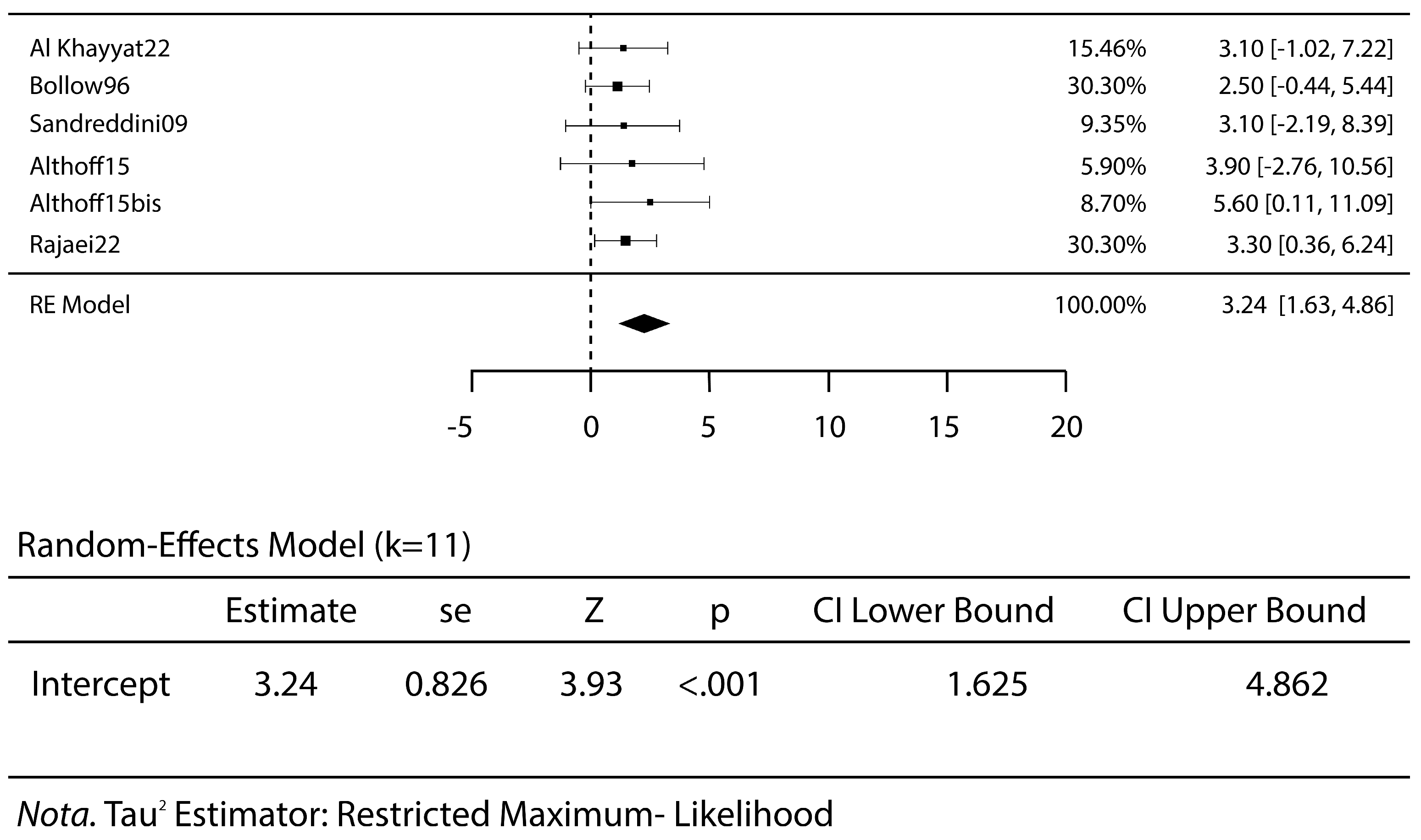
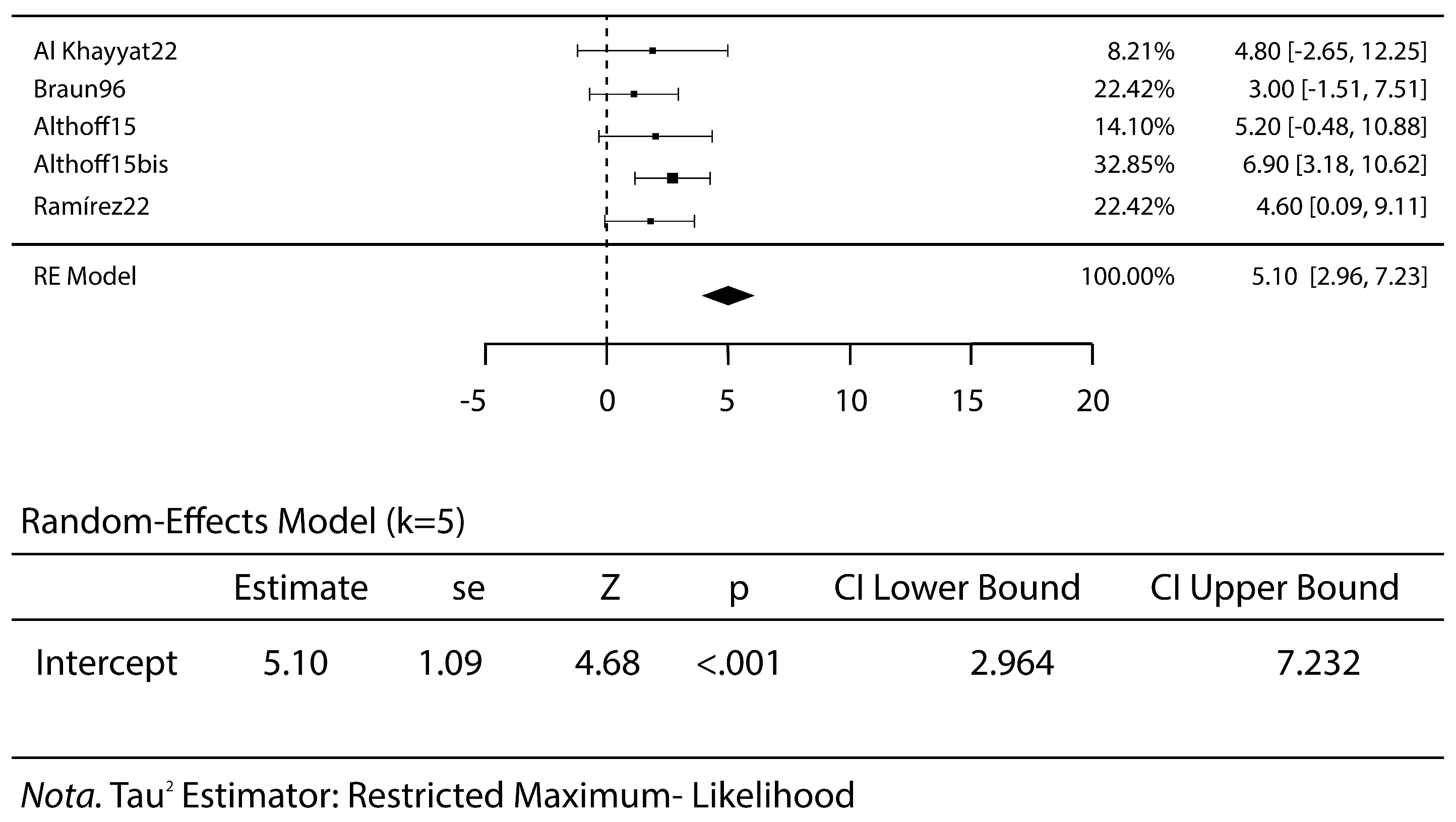
3.2. Meta-Analysis Results
3.3. Risk of Bias and Quality of Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rajaei, E.; Bavieh, K.; Mowla, K.; Ghorbani, A.; Movaffagh, N. The impact of intra-sacroiliac joint methylprednisolone injection in the recovery of patients with spondyloarthropathy: A randomized controlled trial. Reumatologia 2022, 60, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Luukkainen, R.K.; Wennerstrand, P.V.; Kautiainen, H.H.; Sanila, M.T.; Asikainen, E.L. Efficacy of periarticular corticosteroid treatment of the sacroiliac joint in non-spondylarthropathic patients with chronic low back pain in the region of the sacroiliac joint. Clin. Exp. Rheumatol. 2002, 20, 52–54. [Google Scholar] [PubMed]
- Ramiro, S.; Nikiphorou, E.; Sepriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.; van der Heijde, D. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 2023, 82, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Günaydin, I.; Pereira, P.L.; Fritz, J.; König, C.; Kötter, I. Magnetic resonance imaging guided corticosteroid injection of sacroiliac joints in patients with spondylarthropathy. Are multiple injections more beneficial? Rheumatol. Int. 2006, 26, 396–400. [Google Scholar] [CrossRef]
- Al Khayyat, S.G.; Fogliame, G.; Barbagli, S.; Conticini, E.; Fabbroni, M.; D’alessandro, R.; Vitale, A.; Gentileschi, S.; Bardelli, M.; Baldi, C.; et al. Ultrasound guided corticosteroids sacroiliac joint injections (SIJIs) in the management of active sacroiliitis: A real-life prospective experience. J. Ultrasound 2023, 26, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Bollow, M.; Braun, J.; Taupitz, M.; Häberle, J.; Reihauer, B.-H.; Paris, S.; Mutze, S.; Seyrekbasan, F.; Wolf, K.-J.; Hamm, B. CT-guided intraarticular corticosteroid injection into the sacroiliac joints in patients with spondyloarthropathy: Indication and follow-up with contrast-enhanced MRI. J. Comput. Assist. Tomogr. 1996, 20, 512–521. [Google Scholar] [CrossRef]
