Clinical Outcomes for Nasopharyngeal Cancer in Non-Asian Patients: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Toxicity
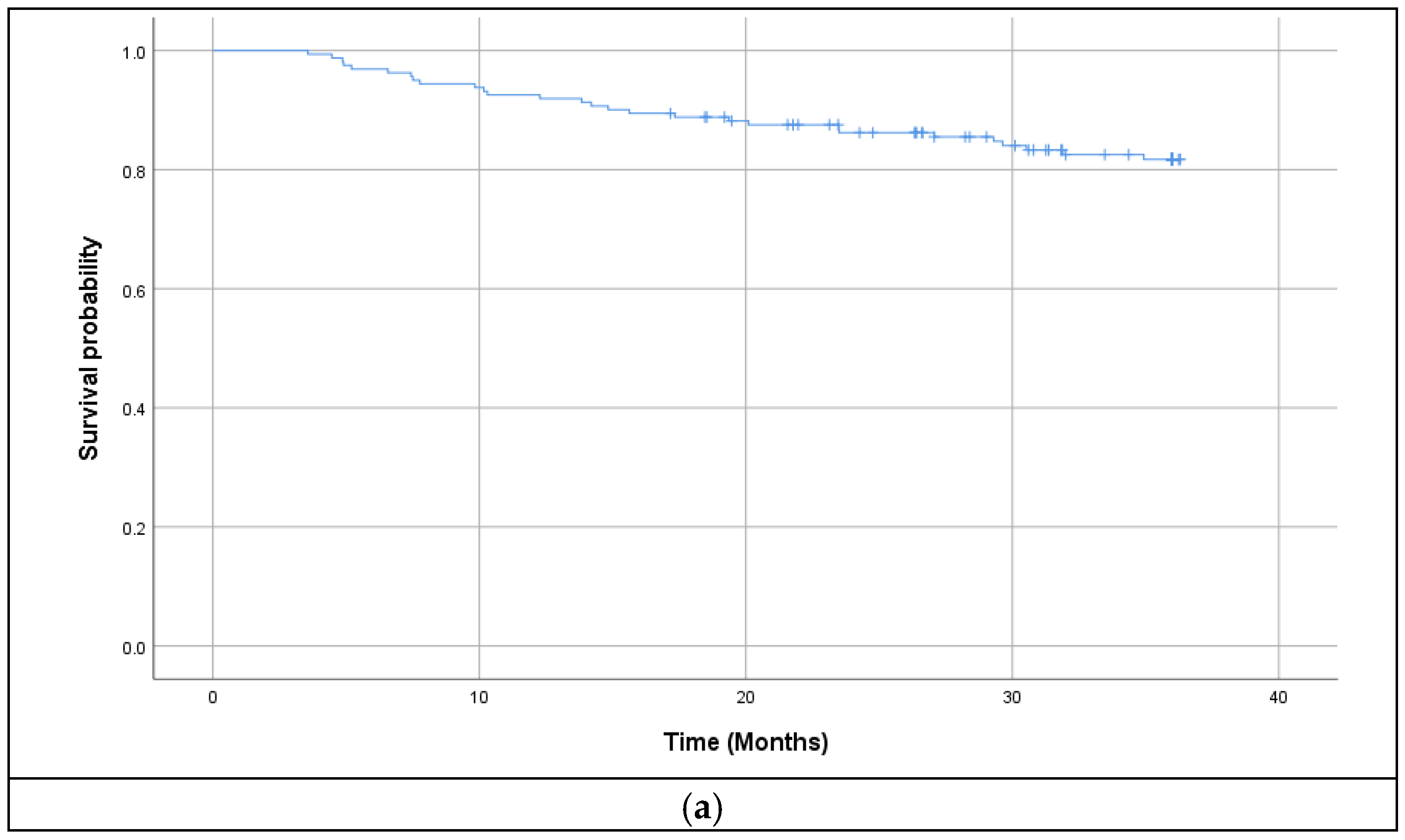
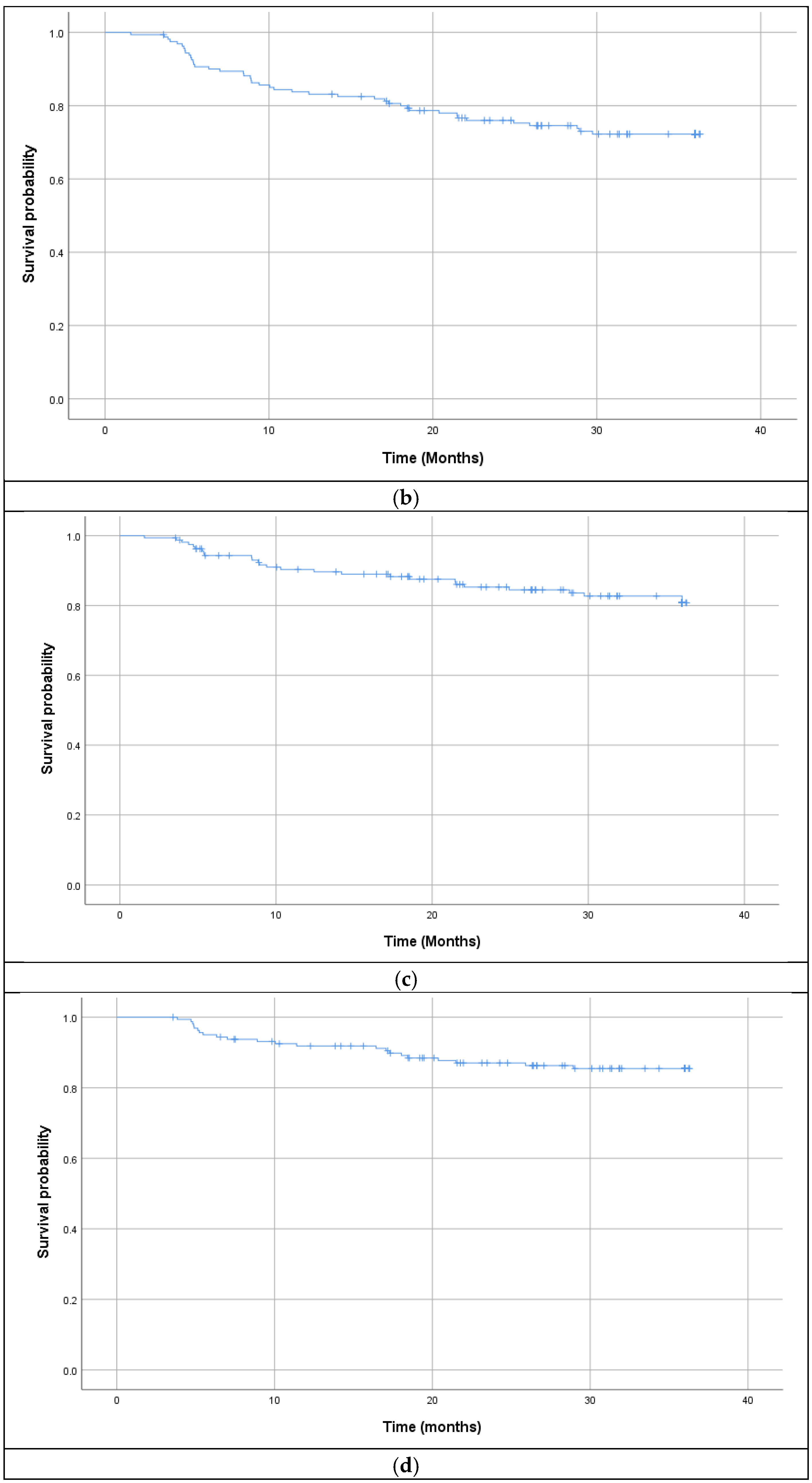
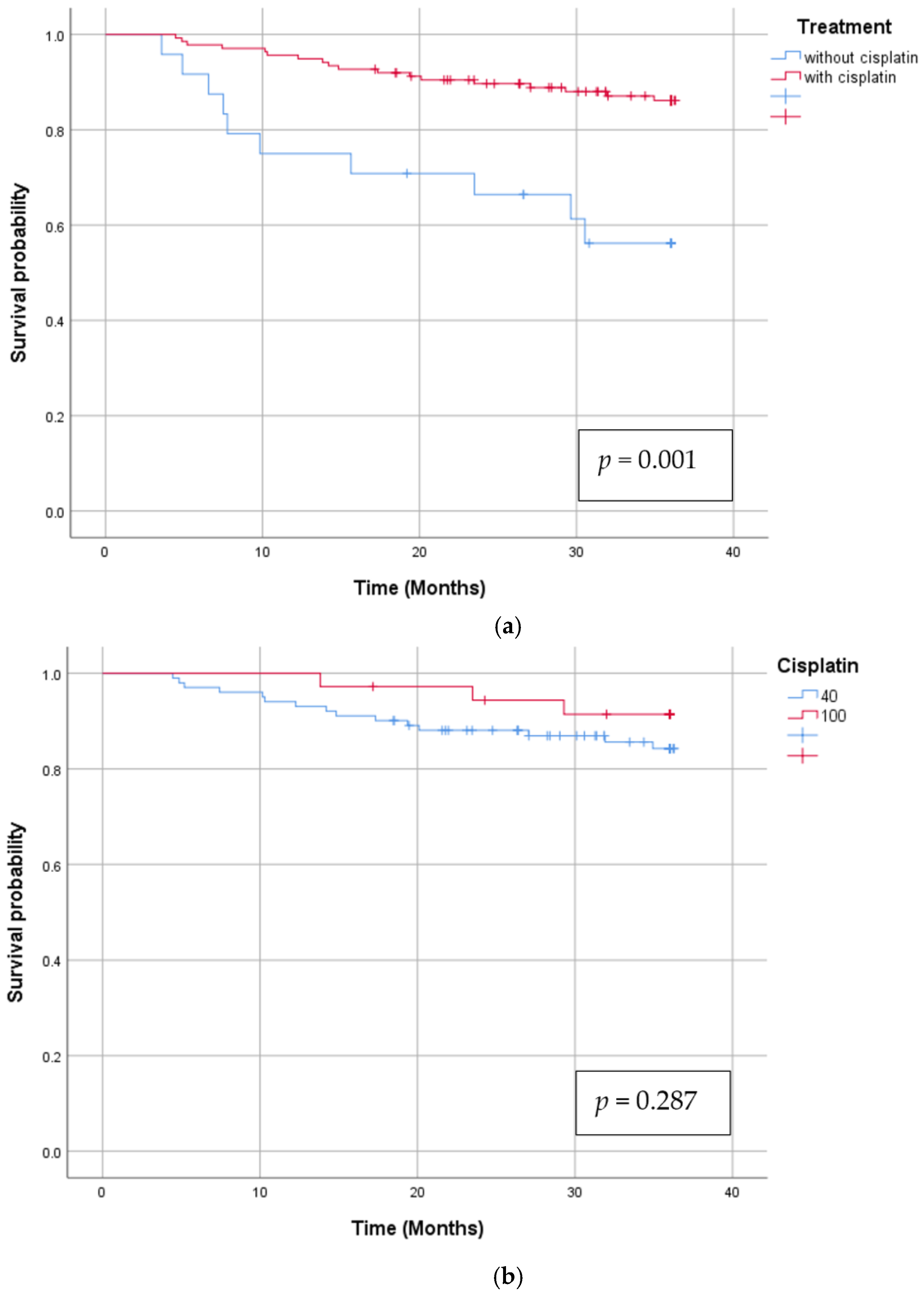
3.2. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPC | Nasopharyngeal Cancer |
| IMRT | Instensity Modulated Radiotherapy |
| EBV | Epstein Bar Virus |
| HPV | Human Papiloma Virus |
| CTV | Clinical Target Volume |
| OS | Overall Survival |
| DFS | Disease Free Survival |
| LRRFS | Locoregional Relapse Free Survival |
| DMFS | Distant Metastasis Free Survival |
| 2D | 2 Dimensional |
| 3D | 3 Dimensional |
References
- Chang, E.T.; Chang, E.T.; Ye, W.; Ye, W.; Zeng, Y.-X.; Zeng, Y.-X.; Adami, H.-O.; Adami, H.-O. The Evolving Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cheng, W.; Li, H.; Liu, X. The global, regional, national burden of nasopharyngeal cancer and its attributable risk factors (1990–2019) and predictions to 2035. Cancer Med. 2022, 11, 4310–4320. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Siak, P.Y.; Leong, C.-O.; Cheah, S.-C. The role of Epstein–Barr virus in nasopharyngeal carcinoma. Front. Microbiol. 2023, 14, 1116143. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.J.; Chen, N.; Resto, V.A. Racial and Ethnic Disparities in Nasopharyngeal Cancer Survival in the United States: A SEER Study. Otolaryngol.–Head Neck Surg. 2017, 156, 122–131. [Google Scholar] [CrossRef]
