Opportunistic Osteoporosis Assessment and Fracture Risk Determination Using Cancellous Density Measurement in Hounsfield Units of Native Lumbar Computed Tomography Images—A Comparative Study with Conventional Bone Density Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
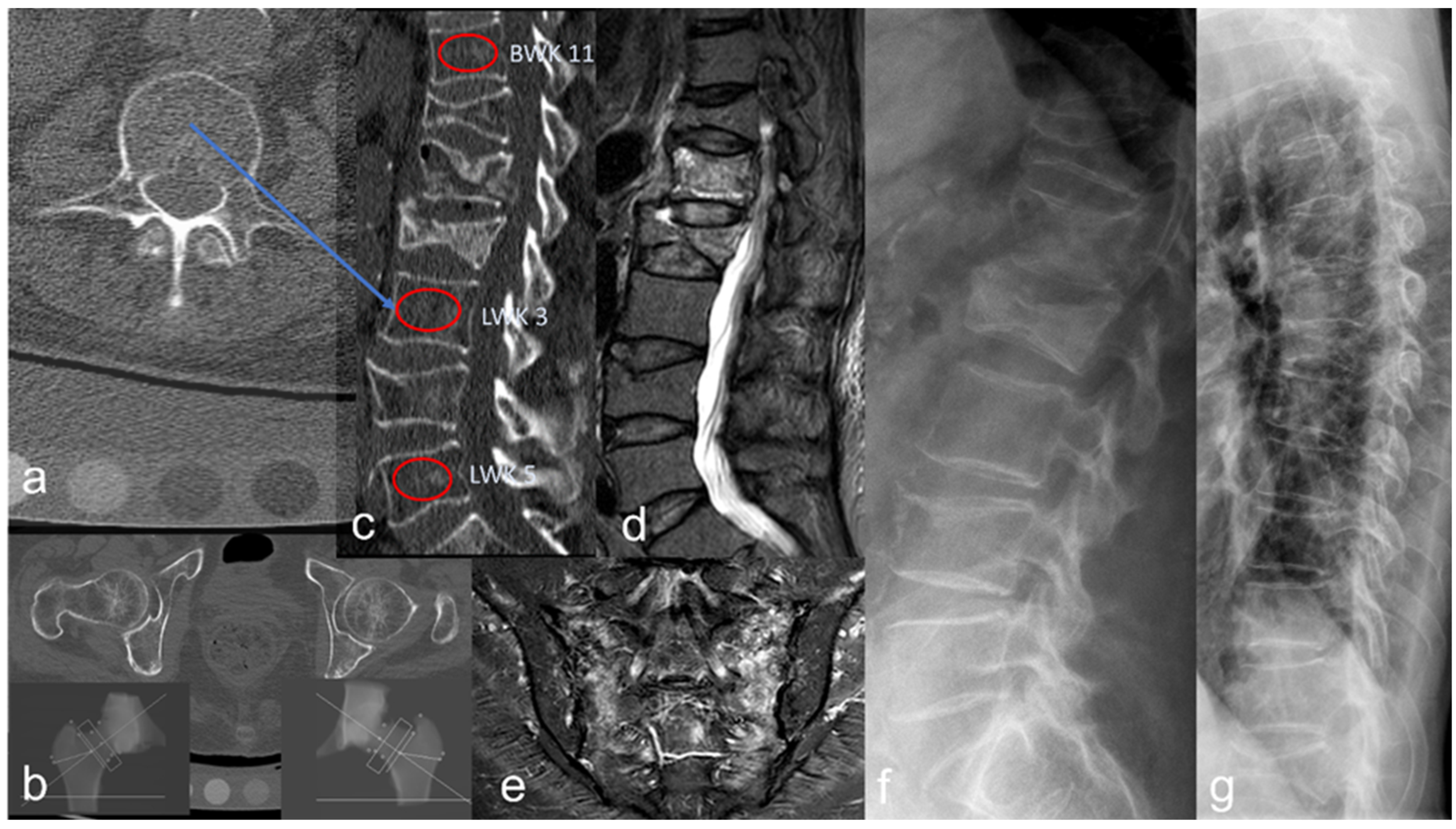
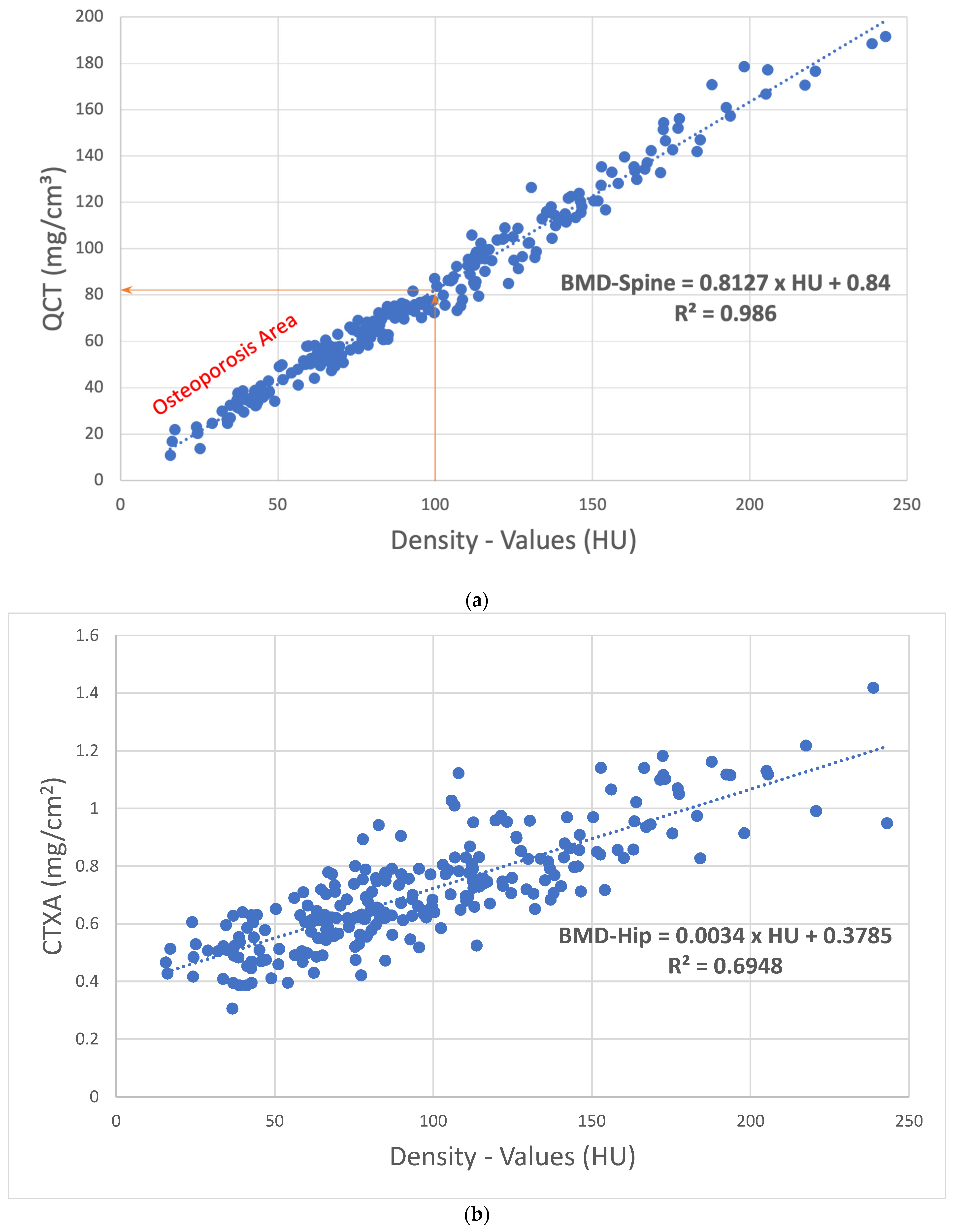
2.3. Bone Density Analysis
2.4. Fracture Detection
2.5. Statistics
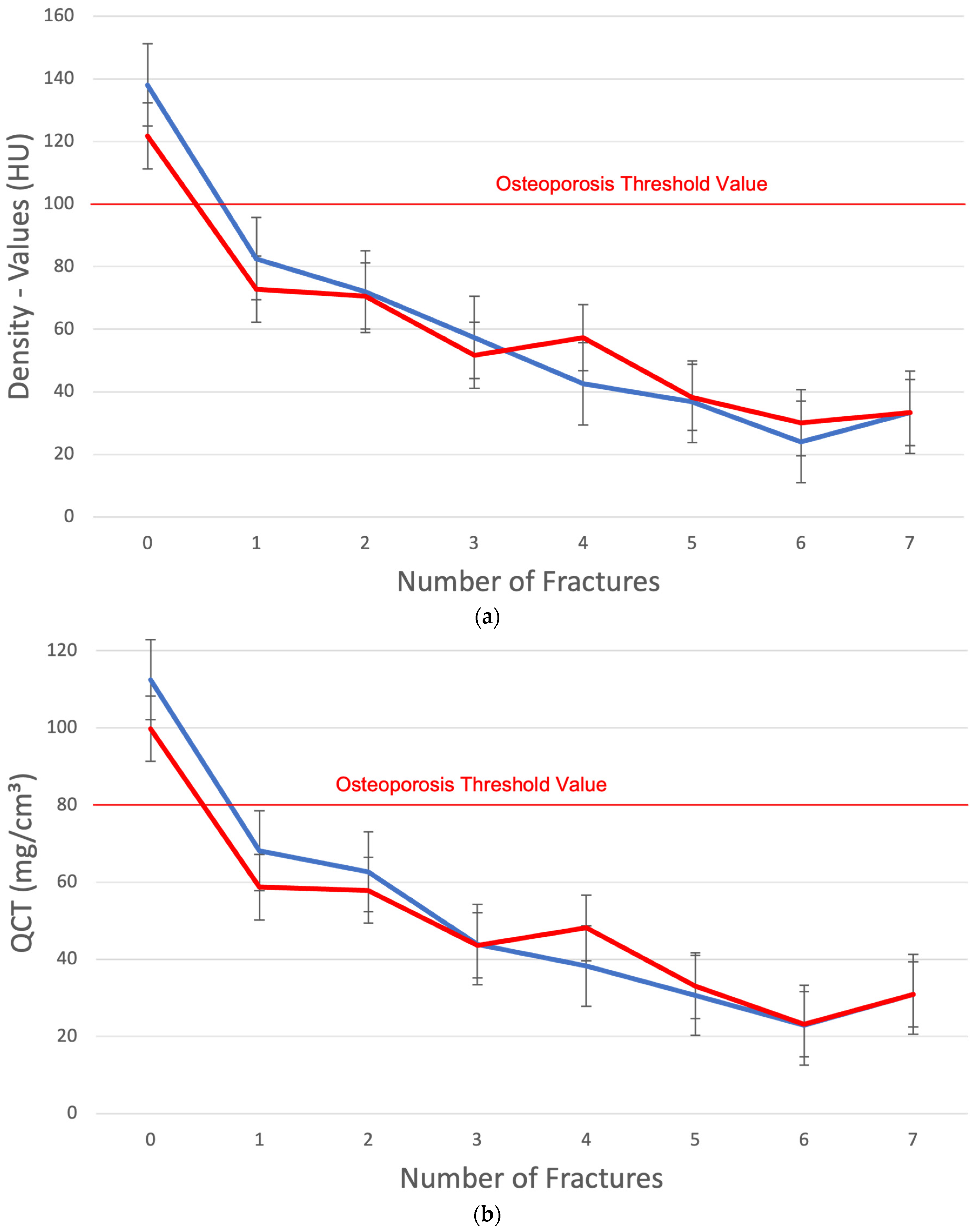
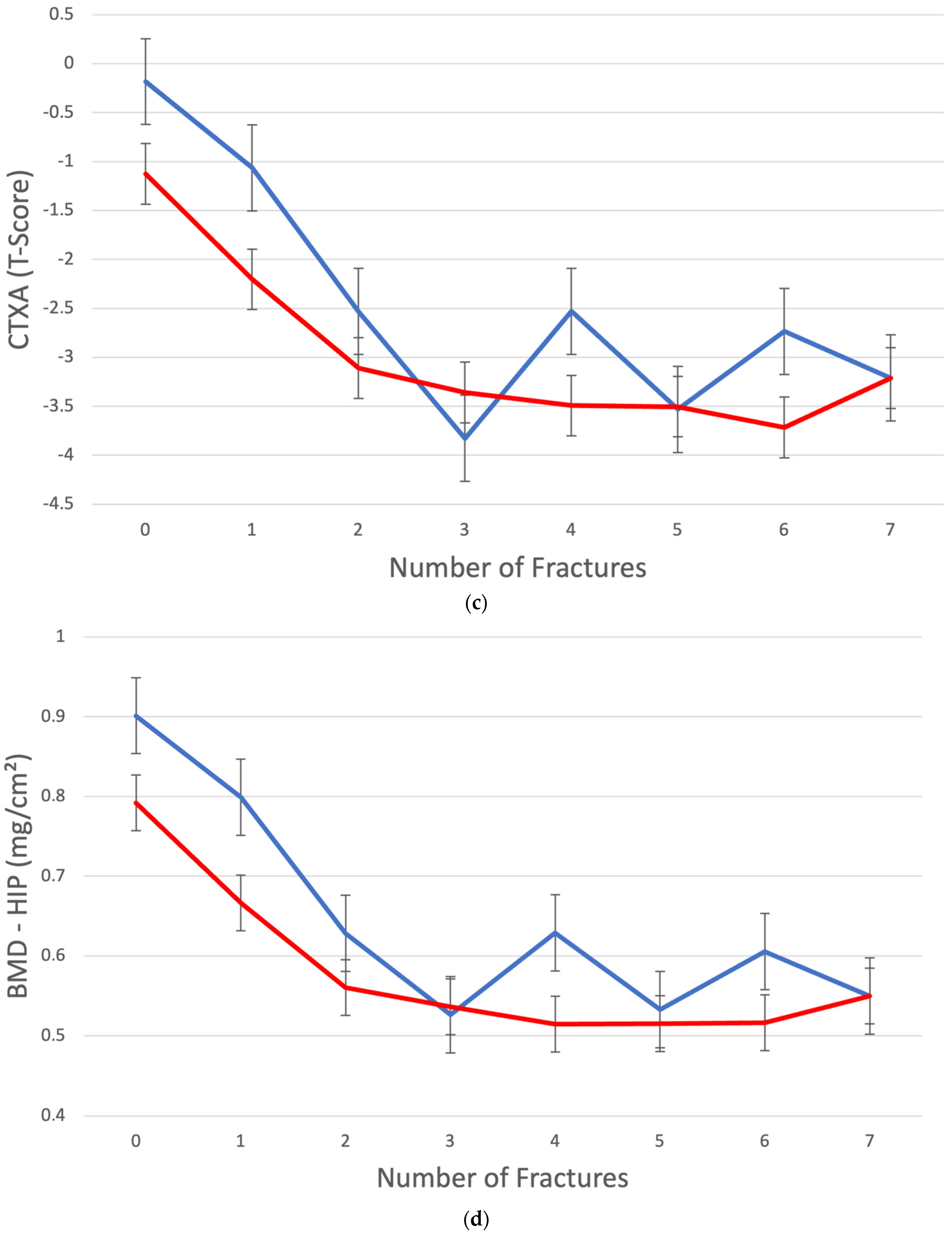
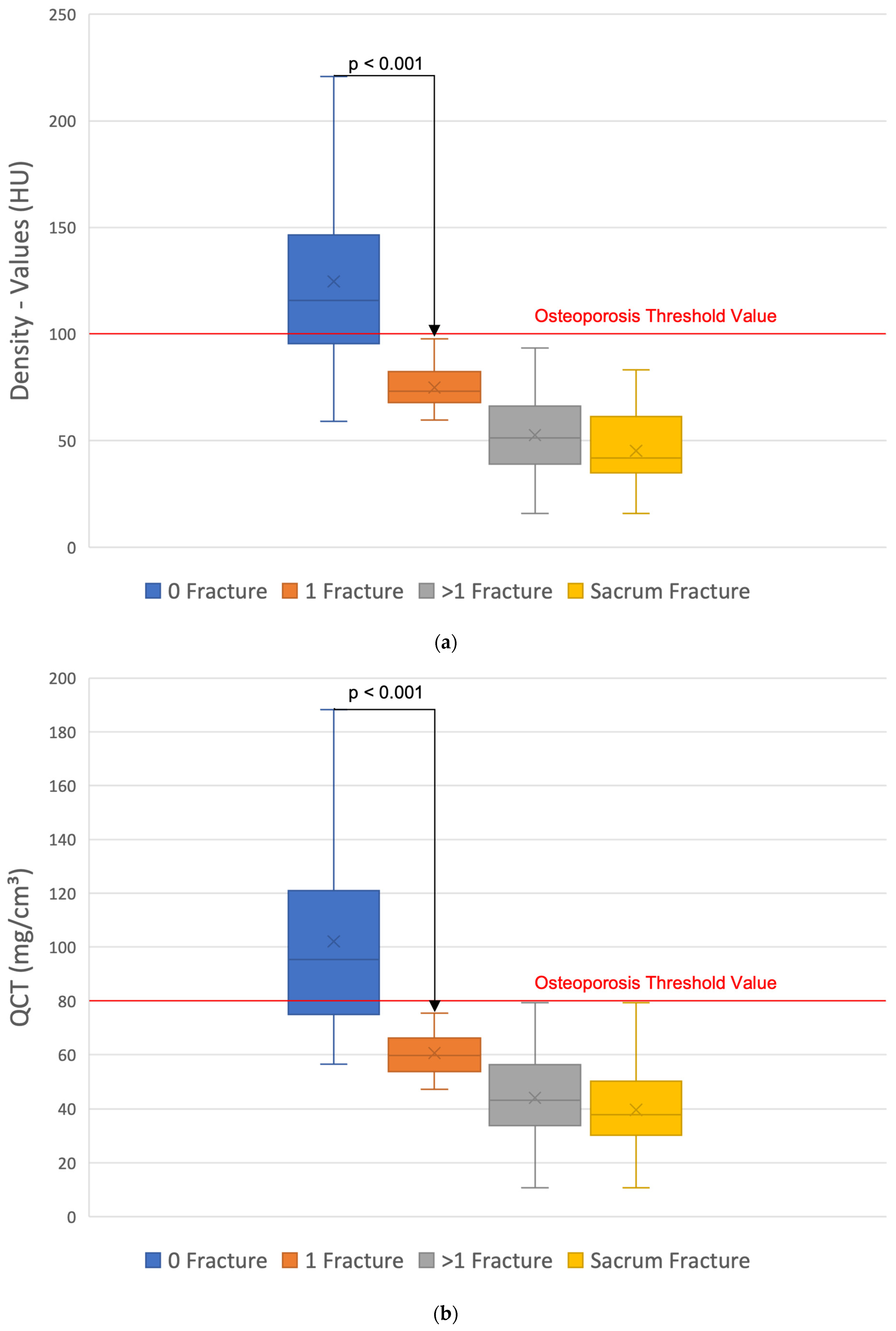
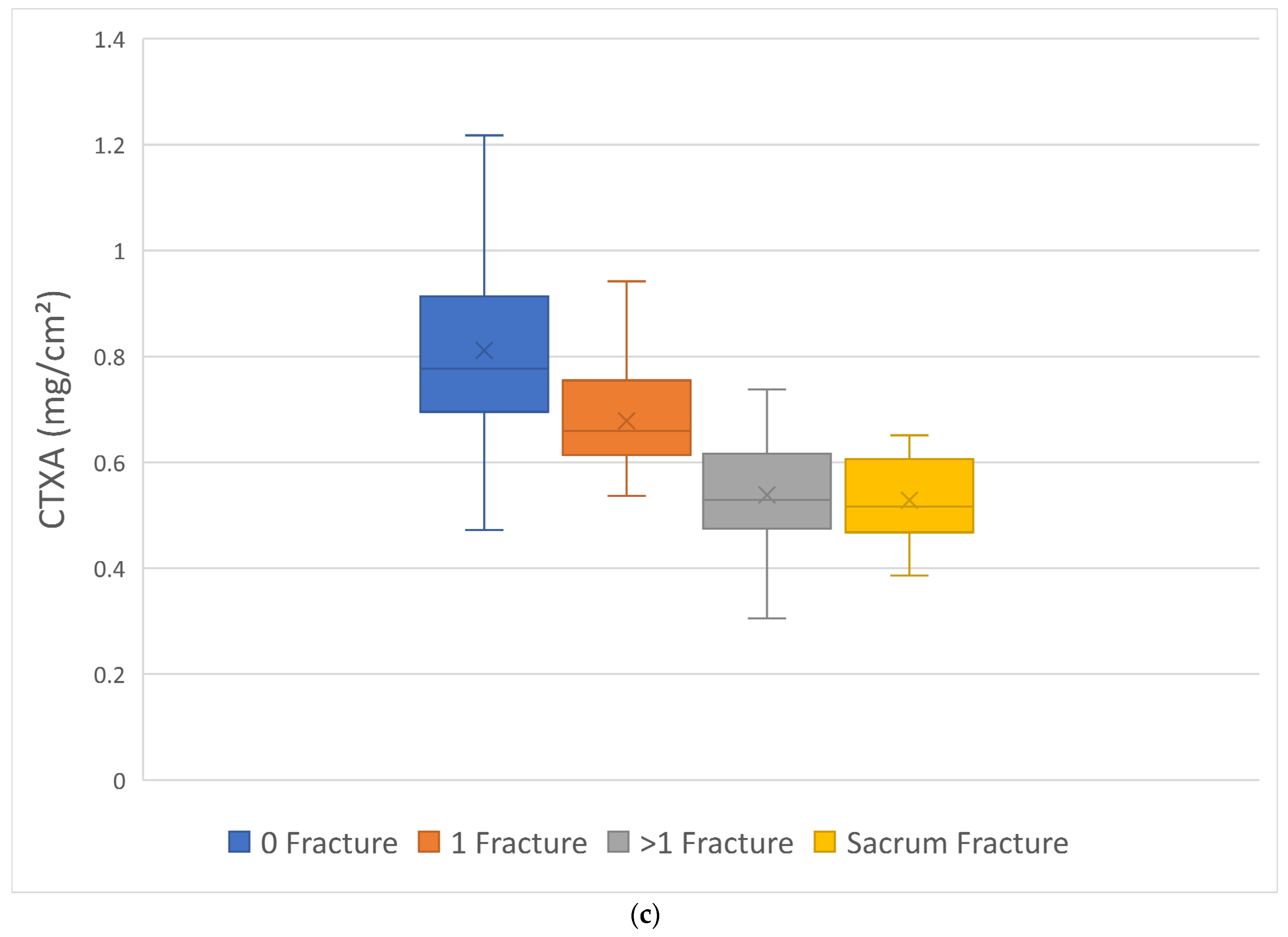
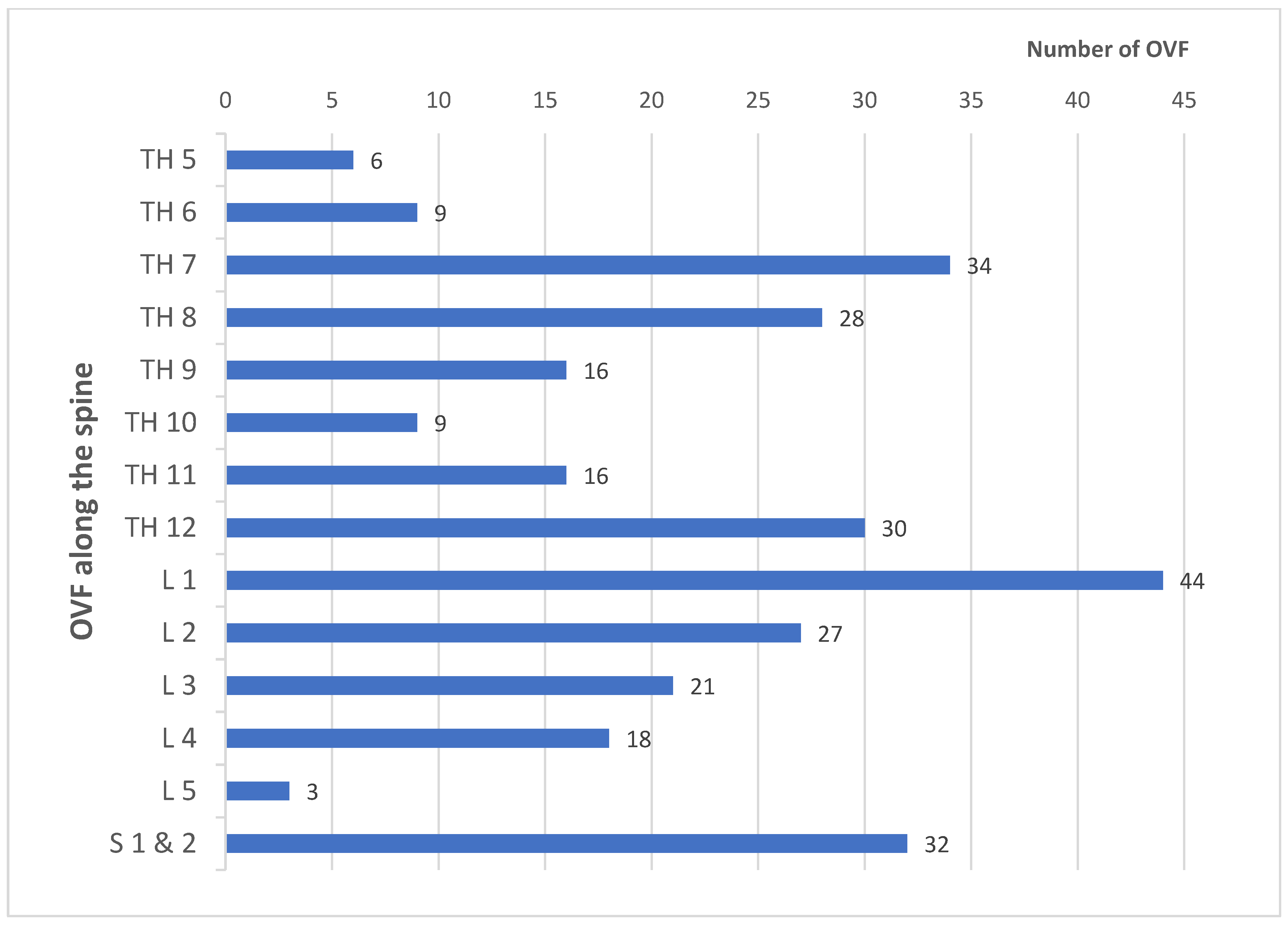
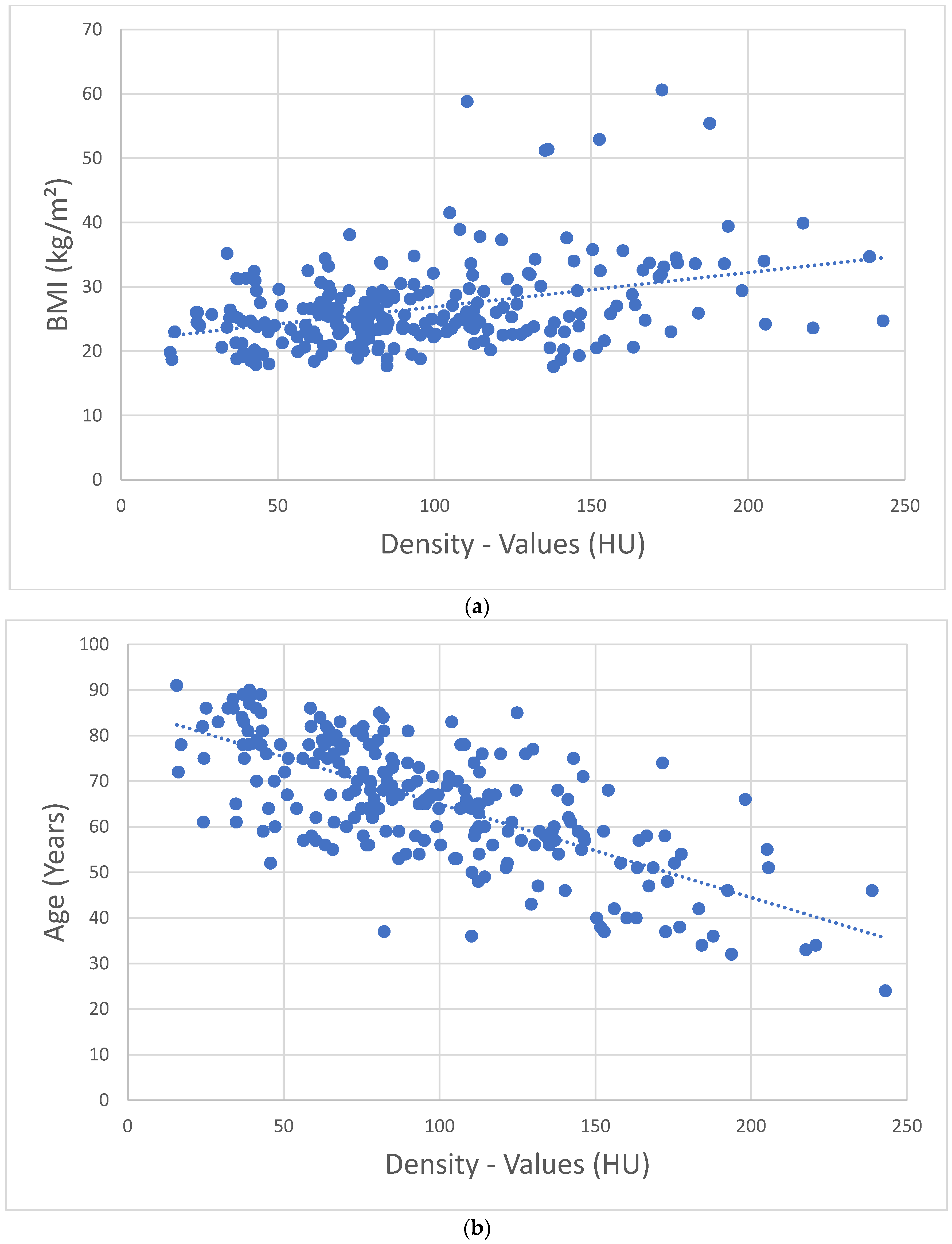
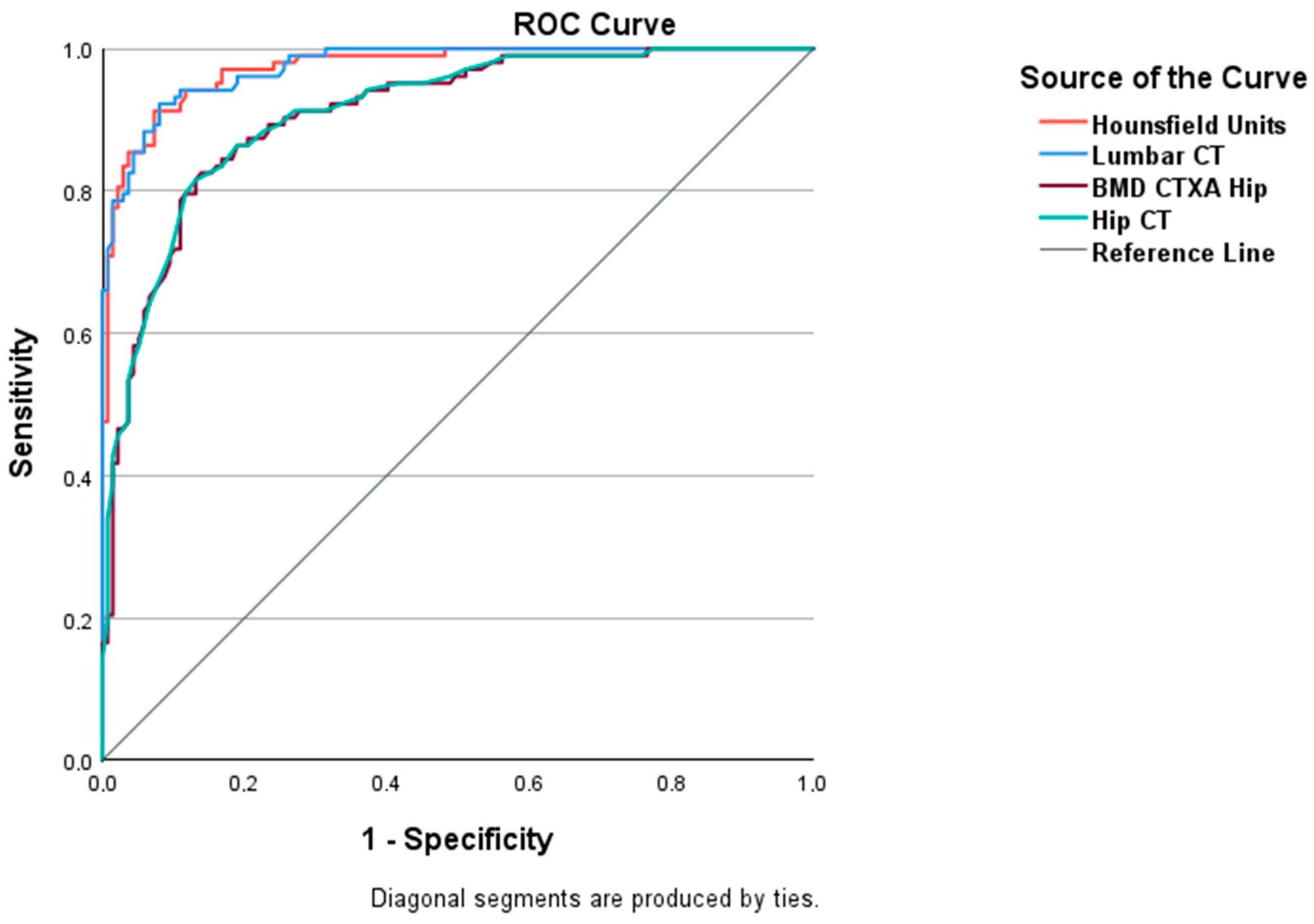
3. Results
4. Discussion
4.1. Further Clinical Significance of HU Density Determination
4.2. Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BMD | Bone mineral density |
| BMI | Body mass index |
| CT | Computed tomography |
| CTXA | Computed tomography X-ray absorptiometry |
| DEQCT | Dual-energy quantitative computed tomography |
| DEXA | Dual-energy X-ray absorptiometry |
| HU | Hounsfield Units |
| LuS | Lumbar spine |
| L | Lumbar vertebral body |
| mg/cm2 | Milligram/Square centimeter |
| mg/cm3 | Milligram/Cubic centimeter |
| OVF | Osteoporotic vertebral fractures |
| QCT | Quantitative computed tomography |
| Pat | Patient |
| ROCs | Receiver operating characteristics |
| ROI | Region of interest |
| ThS | Thoracic spine |
| T | Thoracic vertebral body |
References
- Andresen, R.; Radmer, S.; Banzer, D. Bone Mineral Density and Spongiosa Architecture in Correlation to Vertebral Body Insufficiency Fractures. Acta Radiol. 1998, 39, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An Estimate of the Worldwide Prevalence and Disability Associated with Osteoporotic Fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R. Risk of New Vertebral Fracture in the Year Following a Fracture. JAMA 2001, 285, 320. [Google Scholar] [CrossRef] [PubMed]
- Oleksik, A.; Lips, P.; Dawson, A.; Minshall, M.E.; Shen, W.; Cooper, C.; Kanis, J. Health-Related Quality of Life in Postmenopausal Women With Low BMD With or Without Prevalent Vertebral Fractures. J. Bone Miner. Res. 2000, 15, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Park, S.-J.; Kim, J.-K.; Park, J.-S. Mortality Risk after the First Occurrence of Osteoporotic Vertebral Compression Fractures in the General Population: A Nationwide Cohort Study. PLoS ONE 2023, 18, e0291561. [Google Scholar] [CrossRef] [PubMed]
