Tinnitus and Its Comorbidities: A Comprehensive Analysis of Their Relationships
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Tinnitus Handicap Inventory
2.3. Audiological Examination and Tinnitus Pitch-Matching
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sedley, W.; Friston, K.J.; Gander, P.E.; Kumar, S.; Griffiths, T.D. An Integrative Tinnitus Model Based on Sensory Precision. Trends Neurosci. 2016, 39, 799–812. [Google Scholar] [CrossRef]
- Aldè, M.; Cantarella, G.; Zanetti, D.; Pignataro, L.; La Mantia, I.; Maiolino, L.; Ferlito, S.; Di Mauro, P.; Cocuzza, S.; Lechien, J.R.; et al. Autosomal Dominant Non-Syndromic Hearing Loss (DFNA): A Comprehensive Narrative Review. Biomedicines 2023, 11, 1616. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, B.; O’Brien, K.; Döge, J.; Lackner, K.J.; Beutel, M.E.; Münzel, T.; Pfeiffer, N.; Schulz, A.; Schmidtmann, I.; Wild, P.S.; et al. Tinnitus Prevalence in the Adult Population-Results from the Gutenberg Health Study. Medicina 2023, 59, 620. [Google Scholar] [CrossRef]
- Bhatt, J.M.; Lin, H.W.; Bhattacharyya, N. Prevalence, Severity, Exposures, and Treatment Patterns of Tinnitus in the United States. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 959–965. [Google Scholar] [CrossRef]
- Langguth, B.; Kreuzer, P.M.; Kleinjung, T.; De Ridder, D. Tinnitus: Causes and clinical management. Lancet Neurol. 2013, 12, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, H.J.; An, S.Y.; Sim, S.; Park, B.; Kim, S.W.; Lee, J.S.; Hong, S.K.; Choi, H.G. Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS ONE 2015, 10, e0127578. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Zimmerman, B.; Bido Medina, R.O.; Carpenter-Thompson, J.R.; Husain, F.T. Changes in gray and white matter in subgroups within the tinnitus population. Brain Res. 2018, 1679, 64–74. [Google Scholar] [CrossRef]
- Haider, H.F.; Bojić, T.; Ribeiro, S.F.; Paço, J.; Hall, D.A.; Szczepek, A.J. Pathophysiology of Subjective Tinnitus: Triggers and Maintenance. Front. Neurosci. 2018, 12, 866. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.R.; Azevedo, A.A.; Penido, N.O. Positive Association between Tinnitus and Arterial Hypertension. Front. Neurol. 