- Braun, J.; Bollow, M.; Seyrekbasan, F.; Häberle, H.J.; Eggens, U.; Mertz, A.; Distler, A.; Sieper, J. Computed tomography guided corticosteroid injection of the sacroiliac joint in patients with spondyloarthropathy with sacroiliitis: Clinical outcome and followup by dynamic magnetic resonance imaging. J. Rheumatol. 1996, 23, 659–664. [Google Scholar] [PubMed]
- Sadreddini, S.; Noshad, H.; Molaeefard, M.; Ardalan, M.-R.; Ghojazadeh, M.; Shakouri, S.-K. Unguided sacroiliac injection: Effect on refractory buttock pain in patients with spondyloarthropathies. Presse Med. 2009, 38, 710–716. [Google Scholar] [CrossRef]
- Cui, Y.; Xiao, Z.; Shuxia, W.; Zhenjun, Z.; Hengguo, Z.; Liangyi, F.; Weicheng, G.; Li, L.; Guangfeng, Z.; Yunzhen, S.; et al. Computed tomography guided intra-articular injection of etanercept in the sacroiliac joint is an effective mode of treatment of ankylosing spondylitis. Scand. J. Rheumatol. 2010, 39, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Hartung, W.; Ross, C.J.; Straub, R.; Feuerbach, S.; Schölmerich, J.; Fleck, M.; Herold, T. Ultrasound-guided sacroiliac joint injection in patients with established sacroiliitis: Precise IA injection verified by MRI scanning does not predict clinical outcome. Rheumatology 2010, 49, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Karabacakoglu, A.; Karaköse, S.; Ozerbil, O.M.; Odev, K. Fluoroscopy-guided intraarticular corticosteroid injection into the sacroiliac joints in patients with ankylosing spondylitis. Acta Radiol. 2002, 43, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Kokar, S.; Kayhan, Ö.; Şencan, S.; Gündüz, O.H. The Role of Sacroiliac Joint Steroid Injections in the Treatment of Axial Spondyloarthritis. Arch. Rheumatol. 2021, 36, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Luukkainen, R.; Nissilä, M.; Asikainen, E.; Sanila, M.; Lehtinen, K.; Alanaatu, A.; Kautiainen, H. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondylarthropathy. Clin. Exp. Rheumatol. 1999, 17, 88–90. [Google Scholar]
- Althoff, C.E.; Bollow, M.; Feist, E.; Marticorena-Garcia, S.R.; Eshed, I.; Diekhoff, T.; Hamm, B.; Hermann, K.G.A. CT-guided corticosteroid injection of the sacroiliac joints: Quality assurance and standardized prospective evaluation of long-term effectiveness over six months. Clin. Rheumatol. 