- Hamilton, S.N.; Ho, C.; Laskin, J.; Zhai, Y.; Mak, P.; Wu, J. Asian Versus Non-Asian Outcomes in Nasopharyngeal Carcinoma: A North American Population-based Analysis. Am. J. Clin. Oncol. 2016, 39, 575. [Google Scholar] [CrossRef]
- Al-Sarraf, M.; LeBlanc, M.; Giri, P.G.; Fu, K.K.; Cooper, J.; Vuong, T.; Forastiere, A.A.; Adams, G.; Sakr, W.A.; Schuller, D.E.; et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J. Clin. Oncol. 1998, 16, 1310–1317. [Google Scholar] [CrossRef]
- Ribassin-Majed, L.; Marguet, S.; Lee, A.W.; Ng, W.T.; Ma, J.; Chan, A.T.; Huang, P.-Y.; Zhu, G.; Chua, D.T.; Chen, Y.; et al. What Is the Best Treatment of Locally Advanced Nasopharyngeal Carcinoma? An Individual Patient Data Network Meta-Analysis. J. Clin. Oncol. 2017, 35, 498–505. [Google Scholar] [CrossRef]
- Lee, N.; Xia, P.; Quivey, J.M.; Sultanem, K.; Poon, I.; Akazawa, C.; Akazawa, P.; Weinberg, V.; Fu, K.K. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF experience. