Beyond the Lungs: Extrapulmonary Effects of Non-Invasive and Invasive Ventilation Strategies
Abstract
1. Introduction
2. Non-Invasive Respiratory Support
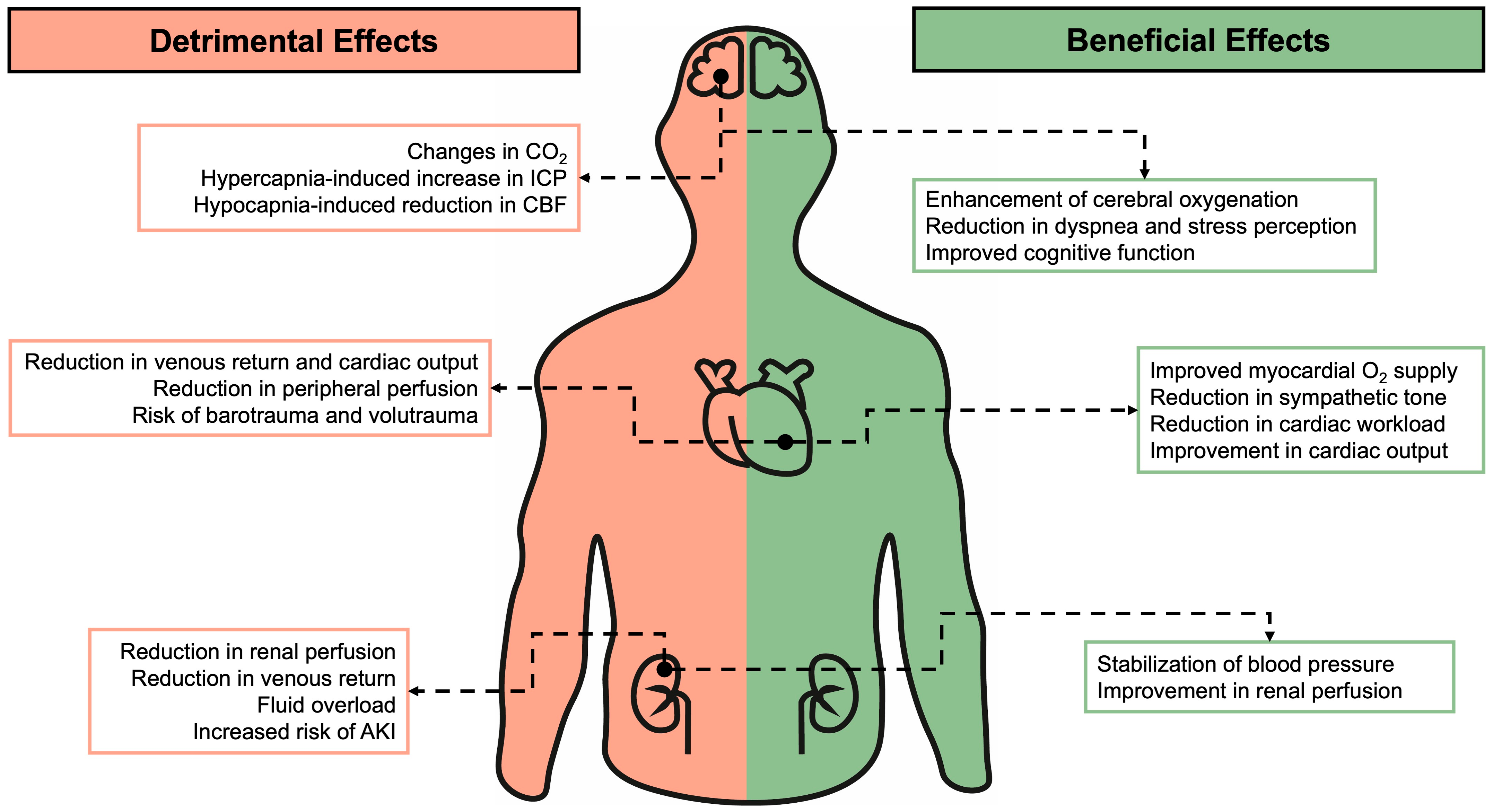
3. Neurological System
3.1. Non-Invasive Respiratory Support
3.2. Invasive Mechanical Ventilation
4. Cardiovascular System
4.1. Non-Invasive Respiratory Support
- Restoration of functional residual capacity (FRC): non-invasive respiratory support helps restore FRC, reducing shunt and improving oxygenation [49].