- Felsenberg, D.; Silman, A.; Lunt, M.; Armbrecht, G.; Ismail, A.; Finn, J.; Cockerill, W.; Banzer, D.; Benevolenskaya, L.; Bhalla, A.; et al. Incidence of Vertebral Fracture in Europe: Results from the European Prospective Osteoporosis Study (EPOS). J. Bone Miner. Res. 2002, 17, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, T.; Han, D.; Gong, W.; Mao, W.; Gan, X.; Shu, D.; Zhou, Q.; Xu, L.; Hou, L.; et al. Risk Factors of Osteoporosis in Elderly Inpatients: A Cross-Sectional Single-Centre Study. Front. Aging 2023, 4, 1126172. [Google Scholar] [CrossRef]
- Lang, S.; Walter, N.; Freigang, V.; Neumann, C.; Loibl, M.; Alt, V.; Rupp, M. Increased Incidence of Vertebral Fractures in German Adults from 2009 to 2019 and the Analysis of Secondary Diagnoses, Treatment, Costs, and in-Hospital Mortality. Sci. Rep. 2023, 13, 6984. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.S.; Seeger, J.B.; Horas, K.; Roth, K.E.; Kurth, A.A.; Maus, U. The Prevalence of Vitamin D Deficiency in Patients with Vertebral Fragility Fractures. Bone Jt. J. 2015, 97-B, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chun, C.; Yang, Y.; Liu, B.; Zhu, Y.; Chen, R.; Rong, L. Vitamin D Deficiency/Insufficiency Is Associated with Risk of Osteoporotic Thoracolumbar Junction Vertebral Fractures. Med. Sci. Monit. 2019, 25, 8260–8268. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Xu, S.; Zong, H.; Pan, M.; Teng, Y.; Xu, J. Prevalence and Risk Factors Associated with Vertebral Osteoporotic Fractures in Patients with Rheumatoid Arthritis. Clin. Rheumatol. 2020, 39, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Renna, M.D.; Conversano, F.; Casciaro, E.; Di Paola, M.; Quarta, E.; Muratore, M.; Casciaro, S. Major Osteoporotic Fragility Fractures: Risk Factor Updates and Societal Impact. World J. Orthop. 2016, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Möller, G.; Andresen, R.; Banzer, D. Shape model-based semi-automatic morphometric assessment of vertebral deformities in clinical practice: Results of a multi-centre medical practice evaluation pilot study in Germany. Osteologie 2011, 20, 239–247. