HLA-B*58 and HLA-C*2 Alleles Are Associated with the Occurrence of Rheumatoid Arthritis Among Omanis
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. DNA Isolation
2.3. HLA Typing
2.4. Statistical Analysis
3. Results
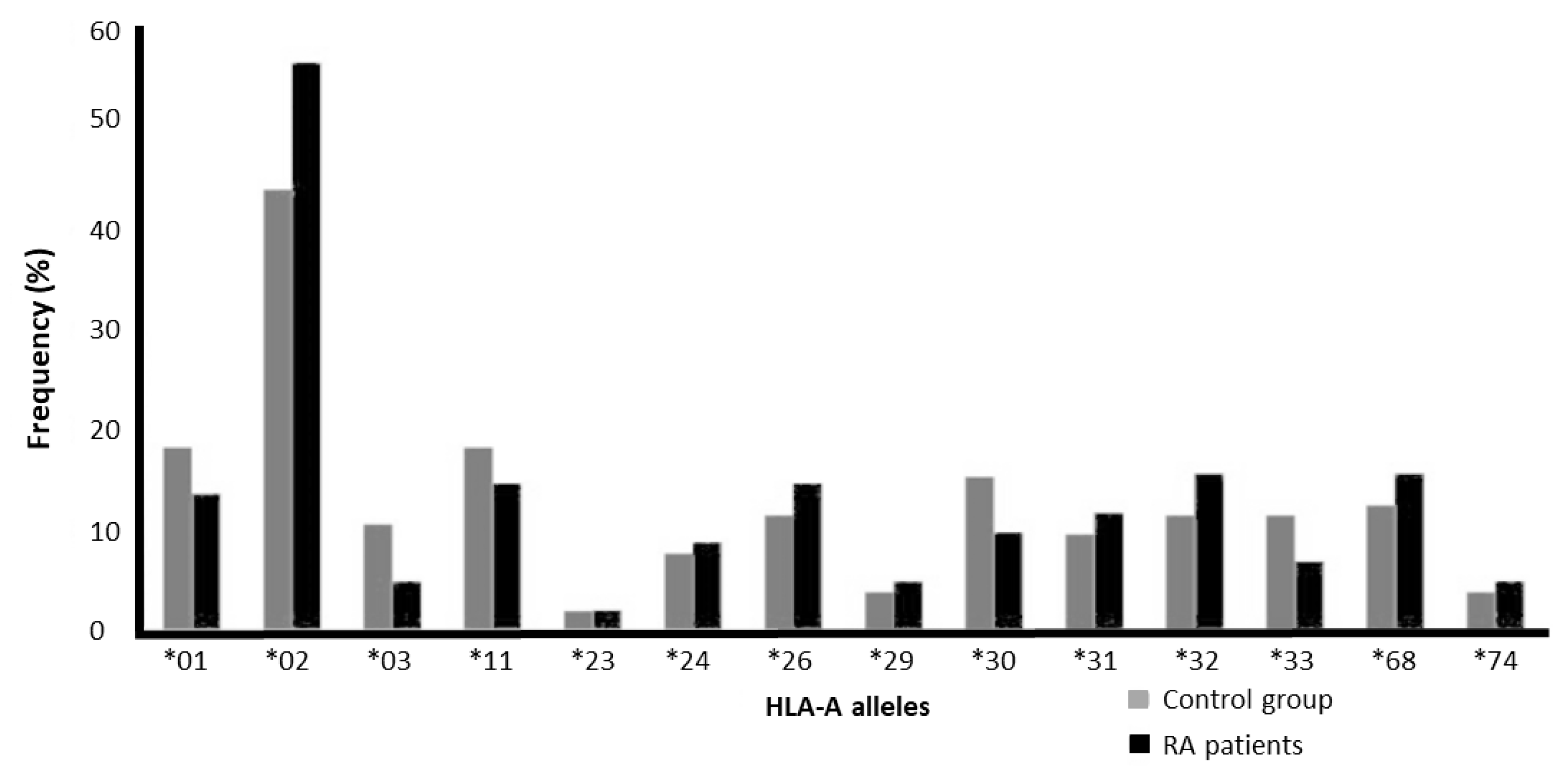
3.1. Absence of Association Between HLA-A Alleles and RA
3.2. The Association Between HLA-B Alleles and RA
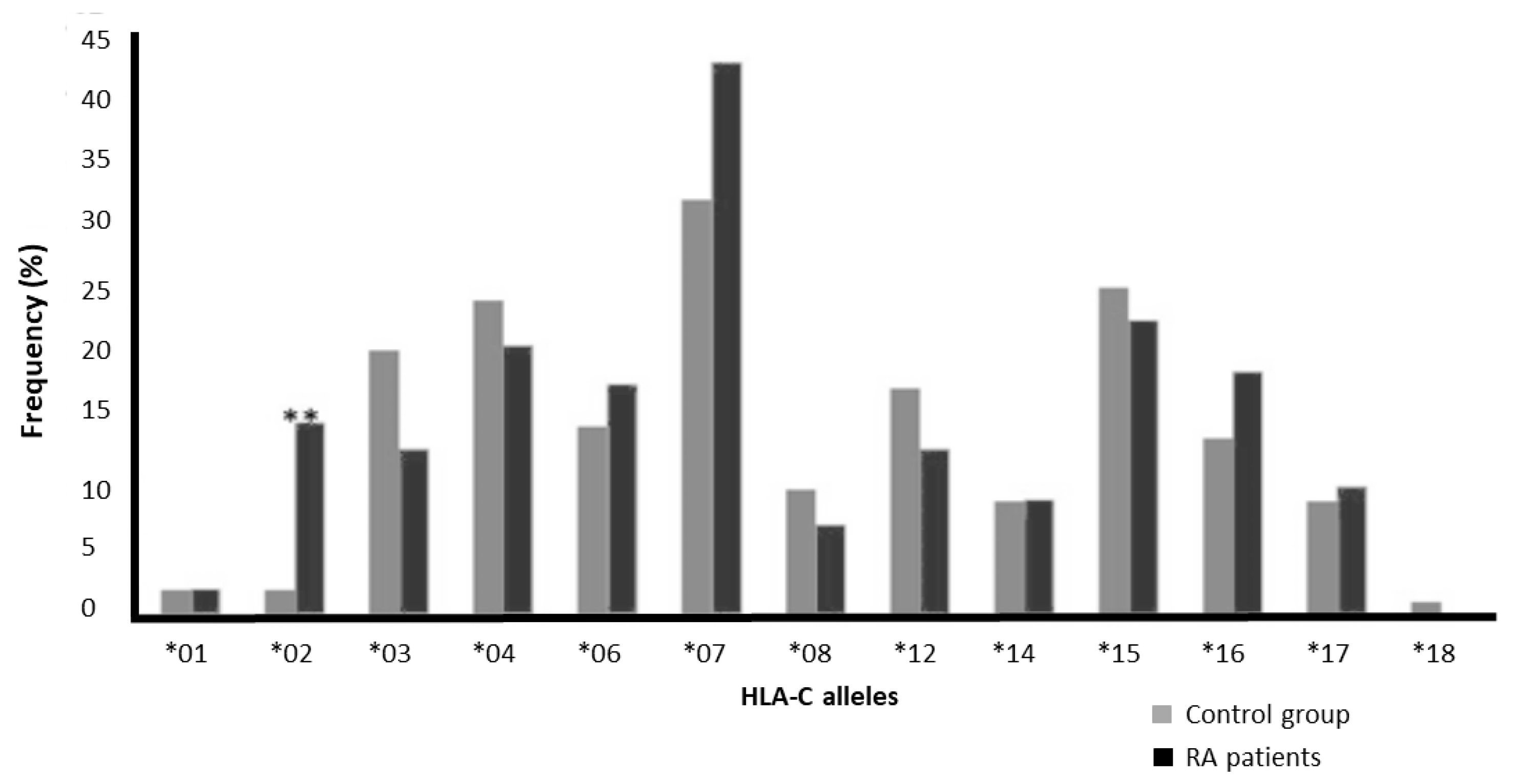
3.3. The Association of HLA-C Alleles with RA
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, D.M.; Weinblatt, M.E. Rheumatoid arthritis. Lancet 2001, 358, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Tobon, G.J.; Youinou, P.; Saraux, A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J. Autoimmun. 2010, 35, 10–14. [Google Scholar] [CrossRef] [PubMed]
- van Drongelen, V.; Holoshitz, J. Human Leukocyte Antigen-Disease Associations in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2017, 43, 363–376. [Google Scholar] [CrossRef]
- Cao, K.; Hollenbach, J.; Shi, X.; Shi, W.; Chopek, M.; Fernandez-Vina, M.A. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum. Immunol. 2001, 62, 1009–1030. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front. Zool. 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Kim, K.; Han, B.; Pillai, N.E.; Ong, R.T.; Saw, W.Y.; Luo, M.; Jiang, L.; Yin, J.; Bang, S.Y.; et al. Risk for ACPA-positive rheumatoid arthritis is driven by shared HLA amino acid polymorphisms in Asian and European populations. Hum. Mol. Genet. 2014, 23, 6916–6926. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Sandor, C.; Stahl, E.A.; Freudenberg, J.; Lee, H.S.; Jia, X.; Alfredsson, L.; Padyukov, L.; Klareskog, L.; Worthington, J.; et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 2012, 44, 291–296. [Google Scholar] [CrossRef]