- Increase in pleural pressure: elevated pleural pressure reduces left ventricular afterload (the difference between left ventricular systolic pressure and pleural pressure) without compromising the cardiac index [50].
- Reduction in left ventricular end-diastolic volume (Preload): this effect is particularly beneficial in patients with preserved left ventricular function, as it decreases preload and consequently the left ventricular end-diastolic volume [51].
4.2. Invasive Respiratory Support
5. Renal System
5.1. Non-Invasive Respiratory Support
5.2. Invasive Respiratory Support
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury; |
| ARDS | Acute respiratory distress syndrome; |
| BiPAP | Bilevel positive airway pressure; |
| CBF | Cerebral blood flow; |
| COPD | Chronic obstructive pulmonary disease; |
| CPAP | Continuous positive airway pressure; |
| CPP | Cerebral perfusion pressure; |
| Eadyn | Dynamic central arterial elastance; |
| EBC | Exhaled breath condensate; |
| ETCO2 | End-tidal CO2; |
| HFNO | High-flow nasal oxygen; |
| IAP | Intra-abdominal pressure; |
| ICP | Intracranial pressure; |
| IL | Interleukin; |
| IMV | Invasive mechanical ventilation; |
| ITP | Intrathoracic pressure; |
| LV | Left Ventricle; |
| NIV | Non-invasive ventilation; |
| PaCO2 | Arterial partial pressure of carbon dioxide; |
| PEEP | Positive end-expiratory pressure; |
| PPV | Positive pressure ventilation; |
| PVR | Pulmonary vascular resistance; |
| RV | Right ventricle; |
| SVV | Systolic volume variation; |
| TBI | Traumatic brain injury; |
| TNF | Tumor necrosis factor; |
| VT | Tidal volume. |
References
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Ferguson, N.D.; Frutos-Vivar, F.; Anzueto, A.; Putensen, C.; Raymondos, K.; Apezteguia, C.; Desmery, P.; Hurtado, J.; Abroug, F.; et al. Management and Outcome of Mechanically Ventilated Neurologic Patients*. Crit. Care Med. 2011, 39, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Battaglini, D.; Abbas, A.; Sarrió, E.; Cinotti, R.; Asehnoune, K.; Taccone, F.S.; Rocco, P.R.; Schultz, M.J.; Citerio, G.; et al. Clinical Practice and Effect of Carbon Dioxide on Outcomes in Mechanically Ventilated Acute Brain-Injured Patients: A Secondary Analysis of the ENIO Study. Intensive Care Med. 2024, 50, 234–246. [Google Scholar] [CrossRef]
- Bossers, S.M.; Mansvelder, F.; Loer, S.A.; Boer, C.; Bloemers, F.W.; Van Lieshout, E.M.M.; Den Hartog, D.; Hoogerwerf, N.; van der Naalt, J.; Absalom, A.R.; et al. Association between Prehospital End-Tidal Carbon Dioxide Levels and Mortality in Patients with Suspected Severe Traumatic Brain Injury. Intensive Care Med. 2023, 49, 491–504. [Google Scholar] [CrossRef] [PubMed]
- González-López, A.; López-Alonso, I.; Aguirre, A.; Amado-Rodríguez, L.; Batalla-Solís, E.; Astudillo, A.; Tomás-Zapico, C.; Fueyo, A.; dos Santos, C.C.; Talbot, K.; et al. Mechanical Ventilation Triggers Hippocampal Apoptosis by Vagal and Dopaminergic Pathways. Am. J. Respir. Crit. Care Med. 2013, 188, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Korovesi, I.; Papadomichelakis, E.; Orfanos, S.E.; Giamarellos-Bourboulis, E.J.; Livaditi, O.; Pelekanou, A.; Sotiropoulou, C.; Koutsoukou, A.; Dimopoulou, I.; Ekonomidou, F.; et al. Exhaled Breath Condensate in Mechanically Ventilated Brain-Injured Patients with No Lung Injury or Sepsis. Anesthesiology 2011, 114, 1118–1129. [Google Scholar] [CrossRef]