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Bouxsein, M.L. Biomechanics of Vertebral Fractures and the Vertebral Fracture Cascade. Curr. Osteoporos. Rep. 2010, 8, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Schröder, G.; Hiepe, L.; Moritz, M.; Vivell, L.-M.; Schulze, M.; Martin, H.; Götz, A.; Andresen, J.R.; Kullen, C.-M.; Andresen, R.; et al. Warum sich in der Halswirbelsäule auch bei Osteoporose nur selten Insuffizienzfrakturen finden. Z. Für Orthop. Unfallchirurgie 2022, 160, 657–669. [Google Scholar] [CrossRef]
- Andresen, J.R.; Radmer, S.; Andresen, R.; Prokop, A.; Schröder, G.; Nissen, U.; Schober, H.-C. Comparative Outcome of Different Treatment Options for Fragility Fractures of the Sacrum. BMC Musculoskelet. Disord. 2022, 23, 1106. [Google Scholar] [CrossRef] [PubMed]
- Damilakis, J.; Maris, T.G.; Karantanas, A.H. An Update on the Assessment of Osteoporosis Using Radiologic Techniques. Eur. Radiol. 2007, 17, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Dachverband Osteologie e.V. S3-Leitlinie Prophylaxe, Diagnostik Und Therapie Der Osteoporose Bei Postmenopausalen Frauen Und Bei Männern Ab Dem 50. Lebensjahr; Dachverband Osteologie e.V.: Essen, Germany, 2023. [Google Scholar]
- Felsenberg, D.; Gowin, W. Knochendichtemessung Mit Zwei-Spektren-Methoden. Radiologie 1999, 39, 186–193. [Google Scholar] [CrossRef]
- Engelke, K.; Adams, J.E.; Armbrecht, G.; Augat, P.; Bogado, C.E.; Bouxsein, M.L.; Felsenberg, D.; Ito, M.; Prevrhal, S.; Hans, D.B.; et al. Clinical Use of Quantitative Computed Tomography and Peripheral Quantitative Computed Tomography in the Management of Osteoporosis in Adults: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 123–162. [Google Scholar] [CrossRef]
- Cann, C.E.; Adams, J.E.; Brown, J.K.; Brett, A.D. CTXA Hip—An Extension of Classical DXA Measurements Using Quantitative CT. PLoS ONE 2014, 9, e91904. [Google Scholar] [CrossRef]
- Genant, H.K.; Wu, C.Y.; Van Kuijk, C.; Nevitt, M.C. Vertebral Fracture Assessment Using a Semiquantitative Technique. J. Bone Miner. Res. 1993, 8, 1137–1148. [Google Scholar] [CrossRef]
- Buenger, F.; Eckardt, N.; Sakr, Y.; Senft, C.; Schwarz, F. Correlation of Bone Density Values of Quantitative Computed Tomography and Hounsfield Units Measured in Native Computed Tomography in 902 Vertebral Bodies. World Neurosurg. 2021, 151, e599–e606. [Google Scholar] [CrossRef] [PubMed]
