Chondrogenic and Osteogenic In Vitro Differentiation Performance of Unsorted and Sorted CD34+, CD146+, and CD271+ Stem Cells Derived from Microfragmented Adipose Tissue of Patients with Knee Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cell Lines
2.2. Magnetic-Activated Cell Sorting (MACS)
2.3. Flow Cytometry
2.4. Chondrogenic Differentiation
2.5. Chondrogenic Pellet Size
2.6. Histological Assessments of Chondrogenic Pellets
2.7. Osteogenic Differentiation
2.8. Alizarin Red S
2.9. Gene Expression by qPCR
2.10. Statistics
3. Results
3.1. Enrichment of CD34+, CD146+, and CD271+ MFAT-Derived Stem Cells by MACS

3.2. Chondrogenic Differentiation of MACS-Enriched MFAT-Derived Stem Cells
No Effect of Cell Type on Chondrogenic Pellet Size
3.3. Similar Chondrogenic Pellet Morphology Among MFAT-Derived Stem Cell Subtypes
3.4. MFAT-Derived CD271+ Stem Cells Produced More Proteoglycans than the Other Subtypes, but Not More than Unsorted Stem Cells
3.5. Upregulation of Chondrogenic Biomarkers in MFAT-Derived Stem Cells
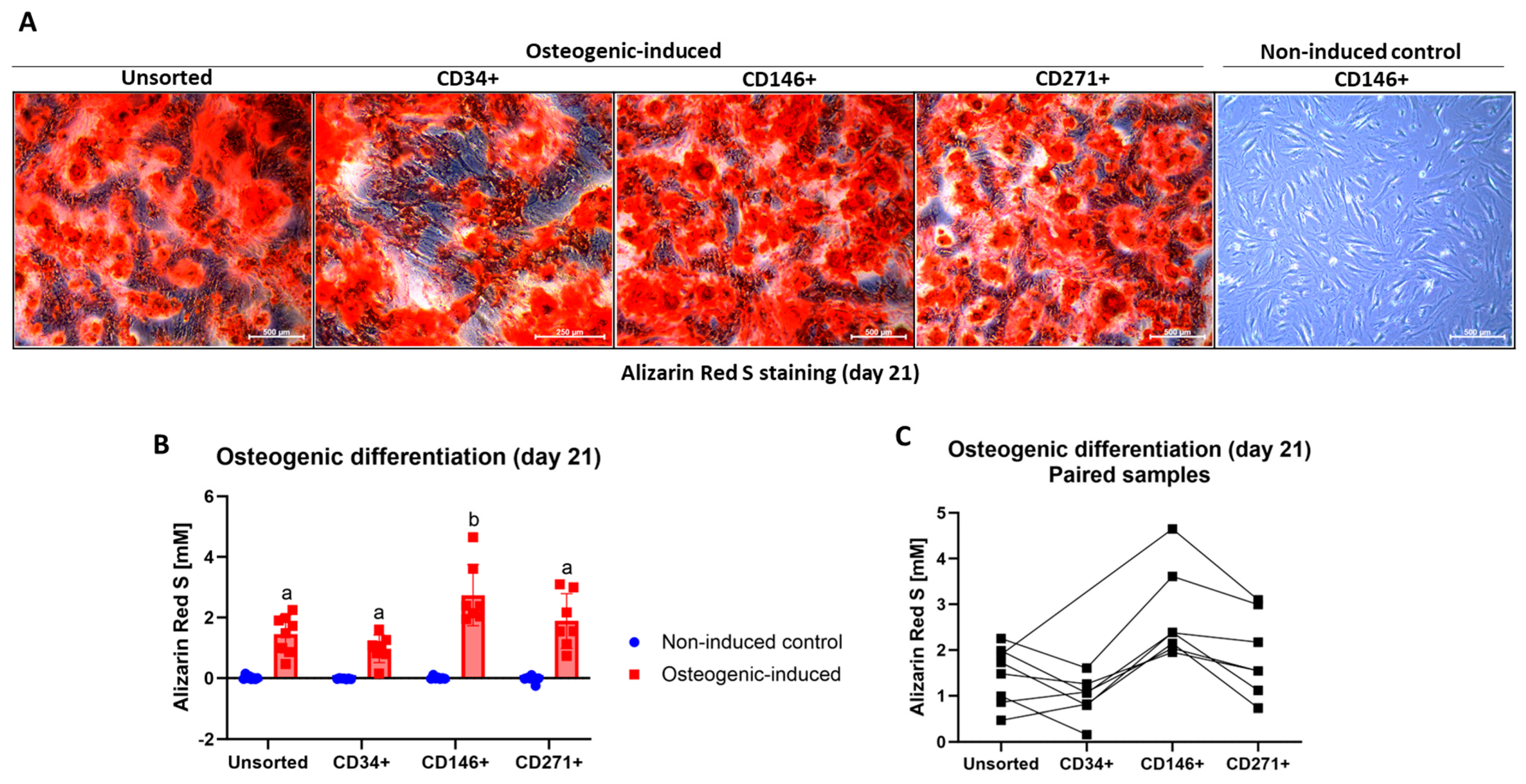
3.6. Osteogenic Differentiation of MFAT-Derived Stem Cells
MFAT-Derived CD146+ Stem Cells Produced More Calcium Deposits than the Other Stem Cell Types
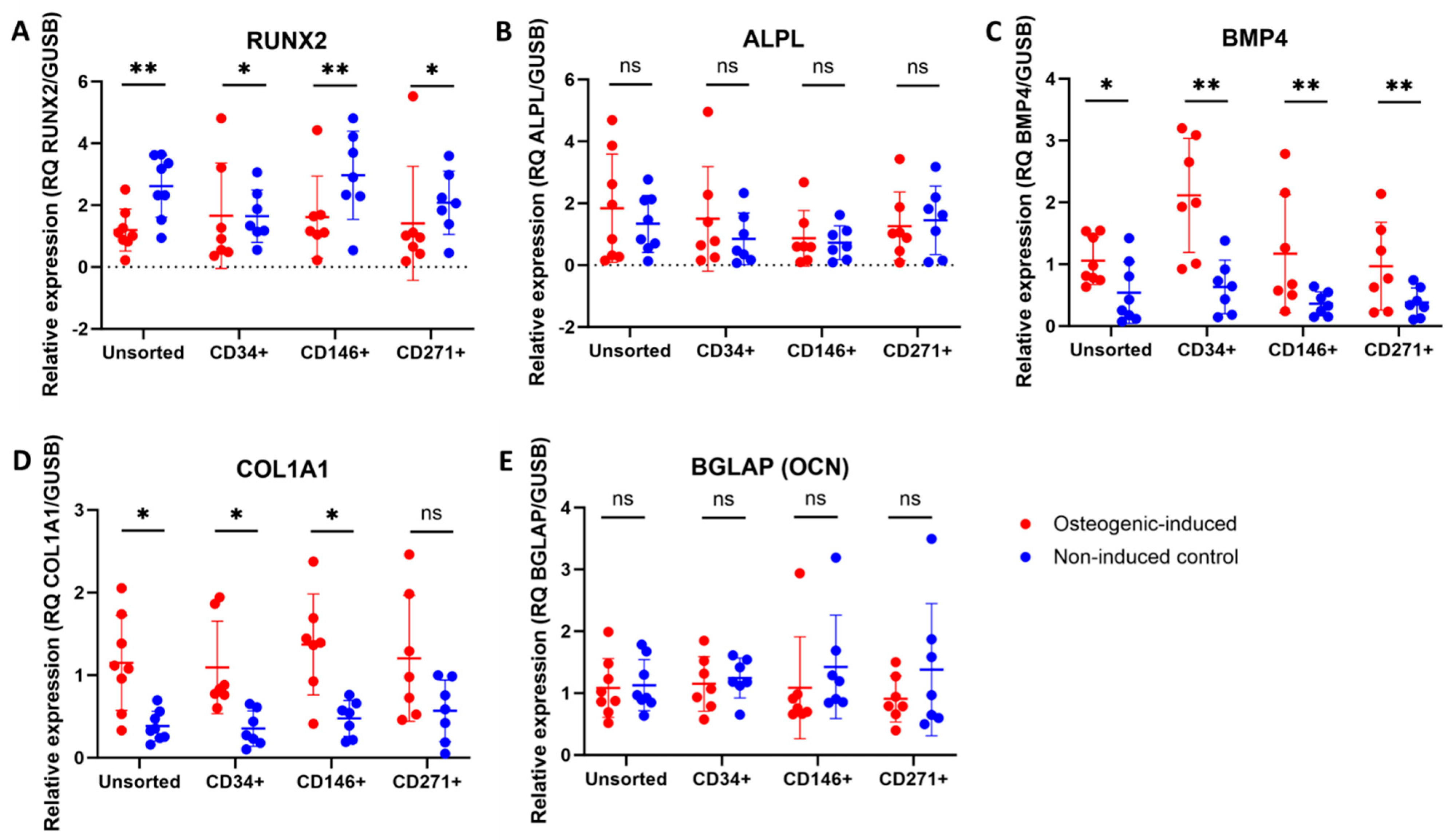
3.7. Upregulation of Some Osteogenic Biomarkers in MFAT-Derived Stem Cells
4. Discussion
4.1. Enrichment of MFAT-Derived Stem Cell Subtypes
4.2. Chondrogenic Potential of MFAT-Derived Stem Cells
4.3. Osteogenic Potential of MFAT-Derived Stem Cells
4.4. General Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, Regional, and National Burden of Osteoarthritis, 1990–2020 and Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis Year in Review 2019: Epidemiology and Therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef]
- Screpis, D.; Natali, S.; Farinelli, L.; Piovan, G.; Iacono, V.; de Girolamo, L.; Viganò, M.; Zorzi, C. Autologous Microfragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: Real-World Data at Two Years Follow-Up. J. Clin. Med. 2022, 11, 1268. [Google Scholar] [CrossRef]
- Barfod, K.W.; Blønd, L. Treatment of Osteoarthritis with Autologous and Microfragmented Adipose Tissue. Dan. Med. J. 2019, 66, A5565. [Google Scholar]
