The Paraspinal Sarcopenia at the Upper Instrumented Vertebra Is a Predictor of Discoligamentous but Not Bony Proximal Junctional Kyphosis
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
4.1. Sarcopenia and PJK
4.2. Bone Density and PJK
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cm | centimeter |
| CSA | cross-sectional area |
| HU | Hounsfield units |
| mm | millimeter |
| PI | pelvic incidence |
| PT | pelvic tilt |
| SS | sacral slope |
| SVA | sagittal vertical axis |
| T1PA | T1-pelvic angle |
| UIV | upper instrumented vertebrae |
| VBQ | vertebral bone quality |
| yr | year |
References
- Schwab, F.; Dubey, A.; Gamez, L.; El Fegoun, A.B.; Hwang, K.; Pagala, M.; Farcy, J.-P. Adult Scoliosis: Prevalence, SF-36, and Nutritional Parameters in an Elderly Volunteer Population. Spine 2005, 30, 1082–1085. [Google Scholar] [CrossRef]
- Diebo, B.G.; Shah, N.V.; Stroud, S.G.; Paulino, C.B.; Schwab, F.J.; Lafage, V. Realignment Surgery in Adult Spinal Deformity. Orthopade 2018, 47, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Li, Y.; Yu, Z. Prevalence and Consequences of the Proximal Junctional Kyphosis After Spinal Deformity Surgery: A Meta-Analysis. Medicine 2016, 95, e3471. [Google Scholar] [CrossRef] [PubMed]
- Theologis, A.A.; Miller, L.; Callahan, M.; Lau, D.; Zygourakis, C.; Scheer, J.K.; Burch, S.; Pekmezci, M.; Chou, D.; Tay, B.; et al. Economic Impact of Revision Surgery for Proximal Junctional Failure After Adult Spinal Deformity Surgery: A Cost Analysis of 57 Operations in a 10-Year Experience at a Major Deformity Center. Spine 2016, 41, E964–E972. [Google Scholar] [CrossRef]
- Lee, B.-J.; Bae, S.S.; Choi, H.Y.; Park, J.H.; Hyun, S.-J.; Jo, D.J.; Cho, Y. Proximal Junctional Kyphosis or Failure After Adult Spinal Deformity Surgery—Review of Risk Factors and Its Prevention. Neurospine 2023, 20, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Cottrill, E.; Ahmed, A.K.; Passias, P.; Protopsaltis, T.; Neuman, B.; Kebaish, K.M.; Ehresman, J.; Westbroek, E.M.; Goodwin, M.L.; et al. Paraspinal Muscle Size as an Independent Risk Factor for Proximal Junctional Kyphosis in Patients Undergoing Thoracolumbar Fusion. J. Neurosurg. Spine 2019, 31, 380–388. [Google Scholar] [CrossRef]