- Schröder, G.; Andresen, J.R.; Hiepe, L.; Schulze, M.; Kullen, C.M.; Kopetsch, C.; Burmeister, J.; Schober, H.-C.; Andresen, R. Interobserver Variability in the Determination of Bone Mineral Density in Hounsfield Units from Differently Configured Fields of Measurement in the Cancellous Bone of Vertebral Bodies from Elderly Body Donors. J. Orthop. 2024, 49, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Andresen, R.; Radmer, S.; Banzer, D.; Felsenberg, D.; Wolf, K.-J. Quantitative Knochenmineralgehaltsbestimmung (QCT)—Systemvergleich baugleicher Computertomographen. RöFo 1994, 160, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Glüer, C.-C.; Reiser, U.J.; Davis, C.A.; Rutt, B.K.; Genant, H.K. Vertebral Mineral Determination by Quantitative Computed Tomography (QCT): Accuracy of Single and Dual Energy Measurements. J. Comput. Assist. Tomogr. 1988, 12, 242–258. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Lauder, T.; Pooler, B.D.; Muñoz Del Rio, A.; Rosas, H.; Bruce, R.J.; Binkley, N. Effect of IV Contrast on Lumbar Trabecular Attenuation at Routine Abdominal CT: Correlation with DXA and Implications for Opportunistic Osteoporosis Screening. Osteoporos. Int. 2016, 27, 147–152. [Google Scholar] [CrossRef]
- Bauer, J.S.; Henning, T.D.; Müeller, D.; Lu, Y.; Majumdar, S.; Link, T.M. Volumetric Quantitative CT of the Spine and Hip Derived from Contrast-Enhanced MDCT: Conversion Factors. Am. J. Roentgenol. 2007, 188, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Baum, T.; Müller, D.; Dobritz, M.; Rummeny, E.J.; Link, T.M.; Bauer, J.S. BMD Measurements of the Spine Derived from Sagittal Reformations of Contrast-Enhanced MDCT without Dedicated Software. Eur. J. Radiol. 2011, 80, e140–e145. [Google Scholar] [CrossRef]
- Buenger, F.; Sakr, Y.; Eckardt, N.; Senft, C.; Schwarz, F. Correlation of Quantitative Computed Tomography Derived Bone Density Values with Hounsfield Units of a Contrast Medium Computed Tomography in 98 Thoraco-Lumbar Vertebral Bodies. Arch. Orthop. Trauma Surg. 2021, 142, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, B.W.; Schwarz, F.; McLean, A.L.; Mendel, T.; Kaden, I.; Hein, E.; Lattauschke, A.; Beyer, J.; Hofmann, G.O.; Klauke, F.; et al. Inter-Rater Reliability of Hounsfield Units as a Measure of Bone Density: Applications in the Treatment of Thoracolumbar Fractures. World Neurosurg. 2022, 158, e711–e716. [Google Scholar] [CrossRef]