- Tabbì, L.; Tonelli, R.; Comellini, V.; Dongilli, R.; Sorgentone, S.; Spacone, A.; Paonessa, M.C.; Sacchi, M.; Falsino, L.; Boni, E.; et al. Delirium Incidence and Risk Factors in Patients Undergoing Non-Invasive Ventilation for Acute Respiratory Failure: A Multicenter Observational Trial. Minerva Anestesiol. 2022, 88, 815–826. [Google Scholar] [CrossRef]
- Tilouche, N.; Hassen, M.F.; Ali, H.B.S.; Jaoued, O.; Gharbi, R.; El Atrous, S.S. Delirium in the Intensive Care Unit: Incidence, Risk Factors, and Impact on Outcome. Indian J. Crit. Care Med. 2018, 22, 144–149. [Google Scholar] [CrossRef]
- Guillamondegui, O.D.; Richards, J.E.; Ely, E.W.; Jackson, J.C.; Archer-Swygert, K.; Norris, P.R.; Obremskey, W.T. Does Hypoxia Affect Intensive Care Unit Delirium or Long-Term Cognitive Impairment After Multiple Trauma Without Intracranial Hemorrhage? J. Trauma Inj. Infect. Crit. Care 2011, 70, 910–915. [Google Scholar] [CrossRef]
- Palakshappa, J.A.; Hough, C.L. How We Prevent and Treat Delirium in the ICU. Chest 2021, 160, 1326–1334. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Whitelaw, W.A.; Sas, R.; Smith, E.R.; Tyberg, J.V.; Belenkie, I. RV Filling Modulates LV Function by Direct Ventricular Interaction during Mechanical Ventilation. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H549–H557. [Google Scholar] [CrossRef] [PubMed]
- Brower, R.; Wise, R.A.; Hassapoyannes, C.; Bromberger-Barnea, B.; Permutt, S. Effect of Lung Inflation on Lung Blood Volume and Pulmonary Venous Flow. J. Appl. Physiol. 1985, 58, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Linde, L.M.; Simmons, D.H.; Ellman, E.L. Pulmonary Hemodynamics during Positive-Pressure Breathing. J. Appl. Physiol. 1961, 16, 644–646. [Google Scholar] [CrossRef]
- Regli, A.; Hockings, L.E.; Musk, G.C.; Roberts, B.; Noffsinger, B.; Singh, B.; van Heerden, P.V. Commonly Applied Positive End-Expiratory Pressures Do Not Prevent Functional Residual Capacity Decline in the Setting of Intra-Abdominal Hypertension: A Pig Model. Crit. Care 2010, 14, R128. [Google Scholar] [CrossRef] [PubMed]
- Regli, A.; Mahendran, R.; Fysh, E.T.; Roberts, B.; Noffsinger, B.; De Keulenaer, B.L.; Singh, B.; van Heerden, P.V. Matching Positive End-Expiratory Pressure to Intra-Abdominal Pressure Improves Oxygenation in a Porcine Sick Lung Model of Intra-Abdominal Hypertension. Crit. Care 2012, 16, R208. [Google Scholar] [CrossRef]
- The ARDS Network. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Mercat, A.; Richard, J.-C.M.; Vielle, B.; Jaber, S.; Osman, D.; Diehl, J.-L.; Lefrant, J.-Y.; Prat, G.; Richecoeur, J.; Nieszkowska, A.; et al. Positive End-Expiratory Pressure Setting in Adults With Acute Lung Injury and Acute Respiratory Distress Syndrome. JAMA 2008, 299, 646. [Google Scholar] [CrossRef] [PubMed]
- Rittayamai, N.; Pravarnpat, C.; Srilam, W.; Bunyarid, S.; Chierakul, N. Safety and Efficacy of Noninvasive Ventilation for Acute Respiratory Failure in General Medical Ward: A Prospective Cohort Study. J. Thorac. Dis. 2023, 15, 5466–5474. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Radovanovic, D.; Saad, M.; Zilianti, C.; Coppola, S.; Chiumello, D.A.; Pecchiari, M. Acute Dyspnea in the Emergency Department: A Clinical Review. Intern. Emerg. Med. 2023, 18, 1491–1507. [Google Scholar] [CrossRef]
- Shah, N.M.; Steier, J.; Hart, N.; Kaltsakas, G. Effects of Non-Invasive Ventilation on Sleep in Chronic Hypercapnic Respiratory Failure. Thorax 2024, 79, 281–288. [Google Scholar] [CrossRef]