2016, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.M.; Curhan, S.G.; Wang, M.; Eavey, R.; Stankovic, K.M.; Curhan, G.C. Hypertension, Diuretic Use, and Risk of Hearing Loss. Am. J. Med. 2016, 129, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Avcı, D. Increased Serum Lipid Levels in Patients with Subjective Tinnitus. Iran. J. Otorhinolaryngol. 2021, 33, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sezer, E.D.; Sozmen, E.Y.; Nart, D.; Onat, T. Effect of atorvastatin therapy on oxidant-antioxidant status and atherosclerotic plaque formation. Vasc. Health Risk Manag. 2011, 7, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.; Tsou, Y.A.; Wang, T.C.; Chang, W.D.; Lin, C.L.; Tyler, R.S. Hypothyroidism and related comorbidities on the risks of developing tinnitus. Sci. Rep. 2022, 12, 3401. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Chiu, C.J.; Chen, P.C.; Chang, T.Y.; Tyler, R.S.; Rojas-Roncancio, E.; Coelho, C.B.; Mancini, P.C.; Lin, C.L.; Lin, C.D.; et al. Increased Incidence of Tinnitus Following a Hyperthyroidism Diagnosis: A Population-Based Longitudinal Study. Front. Endocrinol. 2021, 12, 741719. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; An, M.H.; Ha, S.; Kim, M.G.; Park, T.J.; Kim, H.K.; Sheen, K.; Park, R.W.; Park, S.S. Association between gastroesophageal reflux disease and tinnitus in a nationwide population-based cohort study. Sci. Rep. 2024, 14, 30106. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef]
- Mousavi, S.H.G.; Sajadinejad, B.; Khorsandi, S.; Farhadi, A. Diabetes Mellitus and Tinnitus: An Epidemiology Study. Maedica 2021, 16, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Juhn, S.K.; Jung, M.K.; Hoffman, M.D.; Drew, B.R.; Preciado, D.A.; Sausen, N.J.; Jung, T.T.; Kim, B.H.; Park, S.Y.; Lin, J.; et al. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin. Exp. Otorhinolaryngol. 2008, 1, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; D’Aguanno, V.; Di Stadio, A.; De Virgilio, A.; Croce, A.; Longo, L.; Greco, A.; de Vincentiis, M. Audiovestibular Symptoms in Systemic Autoimmune Diseases. J. Immunol. Res. 2018, 2018, 5798103. [Google Scholar] [CrossRef]