- Lee, S.; Chung, C.K.; Oh, S.H.; Park, S.B. Correlation between Bone Mineral Density Measured by Dual-Energy X-Ray Absorptiometry and Hounsfield Units Measured by Diagnostic CT in Lumbar Spine. J. Korean Neurosurg. Soc. 2013, 54, 384. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Ye, K.; Tian, Y.; Li, W.; Zhou, F.; Zhang, Z.; Lu, Z.; Xu, Z. Characteristics of Vertebral CT Hounsfield Units in Elderly Patients with Acute Vertebral Fragility Fractures. Eur. Spine J. 2020, 29, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Binkley, N.; Lubner, M.G.; Bruce, R.J.; Ziemlewicz, T.J.; Pickhardt, P.J. Opportunistic Screening for Osteoporosis Using the Sagittal Reconstruction from Routine Abdominal CT for Combined Assessment of Vertebral Fractures and Density. Osteoporos. Int. 2016, 27, 1131–1136. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; Del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic Screening for Osteoporosis Using Abdominal Computed Tomography Scans Obtained for Other Indications. Ann. Intern. Med. 2013, 158, 588. [Google Scholar] [CrossRef] [PubMed]
- Marinova, M.; Edon, B.; Wolter, K.; Katsimbari, B.; Schild, H.H.; Strunk, H.M. Use of Routine Thoracic and Abdominal Computed Tomography Scans for Assessing Bone Mineral Density and Detecting Osteoporosis. Curr. Med. Res. Opin. 2015, 31, 1871–1881. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Lee, E.; Lee, J.W. Coronal Plane in Opportunistic Screening of Osteoporosis Using Computed Tomography: Comparison with Axial and Sagittal Planes. Skelet. Radiol. 2024, 53, 1103–1109. [Google Scholar] [CrossRef]
- Scheyerer, M.J.; Ullrich, B.; Osterhoff, G.; Spiegl, U.A.; Schnake, K.J.; Arbeitsgruppe Osteoporotische Frakturen der Sektion Wirbelsäule der Deutschen Gesellschaft für Orthopädie und Unfallchirurgie. „Hounsfield units“ als Maß für die Knochendichte—Anwendungsmöglichkeiten in der Wirbelsäulenchirurgie. Unfallchirurg 2019, 122, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Schröder, G.; Flachsmeyer, D.; Kullen, C.M.; Andresen, J.R.; Schulze, M.; Hiepe, L.; Schober, H.