- Ergan, B.; Nasiłowski, J.; Winck, J.C. How Should We Monitor Patients with Acute Respiratory Failure Treated with Noninvasive Ventilation? Eur. Respir. Rev. 2018, 27, 170101. [Google Scholar] [CrossRef] [PubMed]
- Raux, M.; Straus, C.; Redolfi, S.; Morelot-Panzini, C.; Couturier, A.; Hug, F.; Similowski, T. Electroencephalographic Evidence for Pre-motor Cortex Activation during Inspiratory Loading in Humans. J. Physiol. 2007, 578, 569–578. [Google Scholar] [CrossRef]
- Sharshar, T.; Hopkinson, N.S.; Jonville, S.; Prigent, H.; Carlier, R.; Dayer, M.J.; Swallow, E.B.; Lofaso, F.; Moxham, J.; Polkey, M.I. Demonstration of a Second Rapidly Conducting Cortico-diaphragmatic Pathway in Humans. J. Physiol. 2004, 560, 897–908. [Google Scholar] [CrossRef]
- Raux, M.; Ray, P.; Prella, M.; Duguet, A.; Demoule, A.; Similowski, T. Cerebral Cortex Activation during Experimentally Induced Ventilator Fighting in Normal Humans Receiving Noninvasive Mechanical Ventilation. Anesthesiology 2007, 107, 746–755. [Google Scholar] [CrossRef]
- Hopkinson, N.S.; Sharshar, T.; Dayer, M.J.; Lofaso, F.; Moxham, J.; Polkey, M.I. The Effect of Acute Non-Invasive Ventilation on Corticospinal Pathways to the Respiratory Muscles in Chronic Obstructive Pulmonary Disease. Respir. Physiol. Neurobiol. 2012, 183, 41–47. [Google Scholar] [CrossRef]
- Roberson, S.W.; Patel, M.B.; Dabrowski, W.; Ely, E.W.; Pakulski, C.; Kotfis, K. Challenges of Delirium Management in Patients with Traumatic Brain Injury: From Pathophysiology to Clinical Practice. Curr. Neuropharmacol. 2021, 19, 1519–1544. [Google Scholar] [CrossRef]
- Zhang, R.; Bai, L.; Han, X.; Huang, S.; Zhou, L.; Duan, J. Incidence, Characteristics, and Outcomes of Delirium in Patients with Noninvasive Ventilation: A Prospective Observational Study. BMC Pulm. Med. 2021, 21, 157. [Google Scholar] [CrossRef]
- Frisvold, S.; Coppola, S.; Ehrmann, S.; Chiumello, D.; Guérin, C. Respiratory Challenges and Ventilatory Management in Different Types of Acute Brain-Injured Patients. Crit. Care 2023, 27, 247. [Google Scholar] [CrossRef]
- Godet, T.; Chabanne, R.; Marin, J.; Kauffmann, S.; Futier, E.; Pereira, B.; Constantin, J.-M. Extubation Failure in Brain-Injured Patients. Anesthesiology 2017, 126, 104–114. [Google Scholar] [CrossRef]
- Taran, S.; Diaz-Cruz, C.; Perrot, B.; Alvarez, P.; Godoy, D.A.; Gurjar, M.; Haenggi, M.; Mijangos, J.C.; Pelosi, P.; Robba, C.; et al. Association of Noninvasive Respiratory Support with Extubation Outcomes in Brain-Injured Patients Receiving Mechanical Ventilation: A Secondary Analysis of the ENIO Prospective Observational Study. Am. J. Respir. Crit. Care Med. 2023, 208, 270–279. [Google Scholar] [CrossRef]
- Cinotti, R.; Citerio, G.; Asehnoune, K. Extubation in Neurocritical Care Patients: Lesson Learned. Intensive Care Med. 2023, 49, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Battisti-Charbonney, A.; Fisher, J.; Duffin, J. The Cerebrovascular Response to Carbon Dioxide in Humans. J. Physiol. 2011, 589, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Darkwah Oppong, M.; Wrede, K.H.; Müller, D.; Santos, A.N.; Rauschenbach, L.; Dinger, T.F.; Ahmadipour, Y.; Pierscianek, D.; Chihi, M.; Li, Y.; et al. PaCO2-Management in the Neuro-Critical Care of Patients with Subarachnoid Hemorrhage. Sci. Rep. 2021, 11, 19191. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.W.; Karagiannis, P.; Coletta, M.; Kilgannon, J.H.; Chansky, M.E.; Trzeciak, S. Effects of PaCO2 Derangements on Clinical Outcomes after Cerebral Injury: A Systematic Review. Resuscitation 2015, 91, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.A.; Seifi, A.; Garza, D.; Lubillo-Montenegro, S.; Murillo-Cabezas, F. Hyperventilation Therapy for Control of Posttraumatic Intracranial Hypertension. Front. Neurol. 2017, 8, 250. [Google Scholar] [CrossRef]