2015, 34, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Maugars, Y.; Mathis, C.; Berthelot, J.M.; Charlier, C.; Prost, A. Assessment of the efficacy of sacroiliac corticosteroid injections in spondylarthropathies: A double-blind study. Br. J. Rheumatol. 1996, 35, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Maugars, Y.; Mathis, C.; Vilon, P.; Prost, A. Corticosteroid injection of the sacroiliac joint in patients with seronegative spondylarthropathy. Arthritis Rheum. 1992, 35, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.L.; Günaydin, I.; Trübenbach, J.; Dammann, F.; Remy, C.T.; Kötter, I.; Schick, F.; Koenig, C.W.; Claussen, C.D. Interventional MR imaging for injection of sacroiliac joints in patients with sacroiliitis. AJR Am. J. Roentgenol. 2000, 175, 265–266. [Google Scholar] [CrossRef]
- Huaranga, M.A.R.; Corredor, D.C.; Ezaine, A.E.P.; Huertas, M.P.; Lopez, R.A.; Fernández, J.A.; Rodríguez, C.C.R. First Spanish study on the effectiveness of ultrasound-guided sacroiliac joint injection in patients with spondyloarthritis. Rheumatol. Adv. Pract. 2022, 6, rkac036. [Google Scholar] [CrossRef]
- Soliman, E.; El-Tantawi, G.; Matrawy, K.; Aldawoudy, A.; Naguib, A. Local infliximab injection of sacroiliac joints in non-radiographic axial spondyloarthritis: Impact on clinical and magnetic resonance imaging parameters of disease activity. Mod. Rheumatol. 2015, 25, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Aranke, M.; McCrudy, G.; Rooney, K.; Patel, K.; Lee, C.A.; Hasoon, J.; Kaye, A.D. Minimally Invasive and Conservative Interventions for the Treatment of Sacroiliac Joint Pain: A Review of Recent Literature. Orthop. Rev. 2022, 14, 34098. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Dhir, V.; Naidu, G.S.R.S.N.K.; Khullar, A.; Kumar, V.; Sharma, S.; Sharma, A.; Jain, S. Efficacy of a step-down regimen of oral prednisolone in axial spondyloarthritis: Result of a double-blind randomized controlled trial (COBRA-AS Study). Rheumatology 2021, 60, 1932–1941. [Google Scholar] [CrossRef]