- Biswas, R.; Genitsaridi, E.; Trpchevska, N.; Lugo, A.; Schlee, W.; Cederroth, C.R.; Gallus, S.; Hall, D.A. Low Evidence for Tinnitus Risk Factors: A Systematic Review and Meta-analysis. J. Assoc. Res. Otolaryngol. 2023, 24, 81–94. [Google Scholar] [CrossRef]
- Park, K.W.; Kullar, P.; Malhotra, C.; Stankovic, K.M. Current and Emerging Therapies for Chronic Subjective Tinnitus. J. Clin. Med. 2023, 12, 6555. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 1151–1159. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Nagi, D.; Hambling, C.; Taylor, R. Remission of type 2 diabetes: A position statement from the Association of British Diabetologists (ABCD) and the Primary Care Diabetes Society (PCDS). Br. J. Diabetes 2019, 19, 73–76. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.D.; Stocker, D.J.; Chou, E.L.; Burch, H.B. 2022 Update on Clinical Management of Graves Disease and Thyroid Eye Disease. Endocrinol. Metab. Clin. N. Am. 2022, 51, 287–304. [Google Scholar] [CrossRef] [PubMed]
- McEvoym, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. ESC Scientific Document Group. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart. J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Bencsik, B.; Tamás, L.; Trimmel, K.; Stauder, A. Hungarian adaptation of the Tinnitus Handicap Inventory: Reliability and validity. Eur. Arch. Otorhinolaryngol. 2015, 272, 2243–2248. [Google Scholar] [CrossRef]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 143–148. [Google Scholar] [CrossRef]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol. Head Neck Surg. 2019, 161, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Rabau, S.; Cox, T.; Punte, A.K.; Waelkens, B.; Gilles, A.; Wouters, K.; de Varebeke, S.J.; Van de Heyning, P. Changes over time of psychoacoustic outcome measurements are not a substitute for subjective outcome measurements in acute tinnitus. Eur. Arch. Otorhinolaryngol. 2015, 272, 573–581. [Google Scholar] [CrossRef]
- Oosterloo, B.C.; Croll, P.H.; Baatenburg de Jong, R.J.; Ikram, M.K.; Goedegebure, A. Prevalence of Tinnitus in an Aging Population and Its Relation to Age and Hearing Loss. Otolaryngol. Head Neck Surg. 2021, 164, 859–868. [Google Scholar] [CrossRef]
- Tan, C.M.; Lecluyse, W.; McFerran, D.; Meddis, R. Tinnitus and patterns of hearing loss. J. Assoc. Res. Otolaryngol. 2013, 14, 275–282. [Google Scholar] [CrossRef]