- Aljinovic, J.; Soso, D.; Petric, M.; Perkovic, D.; Marasovic Krstulovic, D.; Kero, D.; Marinovic, I. Clinical Phenotype of HLA B*44 Patients in a Rheumatology Outpatient Clinic Favors Peripheral Arthropathies. J. Clin. Med. 2024, 13, 5440. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, S.; Suzuki, Y.; Kuwana, M.; Sato, S.; Kaneko, Y.; Homma, Y.; Narita, A.; Kashiwase, K.; Okudaira, Y.; Inoue, I.; et al. Associations between six classical HLA loci and rheumatoid arthritis: A comprehensive analysis. Tissue Antigens 2012, 80, 16–25. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Shi, Y.; Li, L. Human leukocyte antigen (HLA)-Cw0303, HLA-Cw04, and HLA-Cw07 polymorphisms are associated with susceptibility of rheumatoid arthritis in Chinese Han patients from Southern China. Iran. J. Basic Med. Sci. 2019, 22, 610–616. [Google Scholar] [CrossRef]
- Ahern, D.J.; Brennan, F.M. The role of Natural Killer cells in the pathogenesis of rheumatoid arthritis: Major contributors or essential homeostatic modulators? Immunol. Lett. 2011, 136, 115–121. [Google Scholar] [CrossRef]
- Carvalheiro, H.; da Silva, J.A.P.; Souto-Carneiro, M.M. Potential roles for CD8+ T cells in rheumatoid arthritis. Autoimmun. Rev. 2013, 12, 401–409. [Google Scholar] [CrossRef]
- Ding, S.-J.; Zhang, Y.; Zhang, X.-M.; Jiang, X.-L.; Pang, B.; Song, Y.-H.; Wang, J.-X.; Pei, Y.-W.; Zhu, C.-F.; Wang, X.-J.; et al. Correlation Between HLA-A, B and DRB1 Alleles and Severe Fever with Thrombocytopenia Syndrome. PLoS Neglected Trop. Dis. 2016, 10, e0005076. [Google Scholar] [CrossRef]
- van Deutekom, H.W.; Kesmir, C. Zooming into the binding groove of HLA molecules: Which positions and which substitutions change peptide binding most? Immunogenetics 2015, 67, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lamothe, P.A.; Ng, R.; Xu, S.; Teng, M.; Walker, B.D.; Wang, J.H. Crystal structure of HLA-B*5801, a protective HLA allele for HIV-1 infection. Protein Cell 2016, 7, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Gough, S.C.L.; Simmonds, M.J. The HLA Region and Autoimmune Disease: Associations and Mechanisms of Action. Curr. Genom. 2007, 8, 453–465. [Google Scholar] [CrossRef]