- Webers, C.; Kiltz, U.; Braun, J.; van der Heijde, D.; Boonen, A. The effect of anti-inflammatory treatment on depressive symptoms in spondyloarthritis: Does the type of drug matter? Rheumatology 2023, 62, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Wendling, D.; Hecquet, S.; Fogel, O.; Letarouilly, J.G.; Verhoeven, F.; Pham, T.; Prati, C.; Molto, A.; Goupille, P.; Dernis, E.; et al. 2022 French Society for Rheumatology (SFR) recommendations on the everyday management of patients with spondyloarthritis, including psoriatic arthritis. Jt. Bone Spine 2022, 89, 105344. [Google Scholar] [CrossRef]
- Wendling, D. Local sacroiliac injections in the treatment of spondyloarthritis. What is the evidence? Jt. Bone Spine 2020, 87, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-W.; Hung, S.-K.; Pan, P.-T.; Kao, M.-C. Diagnosis and interventional pain management options for sacroiliac joint pain. Ci Ji Yi Xue Za Zhi = Tzu-Chi Med. J. 2019, 31, 207–210. [Google Scholar]
- Tantawy, M.F.; Nazim, W.M. Comparison between intraarticular and combined intra and periarticular sacroiliac injection: A prospective randomized controlled clinical trial. J. Neurosurg. Sci. 2022, 68, 294–300. [Google Scholar] [PubMed]
- Fritz, J.; Henes, J.C.; Thomas, C.; Clasen, S.; Fenchel, M.; Claussen, C.D.; Lewin, J.S.; Pereira, P.L. Diagnostic and interventional MRI of the sacroiliac joints using a 1.5-T open-bore magnet: A one-stop-shopping approach. AJR Am. J. Roentgenol. 2008, 191, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Lemos, S.E. Editorial Commentary: Periarticular and Intra-Articular Injections May Do the Right Thing for Patients’ Pain but May Be the Wrong Thing for Their Articular Cartilage: Be Careful. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 1996–1998. [Google Scholar] [CrossRef] [PubMed]
- Kurnutala, L.N.; Ghatol, D.; Upadhyay, A. Clostridium Sacroiliitis (Gas Gangrene) Following Sacroiliac Joint Injection--Case Report and Review of the Literature. Pain Physician 2015, 18, E629–E632. [Google Scholar] [CrossRef] [PubMed]
- Meydani, A.; Schwartz, R.A.; Foye, P.M.; Patel, A.D. Herpes simplex following intra-articular sacroiliac corticosteroid injection. Acta Dermatovenerol. Alp. Pannonica Adriat 2009, 18, 135–137. [Google Scholar] [PubMed]