- Curley, G.; Kavanagh, B.P.; Laffey, J.G. Hypocapnia and the Injured Brain: More Harm than Benefit. Crit. Care Med. 2010, 38, 1348–1359. [Google Scholar] [CrossRef]
- Muizelaar, J.P.; Marmarou, A.; Ward, J.D.; Kontos, H.A.; Choi, S.C.; Becker, D.P.; Gruemer, H.; Young, H.F. Adverse Effects of Prolonged Hyperventilation in Patients with Severe Head Injury: A Randomized Clinical Trial. J. Neurosurg. 1991, 75, 731–739. [Google Scholar] [CrossRef]
- Soustiel, J.F.; Mahamid, E.; Chistyakov, A.; Shik, V.; Benenson, R.; Zaaroor, M. Comparison of Moderate Hyperventilation and Mannitol for Control of Intracranial Pressure Control in Patients with Severe Traumatic Brain Injury—A Study of Cerebral Blood Flow and Metabolism. Acta Neurochir. 2006, 148, 845–851. [Google Scholar] [CrossRef]
- Ziaka, M.; Exadaktylos, A. Pathophysiology of Acute Lung Injury in Patients with Acute Brain Injury: The Triple-Hit Hypothesis. Crit. Care 2024, 28, 71. [Google Scholar] [CrossRef]
- Fisher, A.J.; Donnelly, S.C.; Hirani, N.; Burdick, M.D.; Strieter, R.M.; Dark, J.H.; Corris, P.A. Enhanced Pulmonary Inflammation in Organ Donors Following Fatal Non-Traumatic Brain Injury. Lancet 1999, 353, 1412–1413. [Google Scholar] [CrossRef]
- Young, N.; Rhodes, J.K.; Mascia, L.; Andrews, P.J. Ventilatory Strategies for Patients with Acute Brain Injury. Curr. Opin. Crit. Care 2010, 16, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Quilez, M.E.; Fuster, G.; Villar, J.; Flores, C.; Martí-Sistac, O.; Blanch, L.; López-Aguilar, J. Injurious Mechanical Ventilation Affects Neuronal Activation in Ventilated Rats. Crit. Care 2011, 15, R124. [Google Scholar] [CrossRef]
- Skiöld, B.; Wu, Q.; Hooper, S.B.; Davis, P.G.; McIntyre, R.; Tolcos, M.; Pearson, J.; Vreys, R.; Egan, G.F.; Barton, S.K.; et al. Early Detection of Ventilation-Induced Brain Injury Using Magnetic Resonance Spectroscopy and Diffusion Tensor Imaging: An In Vivo Study in Preterm Lambs. PLoS ONE 2014, 9, e95804. [Google Scholar] [CrossRef] [PubMed]
- Mascia, L.; Fanelli, V.; Mistretta, A.; Filippini, M.; Zanin, M.; Berardino, M.; Mazzeo, A.T.; Caricato, A.; Antonelli, M.; Della Corte, F.; et al. Lung-Protective Mechanical Ventilation in Patients with Severe Acute Brain Injury: A Multicenter Randomized Clinical Trial (PROLABI). Am. J. Respir. Crit. Care Med. 2024, 210, 1123–1131. [Google Scholar] [CrossRef]
- Taran, S.; Stevens, R.D. Does Lung Protective Ventilation Work in Acute Brain Injury? Am. J. Respir. Crit. Care Med. 2024, 210, 1073–1075. [Google Scholar] [CrossRef]
- Ziaka, M.; Exadaktylos, A. ARDS Associated Acute Brain Injury: From the Lung to the Brain. Eur. J. Med. Res. 2022, 27, 150. [Google Scholar] [CrossRef]
- Plaisance, P.; Pirracchio, R.; Berton, C.; Vicaut, E.; Payen, D. A Randomized Study of Out-of-Hospital Continuous Positive Airway Pressure for Acute Cardiogenic Pulmonary Oedema: Physiological and Clinical Effects. Eur. Heart J. 2007, 28, 2895–2901. [Google Scholar] [CrossRef]