- Cammarata, M.; Aubin, C.-É.; Wang, X.; Mac-Thiong, J.-M. Biomechanical Risk Factors for Proximal Junctional Kyphosis. A Detailed Numerical Analysis of Surgical Instrumentation Variables. Spine 2014, 39, E500–E507. [Google Scholar] [CrossRef] [PubMed]
- Dubousset, J.; Diebo, B.G. Proximal Junctional Kyphosis in Modern Spine Surgery: Why Is It So Common? Spine Surg. Relat. Res. 2023, 7, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Rahm, M.; Gaines, R.; Maziad, A.; Ross, T.; Kim, H.J.; Kebaish, K.; Boachie-Adjei, O. Characterization and Surgical Outcomes of Proximal Junctional Failure in Surgically Treated Patients With Adult Spinal Deformity. Spine 2014, 39, E607–E614. [Google Scholar] [CrossRef] [PubMed]
- Mikula, A.L.; Lakomkin, N.; Pennington, Z.; Pinter, Z.W.; Nassr, A.; Freedman, B.; Sebastian, A.S.; Abode-Iyamah, K.; Bydon, M.; Ames, C.P.; et al. Association between Lower Hounsfield Units and Proximal Junctional Kyphosis and Failure at the Upper Thoracic Spine. J. Neurosurg. Spine 2022, 37, 694–702. [Google Scholar] [CrossRef]
- Pennington, Z.; Mikula, A.L.; Lakomkin, N.; Martini, M.; Clarke, M.J.; Sebastian, A.S.; Freedman, B.A.; Rose, P.S.; Karim, S.M.; Nassr, A.; et al. Comparison of Hounsfield Units and Vertebral Bone Quality Score for the Prediction of Time to Pathologic Fracture in Mobile Spine Metastases Treated with Radiotherapy. J. Neurosurg. Spine 2023, 40, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Pinter, Z.W.; Bernatz, J.; Mikula, A.L.; Lakomkin, N.; Pennington, Z.A.; Michalopoulos, G.D.; Nassr, A.; Freedman, B.A.; Bydon, M.; Fogelson, J.; et al. Paraspinal Sarcopenia and Lower Hounsfield Units Are Independent Predictors of Increased Risk for Proximal Junctional Complications Following Thoracolumbar Fusions Terminating in the Upper Thoracic Spine. Glob. Spine J. 2024, 21925682241270096. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Yamanouchi, K.; Fujita, N.; Funao, H.; Ebata, S. Proximal Junctional Failure in Adult Spinal Deformity Surgery: An In-Depth Review. Neurospine 2023, 20, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Mikula, A.L.; Pennington, Z.; Lakomkin, N.; Clarke, M.J.; Rose, P.S.; Bydon, M.; Freedman, B.; Sebastian, A.S.; Lu, L.; Kowalchuk, R.O.; et al. Independent