- Ferracini, R.; Alessio-Mazzola, M.; Sonzogni, B.; Stambazzi, C.; Ursino, C.; Roato, I.; Mussano, F.; Bistolfi, A.; Furlan, S.; Godio, L.; et al. Age and Synovitis Affect the Results of the Treatment of Knee Osteoarthritis with Microfragmented Autologous Fat Tissue. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 3655–3664. [Google Scholar] [CrossRef]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-like Elements by Mild Mechanical Forces from Human Lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef]
- Hammer, F.; Hölmich, P.; Nehlin, J.; Vomstein, K.; Blønd, L.; Hölmich, L.; Barfod, K.; Bagge, J. Microfragmented Abdominal Adipose Tissue—Derived Stem Cells from Knee Osteoarthritis Patients Aged 29–65 Years Demonstrate in Vitro Stemness and Low Levels of Cellular Senescence. J. Exp. Orthop. 2024, 11, e12056. [Google Scholar] [CrossRef]
- Bagge, J.; Hölmich, P.; Hammer, F.; Nehlin, J.; Blønd, L.; Hölmich, L.; Barfod, K. Successful Isolation of Viable Stem Cells from Cryopreserved Microfragmented Human Abdominal Adipose Tissue from Patients with Knee Osteoarthritis—A Comparative Study of Isolation by Tissue Explant Culture and Enzymatic Digestion. J. Exp. Orthop. 2023, 10, 31. [Google Scholar] [CrossRef]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Péault, B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef]
- Meyers, C.A.; Casamitjana, J.; Chang, L.; Zhang, L.; James, A.W.; Péault, B. Pericytes for Therapeutic Bone Repair. Adv. Exp. Med. Biol. 2018, 1109, 21–32. [Google Scholar] [CrossRef]
- Hardy, W.R.; Moldovan, N.I.; Moldovan, L.; Livak, K.J.; Datta, K.; Goswami, C.; Corselli, M.; Traktuev, D.O.; Murray, I.R.; Péault, B.; et al. Transcriptional Networks in Single Perivascular Cells Sorted from Human Adipose Tissue Reveal a Hierarchy of Mesenchymal Stem Cells. Stem Cells 2017, 35, 1273–1289. [Google Scholar] [CrossRef]
- James, A.W.; Hindle, P.; Murray, I.R.; West, C.C.; Tawonsawatruk, T.; Shen, J.; Asatrian, G.; Zhang, X.; Nguyen, V.; Simpson, A.H.; et al. Pericytes for the Treatment of Orthopedic Conditions. Pharmacol. Ther. 2017, 171, 93–103. [Google Scholar] [CrossRef]
- Beckenkamp, L.R.; Souza, L.E.B.; Melo, F.U.F.; Thomé, C.H.; Magalhães, D.A.R.; Palma, P.V.B.; Covas, D.T. Comparative Characterization of CD271+ and CD271− Subpopulations of CD34+ Human Adipose-Derived Stromal Cells. J. Cell. Biochem. 2018, 119, 3873–3884. [Google Scholar] [CrossRef]