- Roessler, M.S.; Schmid, D.S.; Michels, P.; Schmid, O.; Jung, K.; Stöber, J.; Neumann, P.; Quintel, M.; Moerer, O. Early Out-of-Hospital Non-Invasive Ventilation Is Superior to Standard Medical Treatment in Patients with Acute Respiratory Failure: A Pilot Study. Emerg. Med. J. 2012, 29, 409–414. [Google Scholar] [CrossRef]
- Katz, J.A.; Marks, J.D. Inspiratory Work with and without Continuous Positive Airway-Pressure in Patients with Acute Respiratory Failure. Anesthesiology 1985, 63, 598–607. [Google Scholar] [CrossRef]
- Naughton, M.T.; Rahman, M.A.; Hara, K.; Floras, J.S.; Bradley, T.D. Effect of Continuous Positive Airway Pressure on Intrathoracic and Left Ventricular Transmural Pressures in Patients With Congestive Heart Failure. Circulation 1995, 91, 1725–1731. [Google Scholar] [CrossRef]
- Bendjelid, K.; Schütz, N.; Suter, P.M.; Fournier, G.; Jacques, D.; Fareh, S.; Romand, J.-A. Does Continuous Positive Airway Pressure by Face Mask Improve Patients With Acute Cardiogenic Pulmonary Edema Due to Left Ventricular Diastolic Dysfunction? Chest 2005, 127, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- De Santis Santiago, R.; Teggia Droghi, M.; Fumagalli, J.; Marrazzo, F.; Florio, G.; Grassi, L.G.; Gomes, S.; Morais, C.C.A.; Ramos, O.P.S.; Bottiroli, M.; et al. High Pleural Pressure Prevents Alveolar Overdistension and Hemodynamic Collapse in Acute Respiratory Distress Syndrome with Class III Obesity. A Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 203, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Cappio Borlino, S.; Hagry, J.; Lai, C.; Rocca, E.; Fouqué, G.; Rosalba, D.; Fasan, M.; Shi, R.; Recanatini, A.; Cisterna, I.; et al. The Effect of Positive End-Expiratory Pressure on Pulmonary Vascular Resistance Depends on Lung Recruitability in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2024, 210, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Conti, G.; Rocco, M.; Bufi, M.; De Blasi, R.A.; Vivino, G.; Gasparetto, A.; Meduri, G.U. A Comparison of Noninvasive Positive-Pressure Ventilation and Conventional Mechanical Ventilation in Patients with Acute Respiratory Failure. N. Engl. J. Med. 1998, 339, 429–435. [Google Scholar] [CrossRef]
- Bishopp, A.; Sayeed, N.; Oakes, A.; Beauchamp, B.; Chakraborty, B.; Mukherjee, R. P42 Factors Affecting the Duration of Acute Non Invasive Ventilation Required in Patients with Acute Hypercapnic Respiratory Failure. Thorax 2015, 70, A96.2–A97. [Google Scholar] [CrossRef]
- Chen, P.-K.; Shih, C.-C.; Lin, F.-C.; Perng, D.-W.; Chou, K.-T.; Kou, Y.R.; Ko, H.-K. Prolonged Use of Noninvasive Positive Pressure Ventilation after Extubation among Patients in the Intensive Care Unit Following Cardiac Surgery: The Predictors and Its Impact on Patient Outcome. Sci. Rep. 2019, 9, 9539. [Google Scholar] [CrossRef]
- Walcher, A.; Faubel, S.; Keniston, A.; Dennen, P. In Critically Ill Patients Requiring CRRT, AKI Is Associated with Increased Respiratory Failure and Death Versus ESRD. Ren. Fail. 2011, 33, 935–942. [Google Scholar] [CrossRef]
- Regli, A.; Chakera, J.; De Keulenaer, B.L.; Roberts, B.; Noffsinger, B.; Singh, B.; van Heerden, P.V. Matching Positive End-Expiratory Pressure to Intra-Abdominal Pressure Prevents End-Expiratory Lung Volume Decline in a Pig Model of Intra-Abdominal Hypertension*. Crit. Care Med. 2012, 40, 1879–1886. [Google Scholar] [CrossRef]