Predictors of Vertebral Compression Fracture Following Radiation for Metastatic Spine Disease. J. Neurosurg. Spine 2022, 37, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.L.; Mikula, A.L.; Lakomkin, N.; Pennington, Z.; Everson, M.C.; Hamouda, A.M.; Bydon, M.; Freedman, B.; Sebastian, A.S.; Nassr, A.; et al. Opportunistic CT-Based Hounsfield Units Strongly Correlate with Biomechanical CT Measurements in the Thoracolumbar Spine. Spine 2023, 49, 1021–1028. [Google Scholar] [CrossRef]
- Ehresman, J.; Pennington, Z.; Schilling, A.; Lubelski, D.; Ahmed, A.K.K.; Cottrill, E.; Khan, M.; Sciubba, D.M. Novel MRI-Based Score for Assessment of Bone Density in Operative Spine Patients. Spine J. 2020, 20, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Creze, M.; Soubeyrand, M.; Gagey, O. The Paraspinal Muscle-Tendon System: Its Paradoxical Anatomy. PLoS ONE 2019, 14, e0214812. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, K.J.; Kim, J.S.; Park, S.J.; Baek, H. Sarcopenia in Paraspinal Muscle as a Risk Factor of Proximal Junctional Kyphosis and Proximal Junctional Failure after Adult Spinal Deformity Surgery. J. Neurosurg. Spine 2024, 40, 324–330. [Google Scholar] [CrossRef]
- Pinter, Z.W.; Mikula, A.L.; Townsley, S.E.; Salmons IV, H.I.; Lakomkin, N.; Michalopoulos, G.D.; Nassr, A.; Freedman, B.A.; Bydon, M.; Fogelson, J.; et al. Lower Hounsfield Units and Severe Multifidus Sarcopenia Are Independent Predictors of Increased Risk for Proximal Junctional Kyphosis and Failure Following Thoracolumbar Fusion. Spine 2023, 48, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Hosogane, N.; Watanabe, K.; Asazuma, T.; Matsumoto, M. The Paravertebral Muscle and Psoas for the Maintenance of Global Spinal Alignment in Patient with Degenerative Lumbar Scoliosis. Spine J. 2016, 16, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Babu, J.M.; Wang, K.Y.; Jami, M.; Durand, W.; Neuman, B.J.; Kebaish, K.M. Sarcopenia as a Risk Factor for Complications Following Pedicle Subtraction Osteotomy. Clin. Spine Surg. 2023, 36, 190–194. [Google Scholar] [CrossRef]
- Hu, Y.; Leung, H.B.; Lu, W.W.; Luk, K.D.K. Histologic and Electrophysiological Changes of the Paraspinal Muscle After Spinal Fusion. Spine 2008, 33, 1418–1422. [Google Scholar] [CrossRef]