- Cognet, C.; Farnarier, C.; Gauthier, L.; Frassati, C.; Andre, P.; Magerus-Chatinet, A.; Anfossi, N.; Rieux-Laucat, F.; Vivier, E.; Schleinitz, N. Expression of the HLA-C2-specific activating killer-cell Ig-like receptor KIR2DS1 on NK and T cells. Clin. Immunol. 2010, 135, 26–32. [Google Scholar] [CrossRef]
- Williams, R.C.; Jacobsson, L.T.; Knowler, W.C.; del Puente, A.; Kostyu, D.; McAuley, J.E.; Bennett, P.H.; Pettitt, D.J. Meta-analysis reveals association between most common class II haplotype in full-heritage Native Americans and rheumatoid arthritis. Hum. Immunol. 1995, 42, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Nordang, G.B.; Flam, S.T.; Maehlen, M.T.; Kvien, T.K.; Viken, M.K.; Lie, B.A. HLA-C alleles confer risk for anti-citrullinated peptide antibody-positive rheumatoid arthritis independent of HLA-DRB1 alleles. Rheumatology 2013, 52, 1973–1982. [Google Scholar] [CrossRef]
- Coyle, C.; Ma, M.; Abraham, Y.; Mahony, C.B.; Steel, K.; Simpson, C.; Guerra, N.; Croft, A.P.; Rapecki, S.; Cope, A.; et al. NK cell subsets define sustained remission in rheumatoid arthritis. JCI Insight 2024, 9, e182390. [Google Scholar] [CrossRef]
- Horowitz, A.; Guethlein, L.A.; Nemat-Gorgani, N.; Norman, P.J.; Cooley, S.; Miller, J.S.; Parham, P. Regulation of Adaptive NK Cells and CD8 T Cells by HLA-C Correlates with Allogeneic Hematopoietic Cell Transplantation and with Cytomegalovirus Reactivation. J. Immunol. 2015, 195, 4524–4536. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Huang, J. CD8(+) T Cells and NK Cells: Parallel and Complementary Soldiers of Immunotherapy. Curr. Opin. Chem. Eng. 2018, 19, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Costenbader, K.H.; Karlson, E.W. Epstein-Barr virus and rheumatoid arthritis: Is there a link? Arthritis Res. Ther. 2006, 8, 204. [Google Scholar] [CrossRef] [PubMed]

| Study Population | Total (n) | Female/Male | Age |
|---|---|---|---|
| RA patient group | 102 | 85/17 | 43 ± 16.7 (15–88) |
| Control group | 104 | 87/17 | 42.9 ± 16.9 (15–96) |