- Kohli, N.; Al-Delfi, I.R.T.; Snow, M.; Sakamoto, T.; Miyazaki, T.; Nakajima, H.; Uchida, K.; Johnson, W.E.B. CD271-Selected Mesenchymal Stem Cells from Adipose Tissue Enhance Cartilage Repair and Are Less Angiogenic than Plastic Adherent Mesenchymal Stem Cells. Sci. Rep. 2019, 9, 3194. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Chang, L.; Meyers, C.A.; Zhang, L.; Broderick, K.; Lee, M.; Peault, B.; James, A.W. Relative Contributions of Adipose-Resident CD146+ Pericytes and CD34+ Adventitial Progenitor Cells in Bone Tissue Engineering. npj Regen. Med. 2019, 4. [Google Scholar] [CrossRef]
- Herrmann, M.; Bara, J.J.; Sprecher, C.M.; Menzel, U.; Jalowiec, J.M.; Osinga, R.; Scherberich, A.; Alini, M.; Verrier, S. Pericyte Plasticity—Comparative Investigation of the Angiogenic and Multilineage Potential of Pericytes from Different Human Tissues. Eur. Cells Mater. 2016, 31, 236–249. [Google Scholar] [CrossRef]
- Kilinc, M.O.; Santidrian, A.; Minev, I.; Toth, R.; Draganov, D.; Nguyen, D.; Lander, E.; Berman, M.; Minev, B.; Szalay, A.A. The Ratio of ADSCs to HSC-progenitors in Adipose Tissue Derived SVF May Provide the Key to Predict the Outcome of Stem-cell Therapy. Clin. Transl. Med. 2018, 7, e5. [Google Scholar] [CrossRef]
- Mikkelsen, R.K.; Blønd, L.; Hölmich, L.R.; Mølgaard, C.; Troelsen, A.; Hölmich, P.; Barfod, K.W. Treatment of Osteoarthritis with Autologous, Micro-Fragmented Adipose Tissue: A Study Protocol for a Randomized Controlled Trial. Trials 2021, 22, 748. [Google Scholar] [CrossRef]
- Bagge, J.; Berg, L.C.; Janes, J.; Macleod, J.N. Donor Age Effects on in Vitro Chondrogenic and Osteogenic Differentiation Performance of Equine Bone Marrow- and Adipose Tissue-Derived Mesenchymal Stromal Cells. BMC Vet. Res. 2022, 18, 388. [Google Scholar] [CrossRef]
- Adam, E.N.; Janes, J.; Lowney, R.; Lambert, J.; Thampi, P.; Stromberg, A.; MacLeod, J.N. Chondrogenic Differentiation Potential of Adult and Fetal Equine Cell Types. Vet. Surg. 2019, 48, 375–387. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin Red-Based Assay of Mineralization by Adherent Cells in Culture: Comparison with Cetylpyridinium Chloride Extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Livak, J.K.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 404–408. [Google Scholar] [CrossRef]
- Andersen, C.; Uvebrant, K.; Mori, Y.; Aarsvold, S.; Jacobsen, S.; Berg, L.C.; Lundgren-Åkerlund, E.; Lindegaard, C. Human Integrin α10β1-Selected Mesenchymal Stem Cells Home to Cartilage Defects in the Rabbit Knee and Assume a Chondrocyte-like Phenotype. Stem Cell Res. Ther. 2022, 13, 206. [Google Scholar] [CrossRef]