- Weber, B.R.; Grob, D.; Dvorák, J.; Müntener, M. Posterior Surgical Approach to the Lumbar Spine and Its Effect on the Multifidus Muscle. Spine 1997, 22, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, T.; Herno, A.; Paljärvi, L.; Airaksinen, O.; Partanen, J.; Tapaninaho, A. Local Denervation Atrophy of Paraspinal Muscles in Postoperative Failed Back Syndrome. Spine 1993, 18, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-M.; Kim, S.-H.; Ha, S.-K.; Kim, S.-D.; Lim, D.-J.; Cha, J.; Kim, B.-J. Paraspinal Muscle Changes after Single-Level Posterior Lumbar Fusion: Volumetric Analyses and Literature Review. BMC Musculoskelet. Disord. 2020, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Gejo, R.; Matsui, H.; Kawaguchi, Y.; Ishihara, H.; Tsuji, H. Serial Changes in Trunk Muscle Performance After Posterior Lumbar Surgery. Spine 1999, 24, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Lee, S.-H.; Chung, S.K.; Lee, H.-Y. Comparison of Multifidus Muscle Atrophy and Trunk Extension Muscle Strength. Spine 2005, 30, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Ehresman, J.; Lubelski, D.; Cottrill, E.; Schilling, A.; Ahmed, A.K.; Feghali, J.; Witham, T.F.; Sciubba, D.M. Assessing Underlying Bone Quality in Spine Surgery Patients: A Narrative Review of Dual-Energy X-Ray Absorptiometry (DXA) and Alternatives. Spine J. 2021, 21, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Fujita, N.; Tsuji, O.; Nagoshi, N.; Asazuma, T.; Ishii, K.; Nakamura, M.; Matsumoto, M.; Watanabe, K. Low Bone-Mineral Density Is a Significant Risk for Proximal Junctional Failure After Surgical Correction of Adult Spinal Deformity. Spine 2018, 43, 485–491. [Google Scholar] [CrossRef]
- Kuo, C.C.; Soliman, M.A.; Aguirre, A.O.; Ruggiero, N.; Kruk, M.; Khan, A.; Ghannam, M.M.; Almeida, N.D.; Jowdy, P.K.; Smolar, D.E.; et al. Vertebral Bone Quality Score Independently Predicts Proximal Junctional Kyphosis and/or Failure After Adult Spinal Deformity Surgery. Neurosurgery 2023, 92, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Mikula, A.L.; Lakomkin, N.; Martini, M.; Pinter, Z.W.; Shafi, M.; Hamouda, A.; Bydon, M.; Clarke, M.J.; Freedman, B.A.; et al. Bone Quality as Measured by Hounsfield Units More Accurately Predicts Proximal Junctional Kyphosis than Vertebral Bone Quality Following Long-Segment Thoracolumbar Fusion. World Neurosurg. 