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 12–22. [Google Scholar] [CrossRef]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Zhang, Y.; Rumgay, H.; Li, M.; Cao, S.; Chen, W. Nasopharyngeal Cancer Incidence and Mortality in 185 Countries in 2020 and the Projected Burden in 2040: Population-Based Global Epidemiological Profiling. JMIR Public Health Surveill. 2023, 9, e49968. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, G.; Psyrri, A.; Giannoulatou, E.; Kouvatseas, G.; Rontogianni, D.; Ciuleanu, E.; Ciuleanu, T.-E.; Resiga, L.; Zaramboukas, T.; Bobos, M.; et al. Mutation profiles of nasopharyngeal carcinomas in South-Eastern European patients. Ann. Oncol. 2016, 27, vi333. [Google Scholar] [CrossRef]
- Marin, M.A.; Closca, R.-M.; Marin, A.; Rakitovan, M.; Nicoara, A.; Poenaru, M.; Militaru, M.; Baderca, F. Clinical, Epidemiological, Morphological, and Immunohistochemical Aspects of Nasopharyngeal Carcinoma-4-Year Retrospective Study in the Western Part of Romania. Diagnostics 2024, 14, 722. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Anghel, A.G.; Dumitru, M.; Soreanu, C.C. Nasopharyngeal carcinoma—Analysis of risk factors and immunological markers. Chirurgia 2012, 107, 640–645. [Google Scholar]
- Lee, A.W.; Lee, V.H.; Ng, W.-T.; Strojan, P.; Saba, N.F.; Rinaldo, A.; Willems, S.M.; Rodrigo, J.P.; Forastiere, A.A.; Ferlito, A. A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma. Eur. J. Cancer 2021, 153, 109–122. [Google Scholar] [CrossRef]