- Nagpal, G.; Flaherty, J.P.; Benzon, H.T. Diskitis, Osteomyelitis, Spinal Epidural Abscess, Meningitis, and Endocarditis Following Sacroiliac Joint Injection for the Treatment of Low-Back Pain in a Patient on Therapy for Hepatitis C Virus. Reg. Anesth. Pain. Med. 2017, 42, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Kurup, V.; Vadivelu, N.; Dai, F.; Zhou, B.; Rajput, K. Does Choice of Steroid Matter for Treatment of Chronic Low Back Pain with Sacroiliac Joint Injections: A Retrospective Study. Curr. Pain. Headache Rep. 2021, 25, 34. [Google Scholar] [CrossRef]
- Previtali, D.; Merli, G.; Di Laura Frattura, G.; Candrian, C.; Zaffagnini, S.; Filardo, G. The Long-Lasting Effects of “Placebo Injections” in Knee Osteoarthritis: A Meta-Analysis. Cartilage 2021, 13 (Suppl. S1), 185S–196S. [Google Scholar] [CrossRef] [PubMed]


| First Author | Year | Study Design | N° Initial Patients (Treated) | Type of Treatment | Injected Amount | Final Follow-up | Scores | N° Failures (Definition) | N° Complications |
|---|---|---|---|---|---|---|---|---|---|
| Al Khayyat S.G. [5] | 2022 | Prospective case series | 26 | Steroid (TAC) | 1 mL | 6 m | VAS | 0 | 0 |
| Bollow M. [6] | 1996 | Prospective case series | 66 | Steroid (TAC) | 1 mL | 1 w | VAS | 5 (not effective) | 0 |
| Braun J. [7] | 1996 | Prospective case series | 30 | Steroid (TAC) | 1 mL | 18 m | VAS | 2 (not effective) | 0 |
| Sadreddini S. [8] | 2009 | Prospective case series | 29 | Steroid (TAC) | 2 mL | 5 m | VAS, 4-level likerty pain score, provocative tests | 0 | 0 |
| Cui Y. [9] | 2010 | Prospective case series | 16 | Biologic (etanercept) | 2 mL | 3 m | VAS, BASDAI | 0 | 0 |
| Günaydin I. [4] | 2006 | Prospective case series | 31 | Steroid (TAC) | 1 mL | 3 m | VAS | 0 | 0 |
| Hartung W. [10] | 2010 | Prospective case series | 14 | Steroid (TAC) | 3 mL | 1 m | VAS | 3 (pain during the procedure) | 0 |
| Karabacakoglu A. [11] | 2002 | Prospective case series | 17 | Steroid (betamethasone) | N.R. | 2 m | Patients’ personal satisfaction | 3 (lost at follow-up) | 0 |
| Kokar S. [12] | 2021 | Retrospective comparative study | 43 | Steroid (TAC) | 3 mL | 1 m | VAS, BASDAI | 10 (not effective) | 0 |
| Luukkainen R. [13] | 1999 | Prospective comparative study | 20 | Steroid (methylprednisone acetate) | 3 mL | 2 m | VAS, pain index | 7 (not effective) | 0 |