2024, 186, e584–e592. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Ai, Y.; Huang, Y.; Zhu, C.; Ding, H.; Feng, G.; Liu, L.; Song, Y. Vertebral Bone Quality Score as a Novel Predictor of Proximal Junctional Kyphosis after Thoracic Adolescent Idiopathic Scoliosis Surgery. Eur. Spine J. 2023, 32, 3996–4002. [Google Scholar] [CrossRef] [PubMed]
- Turbucz, M.; Fayad, J.; Pokorni, A.J.; Varga, P.P.; Eltes, P.E.; Lazary, A. Can Semirigid Fixation of the Rostral Instrumented Segments Prevent Proximal Junctional Kyphosis in the Case of Long Thoracolumbar Fusions? A Finite Element Study. J. Neurosurg. Spine 2023, 38, 662–672. [Google Scholar] [CrossRef]
- Doodkorte, R.J.P.; Roth, A.K.; Arts, J.J.; Lataster, L.M.A.; van Rhijn, L.W.; Willems, P.C. Biomechanical Comparison of Semirigid Junctional Fixation Techniques to Prevent Proximal Junctional Failure after Thoracolumbar Adult Spinal Deformity Correction. Spine J. 2021, 21, 855–864. [Google Scholar] [CrossRef] [PubMed]
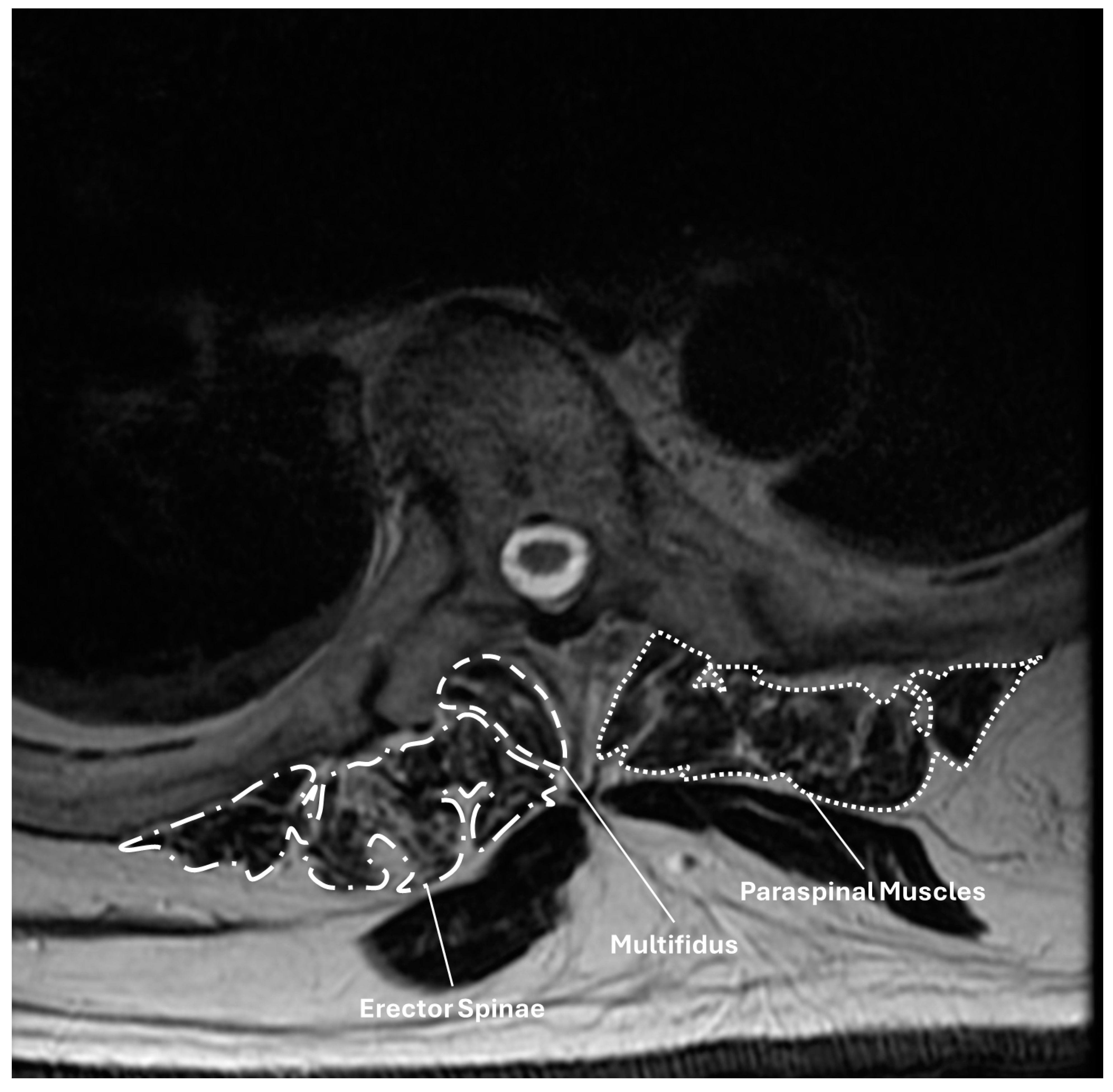
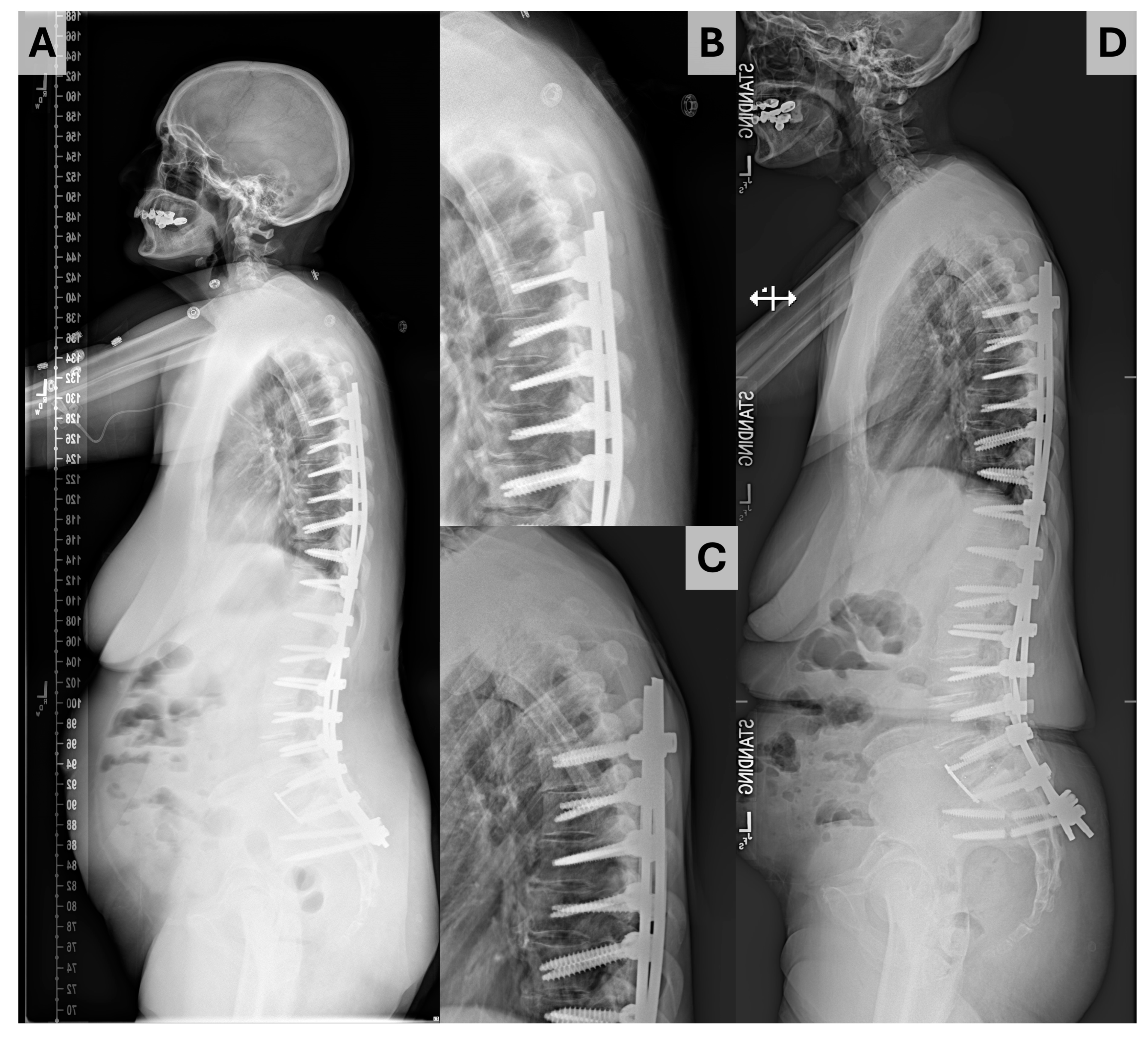
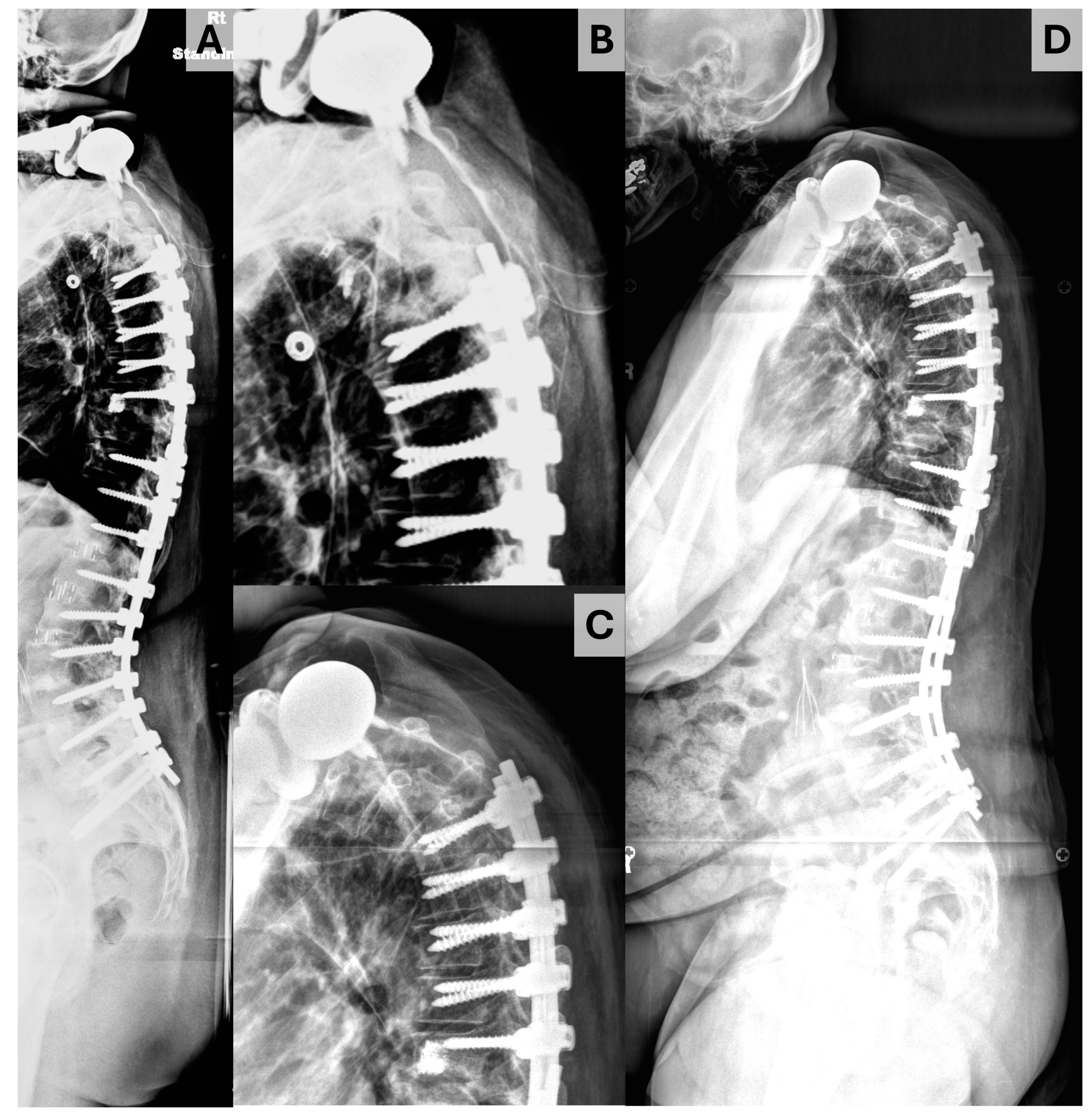
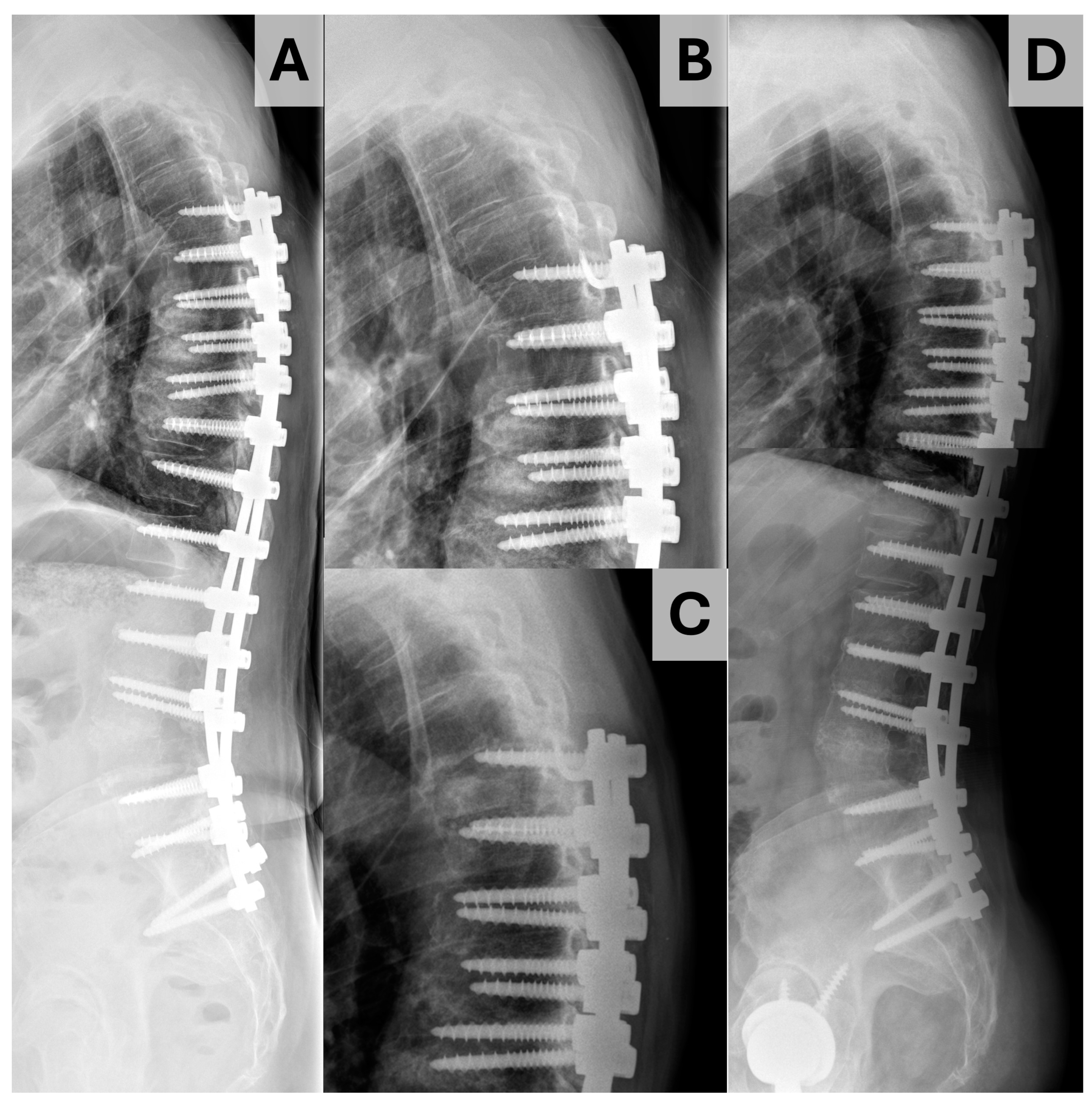
| All | PJK | No PJK | p | |
|---|---|---|---|---|
| Demographics | 76 | 15 | 61 | |
| Age (yr) | 66.0 [61.0, 71.0] | 66 [59, 72] | 66 [61, 71] | 0.86 |
| Sex (Female) | 55 (72.4) | 13 (86.7) | 42 (68.9) | 0.21 |
| Height (m) | 1.63 [1.57, 1.72] | 1.63 [1.55, 1.65] | 1.63 [1.57, 1.73] | 0.34 |
| Weight (kg) | 74.2 [62.3, 90.3] | 68.1 [59.5, 83.9] | 77.7 [63.6, 90.7] | 0.23 |
| BMI (kg/m2) | 28.4 [32.6, 24.9] | 25.5 [24.9, 30.2] | 28.9 [24.9, 32.7] | 0.36 |
| mFI5 | 1 (0, 1.75) | 1 (0, 2) | 0 (0, 1) | 0.09 |
| ASA Class | 1 (1, 2) | 2 (1, 2) | 1 (1, 2) | 0.46 |
| CCI | 3 (2, 3.75) | 2 (2, 4) | 3 (2, 3) | 0.22 |
| Smoking Status | 0.99 | |||
| Current | 5 (6.6) | 1 (6.7) | 4 (6.6) | |
| Previous | 27 (35.5) | 5 (33.3) | 22 (36.1) | |
| None | 44 (57.9) | 9 (60.0) | 36 (47.4) | |
| Governmental Insurance | 44 (57.9) | 11 (73.3) | 33 (54.1) | 0.40 |
| Albumin (g/dL) | 4.0 [3.3, 4.4] | 4.2 [3.5, 4.4] | 3.9 [3.3, 4.3] | 0.51 |