- Gollnast, D.; Tziridis, K.; Krauss, P.; Schilling, A.; Hoppe, U.; Schulze, H. Analysis of Audiometric Differences of Patients with and without Tinnitus in a Large Clinical Database. Front. Neurol. 2017, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Schulze, H.; Tziridis, K. Is phase locking crucial to improve hearing thresholds in tinnitus patients? Eur. J. Neurosci. 2023, 58, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Vîrlan, M.J.R.; Costea, D.E.; Păun, D.L.; Zamfir-Chiru-Anton, A.; Sterian, A.G.; Spînu, A.D.; Nimigean, V.; Nimigean, V.R. Degenerative bony changes in the temporal component of the temporomandibular joint—Review of the literature. Rom. J. Morphol. Embryol. 2022, 63, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Musleh, A.; Alharthy, A.K.H.; Alzahrani, M.Y.M.; Bin Maadhah, S.A.; Al Zehefa, I.A.; AlQahtani, R.Y.; Alshehri, I.S.M.; Alqahtani, F.B.A.; Asiri, K.A.M.; Almushari, A.A. Psychological Impact and Quality of Life in Adults With Tinnitus: A Cross-Sectional Study. Cureus 2024, 16, e51976. [Google Scholar] [CrossRef] [PubMed]
- Al-Swiahb, J.; Park, S.N. Characterization of tinnitus in different age groups: A retrospective review. Noise Health 2016, 18, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, X.; Chai, R.; Fan, J. Progress on mechanisms of age-related hearing loss. Front. Neurosci. 2023, 17, 1253574. [Google Scholar] [CrossRef]
- Langguth, B.; de Ridder, D.; Schlee, W.; Kleinjung, T. Tinnitus: Clinical Insights in Its Pathophysiology—A Perspective. J. Assoc. Res. Otolaryngol. 2024, 25, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, D.C.; Kim, C.O. The Association between Serum Lipid Levels and Tinnitus Prevalence and Severity in Korean Elderly: A Nationwide Population-Based Cross-Sectional Study. Yonsei Med. J. 2024, 65, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Pulec, J.L.; Pulec, M.B.; Mendoza, I. Progressive sensorineural hearing loss, subjective tinnitus and vertigo caused by elevated blood lipids. Ear Nose Throat J. 1997, 76, 716–720. [Google Scholar] [CrossRef]
- Suzuki, K.; Kaneko, M.; Murai, K. Influence of serum lipids on auditory function. Laryngoscope 2000, 110, 1736–1738. [Google Scholar] [CrossRef]