| Alleles in HLA-A Locus | Control (104) | Patients (102) | Exact p Value | RR | 95% CI | Control (104) | Patients (102) | ||
|---|---|---|---|---|---|---|---|---|---|
| No | % | No | % | Allele Frequency | Allele Frequency | ||||
| A*01 | 19 | 18.3 | 14 | 13.7 | 0.449 | 0.712 | 0.336–1.510 | 0.111 | 0.078 |
| A*02 | 46 | 44.2 | 58 | 56.9 | 0.072 | 1.662 | 0.958–2.883 | 0.26 | 0.328 |
| A*03 | 11 | 10.6 | 5 | 4.9 | 0.192 | 0.436 | 0.146–1.302 | 0.053 | 0.029 |
| A*11 | 19 | 18.3 | 15 | 14.7 | 0.575 | 0.771 | 0.368–1.617 | 0.096 | 0.074 |
| A*23 | 2 | 1.9 | 2 | 2 | 1 | 1.02 | 0.141–7.382 | 0.01 | 0.01 |
| A*24 | 8 | 7.7 | 9 | 8.8 | 0.805 | 1.161 | 0.430–3.138 | 0.043 | 0.049 |
| A*26 | 12 | 11.5 | 15 | 14.7 | 0.541 | 1.322 | 0.586–2.982 | 0.067 | 0.074 |
| A*29 | 4 | 3.8 | 5 | 4.9 | 0.747 | 1.289 | 0.336–4.942 | 0.019 | 0.025 |
| A*30 | 16 | 15.4 | 10 | 9.8 | 0.295 | 0.598 | 0.257–1.388 | 0.082 | 0.049 |
| A*31 | 10 | 9.6 | 12 | 11.8 | 0.658 | 1.253 | 0.516–3.045 | 0.048 | 0.059 |
| A*32 | 12 | 11.5 | 16 | 15.7 | 0.421 | 1.426 | 0.638–3.188 | 0.063 | 0.088 |
| A*33 | 12 | 11.5 | 7 | 6.9 | 0.336 | 0.565 | 0.213–1.498 | 0.058 | 0.034 |
| A*68 | 13 | 12.5 | 16 | 15.7 | 0.552 | 1.302 | 0.592–2.867 | 0.072 | 0.078 |
| A*74 | 4 | 3.8 | 5 | 4.9 | 0.747 | 1.289 | 0.336–4.942 | 0.019 | 0.025 |
| Alleles HLA-B Locus | Control (104) | Patients (102) | Exact p Value | RR | 95% CI | Control (104) | Patients (102) | ||
|---|---|---|---|---|---|---|---|---|---|
| No | % | No | % | Allele Frequency | Allele Frequency | ||||
| B*07 | 2 | 1.9 | 4 | 3.9 | 0.443 | 2.082 | 0.373–11.623 | 0.01 | 0.02 |
| B*08 | 14 | 13.5 | 13 | 12.7 | 1 | 0.939 | 0.418–2.110 | 0.067 | 0.074 |
| B*13 | 7 | 6.7 | 6 | 5.9 | 1 | 0.866 | 0.281–2.671 | 0.034 | 0.029 |
| B*14 | 10 | 9.6 | 8 | 7.8 | 0.806 | 0.8 | 0.302–2.006 | 0.048 | 0.039 |
| B*15 | 7 | 6.7 | 20 | 19.6 | 0.007 | 3.38 | 1.361–8.393 | 0.038 | 0.103 |
| B*18 | 15 | 14.4 | 3 | 2.9 | 0.005 | 0.18 | 0.050–0.642 | 0.072 | 0.015 |
| B*23 | 0 | 0 | 1 | 1 | 0.495 | 1.01 | 0.991–1.030 | 0 | 0.005 |