| Radiographic follow-up (d) | 754 (509, 1296) | 594 (217, 1205) | 755 (719, 1308) | 0.27 |
| Preoperative Radiographics | ||||
| SVA (cm) | 9.3 [4.1, 13.9] | 7.7 [4.3, 12.3] | 9.4 [4.0, 14.0] | 0.65 |
| PI (°) | 54.1 [43.4, 62.9] | 57.2 [50.0, 64.6] | 53.5 [41.1, 60.9] | 0.17 |
| PT (°) | 30.1 [23.0, 37.4] | 34.1 [25.2, 38.6] | 28.9 [22.9, 36.6] | 0.22 |
| SS (°) | 24.0 [13.2, 31.7] | 27.4 [15.6, 34.0] | 22.9 [13.0, 31.1] | 0.41 |
| L1-S1 LL (°) | 31.8 [14.3, 45.6] | 31.8 [20.1, 56.5] | 31.8 [12.3, 44.2] | 0.26 |
| L4-S1 LL (°) | 30.8 [23.0, 39.3] | 29.5 [26.6, 40.1] | 31.2 [23.0, 38.8] | 0.78 |
| T1PA (°) | 31.6 [23.3, 40.8] | 33.5 [25.5, 40.7] | 30.0 [22.2, 41.1] | 0.76 |
| Global Tilt (°) | 61.3 [42.1, 78.6] | 57.9 [41.4, 71.3] | 62.2 [44.3, 79.9] | 0.54 |
| Average HU-UIV | 162 [135, 210] | 147 [111, 218] | 163 [139, 209] | 0.31 |
| Average HU-UIV + UIV +1 | 170 [139, 213] | 157 [123, 191] | 175 [143, 213] | 0.27 |
| VBQ UIV | 2.73 [2.35, 3.22] | 2.9 [2.7, 3.2] | 2.6 [2.4, 3.2] | 0.18 |
| Rod Material | 0.33 | |||
| Ti Alloy | 20 (26.3) | 2 (13.3) | 18 (29.5) | |
| CoCr | 56 (73.7) | 13 (86.7) | 43 (70.5) | |
| Postoperative Radiographics | ||||
| SVA (cm) | 2.9 [0.2, 5.8] | 4.5 [1.3, 5.9] | 2.4 [0.1, 5.6] | 0.34 |
| PI (°) | 54.3 [46.4, 61.2] | 57.3 [49.8, 69.9] | 53.4 [46.3, 59.7] | 0.16 |
| PT (°) | 20.2 [13.8, 26.1] | 25.8 [19.2, 29.1] | 19.1 [13.4, 24.8] | 0.04 |
| SS (°) | 35.1 [26.6, 42.0] | 35.3 [26.0, 42.1] | 33.9 [26.8, 41.6] | 0.75 |
| L1-S1 LL (°) | 48.5 [40.5, 60.1] | 47.1 [35.8, 59.7] | 49.3 [41.1, 60.3] | 0.56 |
| L4-S1 LL (°) | 37.0 [29.7, 43.2] | 35.6 [28.4, 41.9] | 37.0 [30.0, 43.2] | 0.74 |
| T1PA (°) | 16.1 [10.1, 23.2] | 21.6 [16.1, 28.9] | 15.0 [9.5, 21.0] | 0.04 |
| Global Tilt (°) | 41.1 [24.6, 56.6] | 38.6 [27.0, 58.5] | 41.5 [22.9, 56.6] | 0.49 |
| Morphometrics | ||||
| CSA Multifidus-L3 (mm2) | 485 [295, 607] | 346 [295, 490] | 543 [311, 632] | 0.16 |
| CSA All Paraspinals-L3 (mm2) | 2869 [2282, 3369] | 2589 [1797, 3025] | 2993 [2395, 3442] | 0.07 |
| CSA Multifidus-UIV (mm2) | 394 [280, 478] | 382 [256, 455] | 395 [289, 486] | 0.34 |
| CSA All Paraspinals-UIV (mm2) | 945 [814, 1170] | 833 [704, 1050] | 964 [859, 1227] | 0.04 |
| PJK | Type 1 | Type 2 | ||||
|---|---|---|---|---|---|---|
| 15 | 10 | 4 | ||||