- Lenoci, D.; Resteghini, C.; Serafini, M.S.; Pistore, F.; Canevari, S.; Ma, B.; Cavalieri, S.; Alfieri, S.; Trama, A.; Licitra, L.; et al. Tumor molecular landscape of Epstein-Barr virus (EBV) related nasopharyngeal carcinoma in EBV-endemic and non-endemic areas: Implications for improving treatment modalities. Transl. Res. 2024, 265, 1–16. [Google Scholar] [CrossRef]
- Chan, A.T.; Hui, E.P.; Ngan, R.K.; Tung, S.Y.; Cheng, A.C.; Ng, W.T.; Lee, V.H.; Ma, B.B.; Cheng, H.C.; Wong, F.C.; et al. Analysis of Plasma Epstein-Barr Virus DNA in Nasopharyngeal Cancer After Chemoradiation to Identify High-Risk Patients for Adjuvant Chemotherapy: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 3091–3100. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Ma, J. Induction gemcitabine and cisplatin in locoregionally advanced nasopharyngeal carcinoma. Cancer Commun. 2019, 39, 39. [Google Scholar] [CrossRef]
- Bossi, P.; Trama, A.; Bernasconi, A.; Grisanti, S.; Mohamad, I.; Galiana, I.L.; Ozyar, E.; Franco, P.; Vecchio, S.; Bonomo, P.; et al. Nasopharyngeal cancer in non-endemic areas: Impact of treatment intensity within a large retrospective multicentre cohort. Eur. J. Cancer 2021, 159, 194–204. [Google Scholar] [CrossRef]