| B*27 | 1 | 1 | 3 | 2.9 | 0.366 | 3.121 | 0.319–30.513 | 0.005 | 0.015 |
| B*35 | 26 | 25 | 26 | 25 | 1 | 1.026 | 0.547–1.925 | 0.12 | 0.127 |
| B*37 | 1 | 1 | 4 | 3.9 | 0.21 | 4.204 | 0.462–38.273 | 0 | 0.02 |
| B*38 | 0 | 0 | 1 | 1 | 0.495 | 1.01 | 0.991–1.030 | 0.005 | 0.005 |
| B*39 | 8 | 7.7 | 2 | 2 | 0.101 | 0.24 | 0.050–1.159 | 0.038 | 0.01 |
| B*40 | 19 | 18.3 | 16 | 15.7 | 0.712 | 0.832 | 0.401–1.726 | 0.091 | 0.093 |
| B*41 | 0 | 0 | 3 | 2.9 | 0.12 | 1.03 | 0.996–1.066 | 0 | 0.015 |
| B*42 | 2 | 1.9 | 4 | 3.9 | 0.443 | 2.082 | 0.373–11.623 | 0.01 | 0.025 |
| B*44 | 2 | 1.9 | 8 | 7.8 | 0.057 | 4.34 | 0.899–20.960 | 0.01 | 0.039 |
| B*45 | 2 | 1.9 | 2 | 2 | 1 | 1.02 | 0.141–7.382 | 0.01 | 0.01 |
| B*49 | 2 | 1.9 | 2 | 2 | 1 | 1.02 | 0.141–7.382 | 0.01 | 0.01 |
| B*50 | 8 | 7.7 | 5 | 4.9 | 0.569 | 0.619 | 0.195–1.958 | 0.038 | 0.025 |
| B*51 | 25 | 24 | 28 | 27.5 | 0.634 | 1.196 | 0.640–2.235 | 0.13 | 0.167 |
| B*52 | 6 | 5.8 | 7 | 6.9 | 0.782 | 1.204 | 0.390–3.712 | 0.029 | 0.039 |
| B*53 | 4 | 3.8 | 6 | 5.9 | 0.536 | 1.563 | 0.428–5.709 | 0.019 | 0.029 |
| B*55 | 5 | 4.8 | 4 | 3.9 | 1 | 0.808 | 0.211–3.099 | 0.024 | 0.02 |
| B*57 | 7 | 6.7 | 1 | 1 | 0.065 | 0.137 | 0.017–1.136 | 0.034 | 0.005 |
| B*58 | 30 | 28.8 | 11 | 10.8 | 0.002 | 0.298 | 0.140–0.635 | 0.144 | 0.054 |
| B*73 | 0 | 0 | 1 | 1 | 0.495 | 1.01 | 0.991–1.030 | 0 | 0.005 |
| B*81 | 1 | 1 | 0 | 0 | 1 | 0.99 | 0.972–1.09 | 0.005 | 0 |
| Alleles in HLA-C Locus | Control (104) | Patients (102) | Exact p Value | RR | 95% CI | Control (104) | Patients (102) | ||
|---|---|---|---|---|---|---|---|---|---|
| No | % | No | % | Allele Frequency | Allele Frequency | ||||
| C*01 | 2 | 1.9 | 2 | 2 | 1 | 1.02 | 0.141–7.382 | 0.01 | 0.01 |
| C*02 | 2 | 1.9 | 15 | 15 | 0.001 | 8.79 | 1.956–39.522 | 0.01 | 0.074 |
| C*03 | 21 | 20 | 13 | 13 | 0.189 | 0.58 | 0.272–1.227 | 0.13 | 0.064 |
| C*04 | 25 | 24 | 21 | 21 | 0.617 | 0.82 | 0.424–1.582 | 0.149 | 0.103 |
| C*06 | 15 | 14 | 18 | 18 | 0.572 | 1.27 | 0.602–2.684 | 0.072 | 0.093 |