| Variable | HR [95% CI] | p | HR [95% CI] | p | HR [95% CI] | p |
| BMI (per kg/m2) | 0.97 [0.88, 1.08] | 0.61 | 0.94 [0.82, 1.07] | 0.32 | 1.13 [0.92, 1.38] | 0.24 |
| UIV | ||||||
| T1 | 0.17 [0.01, 3.03] | 0.23 | 0.17 [0.01, 3.02] | 0.22 | 1.01 [0, ∞] | 1.00 |
| T2 | 0.10 [0.01, 1.60] | 0.10 | 0.10 [0.01, 1.76] | 0.12 | 1.01 [0, ∞] | 1.00 |
| T3 | 0.30 [0.03, 2.78] | 0.29 | 0.17 [0.01, 2.00] | 0.16 | 3816 [0, ∞] | 0.96 |
| T4 | 0.18 [0.02, 1.67] | 0.13 | 0.10 [0.01, 1.17] | 0.07 | 2443 [0, ∞] | 0.96 |
| T5 | 0.27 [0.03, 2.51] | 0.25 | 0.23 [0.02, 2.31] | 0.21 | 1.01 [0, ∞] | 1.00 |
| T7 | Ref | — | Ref | — | Ref | — |
| Preoperative Radiographics | ||||||
| Average HU-UIV (per unit) | 0.97 [0.99, 1.01] | 0.55 | 1.00 [0.99, 10.2] | 0.57 | 0.99 [0.96, 1.01] | 0.22 |
| Average HU-UIV + UIV + 1 (per unit) | 1.00 [0.99, 1.01] | 0.39 | 1.00 [0.99, 1.01] | 0.72 | 0.98 [0.96, 1.01] | 0.16 |
| Average HU-UIV/UIV + 1 <164 | 3.05 [1.03, 9.03] | 0.04 | 1.55 [0.44, 5.43] | 0.49 | 84.0 [0.03, 2.9 × 105] | 0.29 |
| VBQ UIV (per unit) | 1.15 [0.85, 1.57] | 0.37 | 1.20 [0.85, 1.70] | 0.30 | 1.10 [0.51, 2.40] | 0.81 |
| Postoperative Radiographics | ||||||
| SVA (per cm) | 1.04 [0.92, 1.18] | 0.54 | 1.03 [0.88, 1.20] | 0.76 | 1.02 [0.80, 1.30] | 0.87 |
| T1PA (per °) | 1.04 [0.99, 1.10] | 0.15 | 1.01 [0.95, 1.08] | 0.72 | 1.12 [0.99, 1.26] | 0.07 |
| Global Tilt (per °) | 1.03 [1.00, 1.05] | 0.09 | 1.03 [0.99, 1.06] | 0.16 | 1.00 [0.96, 1.06] | 0.88 |
| Morphometrics | ||||||
| CSA All Paraspinals-L3 (per 100 mm2) | 0.94 [0.89, 1.01] | 0.07 | 0.94 [0.87, 1.02] | 0.12 | 0.99 [0.89, 1.09] | 0.79 |
| CSA All Paraspinals-UIV (per 100 mm2) | 0.74 [0.57, 0.96] | 0.02 | 0.64 [0.45, 0.92] | 0.02 | 0.82 [0.55, 1.21] | 0.32 |
| Albumin (per g/dL) | 1.13 [0.47, 2.73] | 0.79 | 1.01 [0.36, 2.84] | 0.99 | 1.31 [0.20, 8.44] | 0.78 |
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Postop Global Tilt | — | — | 0.22 |
| Average HU-UIV + UIV + 1 (per unit) | 2.98 | [0.99, 8.92] | 0.05 |
| CSA All Paraspinals-L3 (per 100 cm2) | — | — | 0.10 |
| CSA All Paraspinals-UIV (per 100 cm2) | 0.74 | [0.57, 0.96] | 0.03 |
| Variable | Type 1 | Type 2 | p |
|---|---|---|---|
| Time to PJK (d) | 932 [419, 1452] | 93 [129, 287] | 0.14 |
| Average HU UIV | 168 [106, 270] | 140 [130, 150] | 0.54 |