- Annat, G.; Viale, J.P.; Xuan, B.B.; Aissa, O.H.; Benzoni, D.; Vincent, M.; Gharib, C.; Motin, J. Effect of PEEP Ventilation on Renal Function, Plasma Renin, Aldosterone, Neurophysins and Urinary ADH, and Prostaglandins. Anesthesiology 1983, 58, 136–141. [Google Scholar] [CrossRef]
- van den Akker, J.P.; Egal, M.; Groeneveld, A.J. Invasive Mechanical Ventilation as a Risk Factor for Acute Kidney Injury in the Critically Ill: A Systematic Review and Meta-Analysis. Crit. Care 2013, 17, R98. [Google Scholar] [CrossRef]
| Organs | Possible Physiological Consequences of IMV | Suggested Practices |
|---|---|---|
| Brain | Changes in CO2 (1 mmHg change in PaCO2, CBF changes by 3%) | Mild hypocapnia and normocapnia (PaCO2 32–35 mmHg) was well tolerated in TBI and intracranial hypertension [3] Normocapnia in severe TBI [4] |
| Hypercapnia-induced increases in ICP | Avoid hypercapnia (PaCO2 > 45 mmHg) [3] | |
| Hypocapnia-induced reduction in CBF | Avoid severe (PaCO2 26–31 mmHg), forced (PaCO2 < 26 mmHg) hypocapnia [3] | |
| High VT, continuous or short periods | Adjust VT (6–8 mL/kg) according to predicted body weight [5] | |
| Low (0 cmH2O) or high PEEP (>8 cmH2O) levels | PEEP at 8, compared to 0 cmH2O, reduced the systemic inflammatory response in ABI patients [6] | |
| Occurrence of delirium | Check for key factors (age, cancer, sepsis, excessive use of sedative-hypnotic medication) [7,8] Hypoxic events should be avoided, but up to now, it is not directly linked to delirium [9] Antipsychotic agents remain the most common treatment [10] | |
| Heart | ITP increases and may affect EDV and compliance of RV and LV | Evaluate RV and LV performance by non-invasive techniques, such as echocardiography. Check airway pressure and PEEP levels constantly [11,12] |
| PEEP levels may increase PVR and RV afterload | Adjustment of PEEP with lung recruitment, no changes in PVR [13] Adjustment of PEEP with lung distension, PVR may increase [13] | |
| Kidney | Elevated IAP may impair microvascular blood flow and venous drainage from kidneys | Check for other factors other than IMV (fluid balance, gastric distension) [14] Adjustment of PEEP to match IAP levels has shown positive results, as long no profound hemodynamic changes are observed [15] |
| Increased risk of AKI | Protective VT (6–8 mL/kg) was associated with more renal failure-free days [16] No association of low and high PEEP levels with renal failure-free days [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.L.; Chiumello, D.; Pozzi, T.; Rocco, P.R.M. Beyond the Lungs: Extrapulmonary Effects of Non-Invasive and Invasive Ventilation Strategies. J. Clin. Med. 2025, 14, 1242. https://doi.org/10.3390/jcm14041242
Silva PL, Chiumello D, Pozzi T, Rocco PRM. Beyond the Lungs: Extrapulmonary Effects of Non-Invasive and Invasive Ventilation Strategies. Journal of Clinical Medicine. 2025; 14(4):1242. https://doi.org/10.3390/jcm14041242
Chicago/Turabian StyleSilva, Pedro Leme, Davide Chiumello, Tommaso Pozzi, and Patricia Rieken Macedo Rocco. 2025. "Beyond the Lungs: Extrapulmonary Effects of Non-Invasive and Invasive Ventilation Strategies" Journal of Clinical Medicine 14, no. 4: 1242. https://doi.org/10.3390/jcm14041242
APA StyleSilva, P. L., Chiumello, D., Pozzi, T., & Rocco, P. R. M. (2025). Beyond the Lungs: Extrapulmonary Effects of Non-Invasive and Invasive Ventilation Strategies. Journal of Clinical Medicine, 14(4), 1242. https://doi.org/10.3390/jcm14041242