- Sardroud, H.; Chen, X.; Eames, F. Applied Compressive Strain Governs Hyaline-like Cartilage versus Fibrocartilage-like ECM Produced within Hydrogel Constructs. Int. J. Mol. Sci. 2023, 24, 7410. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The Role of Alkaline Phosphatase in Mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Zhou, Y.; Wu, G. The Roles of Bone Morphogenetic Proteins and Their Signaling in the Osteogenesis of Adipose-Derived Stem Cells. Tissue Eng. Part B Rev. 2014, 20, 84–92. [Google Scholar] [CrossRef]
- McKee, M.D.; Cole, W.G. Bone Martix and Mineralization. In Pediatric Bone; Elsevier: Amsterdam, The Netherlands, 2012; pp. 9–37. [Google Scholar]
- Li, X.; Guo, W.; Zha, K.; Jing, X.; Wang, M.; Zhang, Y.; Hao, C.; Gao, S.; Chen, M.; Yuan, Z.; et al. Enrichment of CD146+ Adipose-Derived Stem Cells in Combination with Articular Cartilage Extracellular Matrix Scaffold Promotes Cartilage Regeneration. Theranostics 2019, 9, 5105–5121. [Google Scholar] [CrossRef]
- Lauvrud, A.T.; Kelk, P.; Wiberg, M.; Kingham, P.J. Characterization of Human Adipose Tissue-Derived Stem Cells with Enhanced Angiogenic and Adipogenic Properties. J. Tissue Eng. Regen. Med. 2017, 11, 2490–2502. [Google Scholar] [CrossRef]
- Tawonsawatruk, T.; West, C.C.; Murray, I.R.; Soo, C.; Peaúlt, B.; Simpson, A.H.R.W. Adipose Derived Pericytes Rescue Fractures from a Failure of Healing—Non-Union. Sci. Rep. 2016, 6, 22779. [Google Scholar] [CrossRef] [PubMed]
- Polancec, D.; Zenic, L.; Hudetz, D.; Boric, I.; Jelec, Z.; Rod, E.; Vrdoljak, T.; Skelin, A.; Plecko, M.; Turkalj, M.; et al. Immunophenotyping of a Stromal Vascular Fraction from Microfragmented Lipoaspirate Used in Osteoarthritis Cartilage Treatment and Its Lipoaspirate Counterpart. Genes 2019, 10, 474. [Google Scholar] [CrossRef]
- Ragni, E.; Vigan, M.; Torretta, E.; Orfei, C.P.; Colombini, A.; Tremolada, C.; Gelfi, C.; Girolamo, L. De Characterization of Microfragmented Adipose Tissue Architecture, Mesenchymal Stromal Cell Content and Release of Paracrine Mediators. J. Clin. Med. 2022, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Deszcz, I.; Lis-Nawara, A.; Grelewski, P.; Dragan, S.; Bar, J. Utility of Direct 3D Co-Culture Model for Chondrogenic Differentiation of Mesenchymal Stem Cells on Hyaluronan Scaffold (Hyaff-11). Regen. Biomater. 2020, 7, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Ravera, F.; Efeoglu, E.; Byrne, H.J. A Comparative Analysis of Stem Cell Differentiation on 2D and 3D Substrates Using Raman Microspectroscopy. Analyst 2024, 149, 4041–4053. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.; Audigié, F.; Mallein-gerin, F.; et al. Cartilage Tissue Engineering: Molecular Control of Chondrocyte Differentiation for Proper Cartilage Matrix Reconstruction. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Berg, L.C.; Koch, T.G.; Heerkens, T.; Besonov, K.; Thomsen, P.D.; Betts, D.H. Chondrogenic Potential of Mesenchymal Stromal Cells Derived from Equine Bone Marrow and Umbilical Cord Blood. Vet. Comp. Orthop. Traumatol. 2009, 22, 363–370. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; Zara, J.N.; Corselli, M.; Askarinam, A.; Zhou, A.M.; Hourfar, A.; Nguyen, A.; Megerdichian, S.; Asatrian, G.; Pang, S.; et al. An Abundant Perivascular Source of Stem Cells for Bone Tissue Engineering. Stem Cells Transl. Med. 2012, 1, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Pennasilico, L.; Di Bella, C.; Sassaroli, S.; Salvaggio, A.; Roggiolani, F.; Piccionello, A.P. Effects of Autologous Microfragmented Adipose Tissue on Healing of Tibial Plateau Levelling Osteotomies in Dogs: A Prospective Clinical Trial. Animals 2023, 13, 2084. [Google Scholar] [CrossRef] [PubMed]
- Grotheer, V.; Skrynecki, N.; Oezel, L.; Grassmann, J.; Windolf, J.; Suschek, C.V. Osteogenic Differentiation of Human Mesenchymal Stromal Cells and Fibroblasts Differs Depending on Tissue Origin and Replicative Senescence. Sci. Rep. 2021, 11, 11968. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.-Y.; Wang, Y.-P.; Wu, Z.-H.; Yu, B. Dynamic Expression Profiles of Marker Genes in Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. Chin. Med. Sci. J. 2015, 30, 108–113. [Google Scholar] [CrossRef]
- Glynn, E.R.A.; Londono, A.S.; Zinn, S.A.; Hoagland, T.A.; Govoni, K.E. Culture Conditions for Equine Bone Marrow Mesenchymal Stem Cells and Expression of Key Transcription Factors during Their Differentiation into Osteoblasts. J. Anim. Sci. Biotechnol. 2013, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.; Lee, S.W.; Lee, M.; Kim, K.H.; Jung, S.H.; Kang, Y.G. Time-Dependent Expression of Osteoblast Marker Genes in Human Primary Cells Cultured on Microgrooved Titanium Substrata. Clin. Oral Implants Res. 2014, 25, 714–722. [Google Scholar] [CrossRef]