| Average HU UIV + 1 | 174 [142, 249] | 130 [141, 152] | 0.11 |
| Average HU UIV and UIV +1 | 177 [124, 266] | 140 [130, 151] | 0.30 |
| UIV VBQ | 3.0 [2.5, 3.7] | 2.8 [2.5, 3.5] | 0.81 |
| Postoperative SVA (cm) | 4.66 [−1.06, 5.89] | 3.88 [2.38, 4.97] | 0.84 |
| Postoperative T1PA (°) | 18.5 [14.0, 23.2] | 26.2 [21.8, 29.1] | 0.24 |
| Global tilt (°) | 38.0 [27.0, 58.5] | 43.7 [29.1, 55.7] | 0.99 |
| CSA All Paraspinals-L3 (cm2) | 340 [295, 490] | 404 [295, 679] | 0.84 |
| CSA Multifidus-L3 (cm2) | 2637 [1797, 3019] | 2834 [2371, 3321] | 0.54 |
| CSA All Paraspinals-UIV (cm2) | 327 [239, 423] | 419 [319, 461] | 0.30 |
| CSA Multifidus-UIV (cm2) | 795 [704, 922] | 866 [714, 1011] | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennington, Z.; Mikula, A.L.; Hamouda, A.; Astudillo Potes, M.; Nassr, A.; Freedman, B.A.; Sebastian, A.S.; Fogelson, J.L.; Elder, B.D. The Paraspinal Sarcopenia at the Upper Instrumented Vertebra Is a Predictor of Discoligamentous but Not Bony Proximal Junctional Kyphosis. J. Clin. Med. 2025, 14, 1207. https://doi.org/10.3390/jcm14041207
Pennington Z, Mikula AL, Hamouda A, Astudillo Potes M, Nassr A, Freedman BA, Sebastian AS, Fogelson JL, Elder BD. The Paraspinal Sarcopenia at the Upper Instrumented Vertebra Is a Predictor of Discoligamentous but Not Bony Proximal Junctional Kyphosis. Journal of Clinical Medicine. 2025; 14(4):1207. https://doi.org/10.3390/jcm14041207
Chicago/Turabian StylePennington, Zach, Anthony L. Mikula, Abdelrahman Hamouda, Maria Astudillo Potes, Ahmad Nassr, Brett A. Freedman, Arjun S. Sebastian, Jeremy L. Fogelson, and Benjamin D. Elder. 2025. "The Paraspinal Sarcopenia at the Upper Instrumented Vertebra Is a Predictor of Discoligamentous but Not Bony Proximal Junctional Kyphosis" Journal of Clinical Medicine 14, no. 4: 1207. https://doi.org/10.3390/jcm14041207
APA StylePennington, Z., Mikula, A. L., Hamouda, A., Astudillo Potes, M., Nassr, A., Freedman, B. A., Sebastian, A. S., Fogelson, J. L., & Elder, B. D. (2025). The Paraspinal Sarcopenia at the Upper Instrumented Vertebra Is a Predictor of Discoligamentous but Not Bony Proximal Junctional Kyphosis. Journal of Clinical Medicine, 14(4), 1207. https://doi.org/10.3390/jcm14041207