-C.; Andresen, R. Insuffizienzfrakturen der Wirbelsäule in Abhängigkeit von der spongiösen Knochendichte: Eine in-vitro-Studie. Orthopädie 2022, 51, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Vanier, A.T.; Colantonio, D.; Saxena, S.K.; Rodkey, D.; Wagner, S. Computed Tomography of the Chest as a Screening Tool for Low Bone Mineral Density. Mil. Med. 2023, 188, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Schröder, G.; Reichel, M.; Spiegel, S.; Schulze, M.; Götz, A.; Bugaichuk, S.; Andresen, J.R.; Kullen, C.M.; Andresen, R.; Schober, H.-C. Breaking Strength and Bone Microarchitecture in Osteoporosis: A Biomechanical Approximation Based on Load Tests in 104 Human Vertebrae from the Cervical, Thoracic, and Lumbar Spines of 13 Body Donors. J. Orthop. Surg. 2022, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Simion, G.; Eckardt, N.; Ullrich, B.W.; Senft, C.; Schwarz, F. Bone Density of the Cervical, Thoracic and Lumbar Spine Measured Using Hounsfield Units of Computed Tomography—Results of 4350 Vertebras. BMC Musculoskelet. Disord. 2024, 25, 200. [Google Scholar] [CrossRef]
- Rommens, P.M.; Drees, P.; Thomczyk, S.; Betz, U.; Wagner, D.; Hofmann, A. Die Fragilitätsfraktur des Beckens ist eine Indikatorfraktur der Osteoporose. Osteologie 2018, 27, 144–153. [Google Scholar] [CrossRef]
- Choi, M.K.; Kim, S.M.; Lim, J.K. Diagnostic Efficacy of Hounsfield Units in Spine CT for the Assessment of Real Bone Mineral Density of Degenerative Spine: Correlation Study between T-Scores Determined by DEXA Scan and Hounsfield Units from CT. Acta Neurochir. 2016, 158, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, Q.; Danisa, O.A.; Cheng, W. Measurement Techniques and Utility of Hounsfield Unit Values for Assessment of Bone Quality Prior to Spinal Instrumentation: A Review of Current Literature. Spine 2019, 44, E239–E244. [Google Scholar] [CrossRef]
- Zou, D.; Li, W.; Deng, C.; Du, G.; Xu, N. The Use of CT Hounsfield Unit Values to Identify the Undiagnosed Spinal Osteoporosis in Patients with Lumbar Degenerative Diseases. Eur. Spine J. 2019, 28, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Chanbour, H.; Steinle, A.M.; Chen, J.W.; Waddell, W.H.; Vickery, J.; LaBarge, M.E.; Longo, M.; Gardocki, R.J.; Abtahi, A.M.; Stephens, B.F.; et al. The Importance of Hounsfield Units in Adult Spinal Deformity Surgery: Finding an Optimal Threshold to Minimize the Risk of Mechanical Complications. J. Spine Surg. 2023, 9, 149–158. [Google Scholar] [CrossRef]