- Ferrà-Cañellas, M.d.M.; Munar-Bestard, M.; Garcia-Sureda, L.; Lejeune, B.; Ramis, J.M.; Monjo, M. BMP4 Micro-Immunotherapy Increases Collagen Deposition and Reduces PGE2 Release in Human Gingival Fibroblasts and Increases Tissue Viability of Engineered 3D Gingiva under Inflammatory Conditions. J. Periodontol. 2021, 92, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Danisovic, L.; Oravcova, L.; Krajciova, L.; Novakova, Z.V.; Bohac, M.; Varga, I.; Vojtassak, J. Effect of Long-Term Culture on the Biological and Morphological Characteristics of Human Adipose Tissue-Derived Stem Cells. J. Physiol. Pharmacol. 2017, 68, 149–158. [Google Scholar]
- Zhao, A.G.; Shah, K.; Freitag, J.; Cromer, B.; Sumer, H. Differentiation Potential of Early- and Late-Passage Adipose-Derived Mesenchymal Stem Cells Cultured under Hypoxia and Normoxia. Stem Cells Int. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, L.; Stensballe, A.; Elbæk, K.J.; Berg, L.C. Mass Spectrometric Analysis of the in Vitro Secretome from Equine Bone Marrow-Derived Mesenchymal Stromal Cells to Assess the Effect of Chondrogenic Differentiation on Response to Interleukin-1β Treatment. Stem Cell Res. Ther. 2020, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Asumda, F.Z.; Chase, P.B. Age-Related Changes in Rat Bone-Marrow Mesenchymal Stem Cell Plasticity. BMC Cell Biol. 2011, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.; Im, G. Chondrogenesis Using Mesenchymal Stem Cells and PCL Scaffolds. J. Biomed. Mater. Res. Part A 2010, 92, 659–666. [Google Scholar] [CrossRef]
- Mautner, K.; Gottschalk, M.; Boden, S.D.; Akard, A.; Bae, W.C.; Black, L.; Boggess, B.; Chatterjee, P.; Chung, C.B.; Easley, K.A.; et al. Cell-Based versus Corticosteroid Injections for Knee Pain in Osteoarthritis: A Randomized Phase 3 Trial. Nat. Med. 2023, 29, 3120–3126. [Google Scholar] [CrossRef]
- Copp, G.; Robb, K.P.; Viswanathan, S. Culture-Expanded Mesenchymal Stromal Cell Therapy: Does It Work in Knee Osteoarthritis? A Pathway to Clinical Success. Cell. Mol. Immunol. 2023, 20, 626–650. [Google Scholar] [CrossRef]




| Panel | Gene | Gene Name(s) | Annotation | Human Assay ID |
|---|---|---|---|---|
| Chondrogenic | SOX9 | SRY-box transcription factor 9 | Early chondrogenic marker [2] | Hs00165814_m1 |
| ACAN | Aggrecan | Proteoglycan found in articular cartilage [2] | Hs00153936_m1 | |
| COL2A1 | Collagen type 2 alpha 1 | Specific marker of articular hyaline cartilage [2] | Hs00264051_m1 | |
| ITGA10 | Integrin subunit alpha 10 | A collagen type II-binding integrin expressed in cartilage tissue [25] | Hs01006910_m1 | |
| COL1A1 | Collagen type 1 alpha 1 | High in fibrocartilage [26] | Hs00164004_m1 | |
| Osteogenic | RUNX2 | Runt-related transcription factor 2 | Early osteogenic transcription factor; master switch for activation of osteoblasts [27] | Hs01047973_m1 |