| Althoff C.E. [14] | 2015 | Prospective comparative study | 29 | Steroid (TAC) | 2 mL | 6 m | VAS, BASDAI | 6 (need steroid injection) | 0 |
| Maugars Y. [15] | 1996 | Randomized controlled study | 10 | Steroid (cortivazol) | 1.5 mL | 6 m | VAS, NSAID intake variation, limping evolution, unipodal jump evolution, sacroiliac | 4 (not effective) | 3 |
| Maugars Y. [16] | 1992 | Prospective case series | 24 | Steroid (cortivazol) | 1.5 mL | 2 m | IMPROVEMENT (very good–good–fair–failure) | 2 (not effective) | 0 |
| Pereira P.L. [17] | 2000 | Prospective case series | 10 | Steroid (TAC) | 1 mL | 3 m | VAS | 0 | 0 |
| Rajaei E. [1] | 2022 | Randomized controlled study | 60 | Steroid (methylprednisolone) | 1 mL | 2 m | VAS, BASDAI, FTF | 0 | 0 |
| Ramírez H.M.A. [18] | 2022 | Prospective case series | 32 | Steroid (bethametasone) | 3 mL | 6 m | VAS, BASDAI, ASDAS | 0 | 0 |
| Soliman E. [19] | 2015 | Prospective case series | 37 | Biologic (infliximab) | 2 mL | 6 m | VAS | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerasoli, T.; Filardo, G.; Favero, A.; Rinaldi, V.G.; Di Maio, L.; Marcheggiani Muccioli, G.M.; Zaffagnini, S. Injective Therapies for Managing Sacroiliac Joint Pain in Spondyloarthropathy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 1294. https://doi.org/10.3390/jcm14041294
Cerasoli T, Filardo G, Favero A, Rinaldi VG, Di Maio L, Marcheggiani Muccioli GM, Zaffagnini S. Injective Therapies for Managing Sacroiliac Joint Pain in Spondyloarthropathy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(4):1294. https://doi.org/10.3390/jcm14041294
Chicago/Turabian StyleCerasoli, Tosca, Giuseppe Filardo, Antongiulio Favero, Vito Gaetano Rinaldi, Laura Di Maio, Giulio Maria Marcheggiani Muccioli, and Stefano Zaffagnini. 2025. "Injective Therapies for Managing Sacroiliac Joint Pain in Spondyloarthropathy: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 4: 1294. https://doi.org/10.3390/jcm14041294
APA StyleCerasoli, T., Filardo, G., Favero, A., Rinaldi, V. G., Di Maio, L., Marcheggiani Muccioli, G. M., & Zaffagnini, S. (2025). Injective Therapies for Managing Sacroiliac Joint Pain in Spondyloarthropathy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(4), 1294. https://doi.org/10.3390/jcm14041294