- Yoshie, N.; Maruo, K.; Arizumi, F.; Kishima, K.; Kusukawa, T.; Tachibana, T. The Relationship between the Hounsfield Units Value of the Upper Instrumented Vertebra and the Severity of Proximal Junctional Fracture after Adult Spinal Deformity Surgery. Medicina 2023, 59, 1086. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Xie, T.; Song, Z.; Song, Y.; Zeng, J. The Preoperative Hounsfield Unit Value at the Position of the Future Screw Insertion Is a Better Predictor of Screw Loosening than Other Methods. Eur. Radiol. 2022, 33, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Muheremu, A.; Ji, Y.; Zhang, R.; Liu, W. Prediction of Lumbar Pedicle Screw Loosening Using Hounsfield Units inComputed Tomography. Curr. Med. Imaging Rev. 2023, 20, e260423216204. [Google Scholar] [CrossRef]
- Deshpande, N.; Hadi, M.S.; Lillard, J.C.; Passias, P.G.; Linzey, J.R.; Saadeh, Y.S.; LaBagnara, M.; Park, P. Alternatives to DEXA for the Assessment of Bone Density: A Systematic Review of the Literature and Future Recommendations. J. Neurosurg. Spine 2023, 38, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Zou, D.; Zhou, F.; Li, W.; Tian, Y. Low Vertebral CT Hounsfield Units: A Risk Factor for New Osteoporotic Vertebral Fractures after the Treatment of Percutaneous Kyphoplasty. Arch. Osteoporos. 2022, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.; Robinson, J.; Gendelberg, D.; Jimenez, J.; Anand, A.; Rao, A.; Khandehroo, B.; Khandehroo, B.; Kahwaty, S.; Anand, N. Do Peri-Operative Parathyroid Hormone (PTH) Analogues Improve Bone Density and Decrease Mechanical Complications in Spinal Deformity Correction?—A Minimum 2-Year Radiological Study Measuring Hounsfield Units. Eur. Spine J. 2023, 32, 3651–3658. [Google Scholar] [CrossRef]
- Alharthy, A. Assessment of Trabecular Bone Hounsfield Units in the Lumbar Spine for Osteoporosis Evaluation in Individuals Aged 65 and above: A Review. Osteoporos. Int. 2024. [Google Scholar] [CrossRef] [PubMed]
| Characterization of the Total Sample | |||
|---|---|---|---|
| Patients (n = 240) | Men (n = 40) | Women (n = 200) | |