| ALPL | Alkaline phosphatase | Early marker of Osteogenesis; high levels in osteoblasts [28] | Hs01029144_m1 | |
| BMP4 | Bone morphogenic factor 4 | Involved in bone mineralization [29] | Hs01041266_m1 | |
| COL1A1 | Collagen type 1 alpha 1 | Major protein component of bone extracellular matrix [30] | Hs00164004_m1 | |
| BGLAP (OCN) | Osteocalcin, bone gamma- Carboxyglutamic acid- containing protein | Secreted by mature osteoblasts; essential for bone mineralization [30] | Hs01587814_g1 | |
| Endogenous controls | GUSB | Glucuronidase-beta | Hs00939627_m1 | |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Hs02786624_g1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagge, J.; Mahmood, H.; Janes, J.; Vomstein, K.; Blønd, L.; Hölmich, L.R.; Freude, K.; Nehlin, J.O.; Barfod, K.W.; Hölmich, P. Chondrogenic and Osteogenic In Vitro Differentiation Performance of Unsorted and Sorted CD34+, CD146+, and CD271+ Stem Cells Derived from Microfragmented Adipose Tissue of Patients with Knee Osteoarthritis. J. Clin. Med. 2025, 14, 1184. https://doi.org/10.3390/jcm14041184
Bagge J, Mahmood H, Janes J, Vomstein K, Blønd L, Hölmich LR, Freude K, Nehlin JO, Barfod KW, Hölmich P. Chondrogenic and Osteogenic In Vitro Differentiation Performance of Unsorted and Sorted CD34+, CD146+, and CD271+ Stem Cells Derived from Microfragmented Adipose Tissue of Patients with Knee Osteoarthritis. Journal of Clinical Medicine. 2025; 14(4):1184. https://doi.org/10.3390/jcm14041184
Chicago/Turabian StyleBagge, Jasmin, Haider Mahmood, Jennifer Janes, Kilian Vomstein, Lars Blønd, Lisbet R. Hölmich, Kristine Freude, Jan O. Nehlin, Kristoffer W. Barfod, and Per Hölmich. 2025. "Chondrogenic and Osteogenic In Vitro Differentiation Performance of Unsorted and Sorted CD34+, CD146+, and CD271+ Stem Cells Derived from Microfragmented Adipose Tissue of Patients with Knee Osteoarthritis" Journal of Clinical Medicine 14, no. 4: 1184. https://doi.org/10.3390/jcm14041184
APA StyleBagge, J., Mahmood, H., Janes, J., Vomstein, K., Blønd, L., Hölmich, L. R., Freude, K., Nehlin, J. O., Barfod, K. W., & Hölmich, P. (2025). Chondrogenic and Osteogenic In Vitro Differentiation Performance of Unsorted and Sorted CD34+, CD146+, and CD271+ Stem Cells Derived from Microfragmented Adipose Tissue of Patients with Knee Osteoarthritis. Journal of Clinical Medicine, 14(4), 1184. https://doi.org/10.3390/jcm14041184