- Hameed, M.K.; Sheikh, Z.A.; Ahmed, A.; Najam, A. Atorvastatin in the management of tinnitus with hyperlipidemias. J. Coll. Physicians Surg. Pak. 2014, 24, 927–930. [Google Scholar] [PubMed]
- Moog, N.K.; Entringer, S.; Heim, C.; Wadhwa, P.D.; Kathmann, N.; Buss, C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 2017, 342, 68–100. [Google Scholar] [CrossRef]
- Uziel, A.; Marot, M.; Rabie, A. Corrective effects of thyroxine on cochlear abnormalities induced by congenital hypothyroidism in the rat. II. Electrophysiological study. Dev. Brain Res. 1985, 19, 123–127. [Google Scholar] [CrossRef]
- Figueiredo, R.R.; de Azevedo, A.A.; Penido Nde, O. Tinnitus and arterial hypertension: A systematic review. Eur. Arch. Otorhinolaryngol. 2015, 272, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Patel, S.; Finberg, A.; Shah, V.N.; Mittal, R.; Eshraghi, A.A. Association Between Tinnitus and Hypertension: A Cross-Sectional Analysis of the National Health and Nutrition Examination Survey. Otol. Neurotol. 2022, 43, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Ramatsoma, H.; Patrick, S.M. Hypertension Associated With Hearing Loss and Tinnitus Among Hypertensive Adults at a Tertiary Hospital in South Africa. Front. Neurol. 2022, 13, 857600. [Google Scholar] [CrossRef]
- Samelli, A.G.; Santos, I.S.; Padilha, F.Y.O.M.M.; Gomes, R.F.; Moreira, R.R.; Rabelo, C.M.; Matas, C.G.; Bensenor, I.M.; Lotufo, P.A. Hearing loss, tinnitus, and hypertension: Analysis of the baseline data from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Clinics 2021, 76, e2370. [Google Scholar] [CrossRef] [PubMed]
- Gun, T.; Özkan, S.; Yavuz, B. Is tinnitus an early voice of masked hypertension? High masked hypertension rate in patients with tinnitus. Clin. Exp. Hypertens. 2019, 41, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Wattamwar, K.; Qian, Z.J.; Otter, J.; Leskowitz, M.J.; Caruana, F.F.; Siedlecki, B.; Spitzer, J.B.; Lalwani, A.K. Association of Cardiovascular Comorbidities With Hearing Loss in the Older Old. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 623–629. [Google Scholar] [CrossRef] [PubMed]


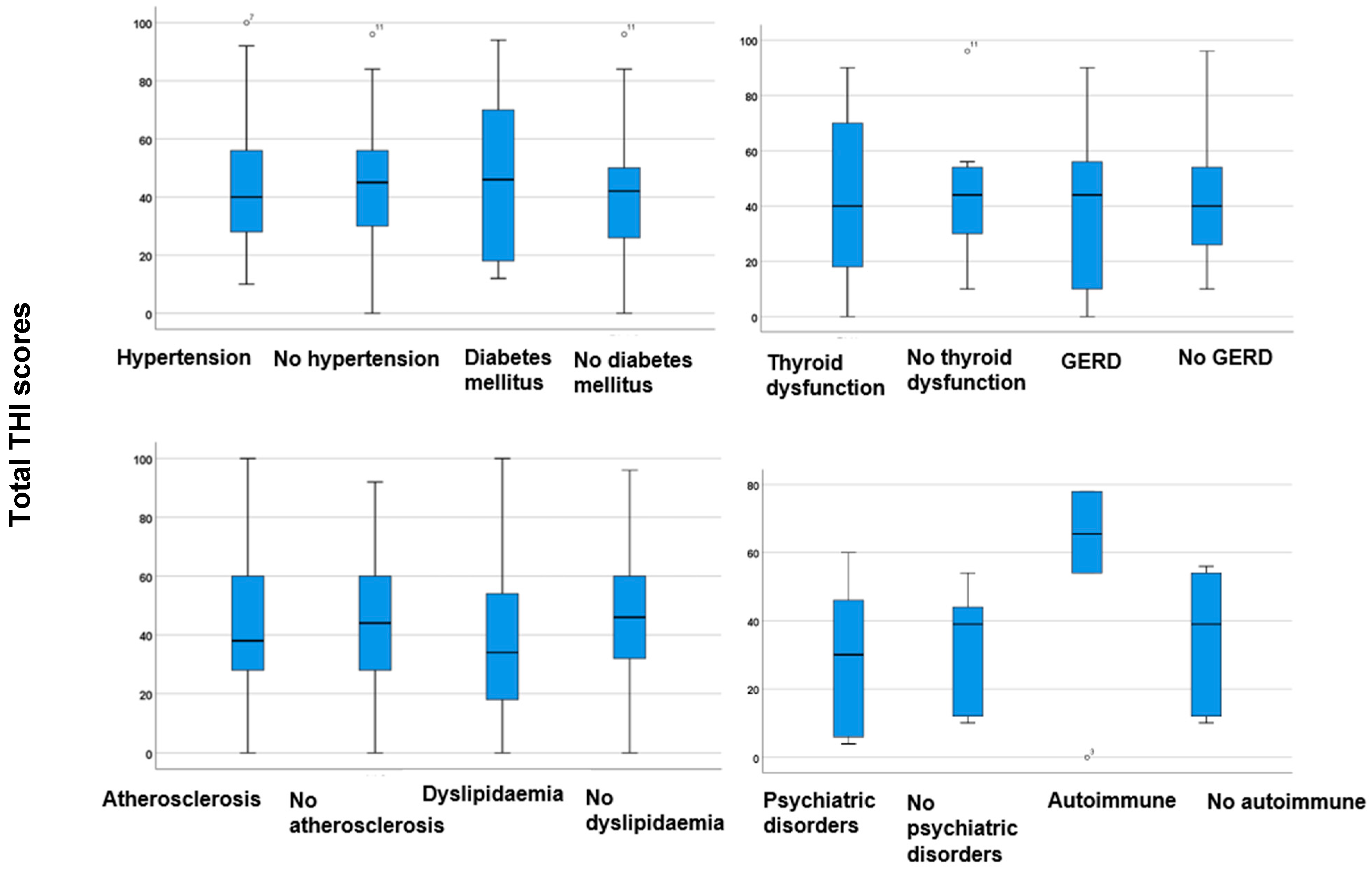
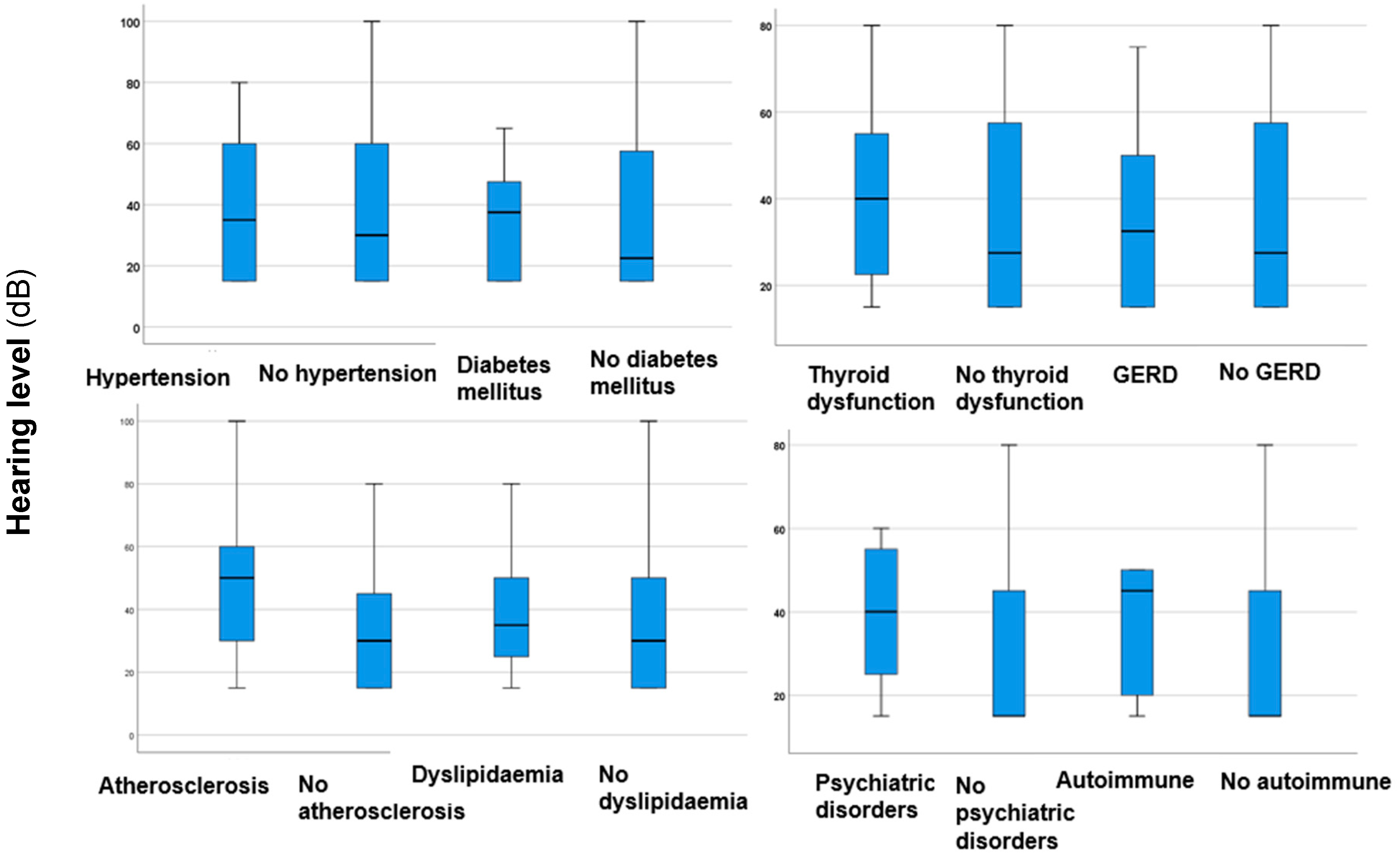