| C*07 | 33 | 32 | 43 | 42 | 0.149 | 1.57 | 0.887–2.773 | 0.178 | 0.24 |
| C*08 | 10 | 9.6 | 7 | 6.9 | 0.614 | 0.69 | 0.253–1.896 | 0.048 | 0.039 |
| C*12 | 18 | 17 | 13 | 13 | 0.437 | 0.7 | 0.322–1.511 | 0.087 | 0.069 |
| C*14 | 9 | 8.7 | 9 | 8.8 | 1 | 1.02 | 0.388–2.687 | 0.058 | 0.044 |
| C*15 | 26 | 25 | 23 | 23 | 0.744 | 0.87 | 0.459–1.660 | 0.139 | 0.118 |
| C*16 | 14 | 14 | 19 | 19 | 0.346 | 1.47 | 0.694–3.122 | 0.067 | 0.093 |
| C*17 | 9 | 8.7 | 10 | 9.8 | 0.814 | 1.15 | 0.446–2.952 | 0.048 | 0.049 |
| C*18 | 1 | 1 | 0 | 0 | 1 | 0.99 | 0.972–1.009 | 0.005 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Balushi, M.S.; Antony, I.; Al-Shirawi, A.H.; Al-Riyami, H.; Al-Busaidi, J.Z.; Koh, C.Y.; Al-Naamani, K.M.; Hasson, S.S.; Al-Jabri, A.A.; Said, E.A. HLA-B*58 and HLA-C*2 Alleles Are Associated with the Occurrence of Rheumatoid Arthritis Among Omanis. J. Clin. Med. 2025, 14, 1219. https://doi.org/10.3390/jcm14041219
Al-Balushi MS, Antony I, Al-Shirawi AH, Al-Riyami H, Al-Busaidi JZ, Koh CY, Al-Naamani KM, Hasson SS, Al-Jabri AA, Said EA. HLA-B*58 and HLA-C*2 Alleles Are Associated with the Occurrence of Rheumatoid Arthritis Among Omanis. Journal of Clinical Medicine. 2025; 14(4):1219. https://doi.org/10.3390/jcm14041219
Chicago/Turabian StyleAl-Balushi, Mohammed S., Irin Antony, Ali H. Al-Shirawi, Hamad Al-Riyami, Jumaa Z. Al-Busaidi, Crystal Y. Koh, Khalid M. Al-Naamani, Sidgi S. Hasson, Ali A. Al-Jabri, and Elias A. Said. 2025. "HLA-B*58 and HLA-C*2 Alleles Are Associated with the Occurrence of Rheumatoid Arthritis Among Omanis" Journal of Clinical Medicine 14, no. 4: 1219. https://doi.org/10.3390/jcm14041219
APA StyleAl-Balushi, M. S., Antony, I., Al-Shirawi, A. H., Al-Riyami, H., Al-Busaidi, J. Z., Koh, C. Y., Al-Naamani, K. M., Hasson, S. S., Al-Jabri, A. A., & Said, E. A. (2025). HLA-B*58 and HLA-C*2 Alleles Are Associated with the Occurrence of Rheumatoid Arthritis Among Omanis. Journal of Clinical Medicine, 14(4), 1219. https://doi.org/10.3390/jcm14041219