| Age (in years) | ⌀ 65.9 | ⌀ 68.5 | ⌀ 65.3 |
| BMI (kg/m2) | ⌀ 26.7 | ⌀ 24.9 | ⌀ 27.1 |
| Fractures | total: 293 G1: 128 G2: 111 G3: 22 Sacrum: 32 | total: 36 G1: 19 G2: 13 G3: 1 Sacrum: 3 | total: 257 G1: 109 G2: 98 G3: 21 Sacrum: 29 |
| Conversion Values of Different Studies | |||
|---|---|---|---|
| Study Group | Average Age (in Years) | Conversion Formula from HU in mg/cm3 | Absolute Value and Percentage Deviation at 100 HU Compared with Own Results |
| Own results | 65.9 | QCT-Value =0.84 + (0.81 × HU) | 81.8 mg/cm3 |
| Buenger et al., 2021 [24] | 66.98 | QCT-Value =17.8 + (0.7 × HU) | 87.8 mg/cm3 about 7.3% higher |
| Schröder et al., 2023 [25] | 81.1 | QCT-Value =13.7 + (0.6 × HU) | 73.7 mg/cm3 about 9.9% lower |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andresen, J.R.; Schröder, G.; Haider, T.; Andresen, R. Opportunistic Osteoporosis Assessment and Fracture Risk Determination Using Cancellous Density Measurement in Hounsfield Units of Native Lumbar Computed Tomography Images—A Comparative Study with Conventional Bone Density Evaluation. J. Clin. Med. 2025, 14, 1226. https://doi.org/10.3390/jcm14041226
Andresen JR, Schröder G, Haider T, Andresen R. Opportunistic Osteoporosis Assessment and Fracture Risk Determination Using Cancellous Density Measurement in Hounsfield Units of Native Lumbar Computed Tomography Images—A Comparative Study with Conventional Bone Density Evaluation. Journal of Clinical Medicine. 2025; 14(4):1226. https://doi.org/10.3390/jcm14041226
Chicago/Turabian StyleAndresen, Julian Ramin, Guido Schröder, Thomas Haider, and Reimer Andresen. 2025. "Opportunistic Osteoporosis Assessment and Fracture Risk Determination Using Cancellous Density Measurement in Hounsfield Units of Native Lumbar Computed Tomography Images—A Comparative Study with Conventional Bone Density Evaluation" Journal of Clinical Medicine 14, no. 4: 1226. https://doi.org/10.3390/jcm14041226
APA StyleAndresen, J. R., Schröder, G., Haider, T., & Andresen, R. (2025). Opportunistic Osteoporosis Assessment and Fracture Risk Determination Using Cancellous Density Measurement in Hounsfield Units of Native Lumbar Computed Tomography Images—A Comparative Study with Conventional Bone Density Evaluation. Journal of Clinical Medicine, 14(4), 1226. https://doi.org/10.3390/jcm14041226