| Parameter | |
|---|---|
| Age (median years, IQR; Q1–Q3) | 52 (21; 43–64) |
| Sex (men/women) | 66/81 |
| Symptom onset (median months, IQR; Q1–Q3) | 16 (55; 5–60) |
| Tinnitus location | |
| Right (n, %) | 34 (23.1%) |
| Left (n, %) | 48 (32.6%) |
| Bilateral (n, %) | 65 (44.21%) |
| Hearing loss (n, %) | 77 (52.38%) |
| Hearing level (median dB, IQR; Q1–Q3) | 30 (30; 15–45) |
| Tinnitus intensity (median dB, IQR; Q1–Q3) | 30 (30; 20–50) |
| Tinnitus pitch (median Hz, IQR; Q1–Q3) | 4000 (6000; 2000–8000) |
| Total THI scores (median, IQR; Q1–Q3) | 44 (38; 28–66) |
| Dependent | Predictor | β | Std. Error | p-Value | OR | 95% CI (Lower Bound) | 95% CI (Upper Bound) |
|---|---|---|---|---|---|---|---|
| Bilateral tinnitus | Dyslipidaemia | −1.282 | 0.428 | 0.003 * | 0.278 | 0.120 | 0.642 |
| Sensorineural hearing loss | Atherosclerosis | 1.400 | 0.513 | 0.006 * | 4.054 | 1.483 | 11.086 |
| Moderate to severe tinnitus | Psychiatric comorbidities | 2.184 | 0.687 | 0.001 * | 8.881 | 2.312 | 34.123 |
| Right-sided tinnitus | Thyroid dysfunction | −2.430 | 0.933 | 0.009 * | 0.088 | 0.014 | 0.548 |
| Atherosclerosis | 1.038 | 0.516 | 0.044 * | 2.823 | 1.026 | 7.766 | |
| Left-sided tinnitus | Dyslipidaemia | 0.927 | 0.406 | 0.023 * | 2.527 | 1.140 | 5.605 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maihoub, S.; Mavrogeni, P.; Molnár, V.; Molnár, A. Tinnitus and Its Comorbidities: A Comprehensive Analysis of Their Relationships. J. Clin. Med. 2025, 14, 1285. https://doi.org/10.3390/jcm14041285
Maihoub S, Mavrogeni P, Molnár V, Molnár A. Tinnitus and Its Comorbidities: A Comprehensive Analysis of Their Relationships. Journal of Clinical Medicine. 2025; 14(4):1285. https://doi.org/10.3390/jcm14041285
Chicago/Turabian StyleMaihoub, Stefani, Panayiota Mavrogeni, Viktória Molnár, and András Molnár. 2025. "Tinnitus and Its Comorbidities: A Comprehensive Analysis of Their Relationships" Journal of Clinical Medicine 14, no. 4: 1285. https://doi.org/10.3390/jcm14041285
APA StyleMaihoub, S., Mavrogeni, P., Molnár, V., & Molnár, A. (2025). Tinnitus and Its Comorbidities: A Comprehensive Analysis of Their Relationships. Journal of Clinical Medicine, 14(4), 1285. https://doi.org/10.3390/jcm14041285