- Zhao, M.; Cai, H.; Li, X.; Zheng, H.; Yang, X.; Fang, W.; Zhang, L.; Wei, G.; Li, M.; Yao, K. Further evidence for the existence of major susceptibility of nasopharyngeal carcinoma in the region near HLA-A locus in Southern Chinese. J. Transl. Med. 2012, 10, 57. [Google Scholar] [CrossRef]
- Li, X.; Fasano, R.; Wang, E.; Yao, K.-T.; Marincola, F.M. HLA Associations with Nasopharyngeal Carcinoma. Curr. Mol. Med. 2009, 9, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ghandri, N.; Piancatelli, D.; Adams, S.; Chen, D.; Robbins, F.-M.; Wang, E.; Monaco, A.; Selleri, S.; Bouaouina, N.; et al. Associations between HLA Class I alleles and the prevalence of nasopharyngeal carcinoma (NPC) among Tunisians. J. Transl. Med. 2007, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Jacinto, J.C.K.; O’sullivan, B.; Su, J.; Kim, J.; Ringash, J.; Spreafico, A.; Yu, E.; Perez-Ordonez, B.; Weinreb, I.; et al. Clinical presentation and outcome of human papillomavirus-positive nasopharyngeal carcinoma in a North American cohort. Cancer 2022, 128, 2908–2921. [Google Scholar] [CrossRef]
- Wotman, M.; Oh, E.J.; Ahn, S.; Kraus, D.; Costantino, P.; Tham, T. HPV status in patients with nasopharyngeal carcinoma in the United States: A SEER database study. Am. J. Otolaryngol. 2019, 40, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, M.; Irjala, H.; Minn, H.; Vahlberg, T.; Randen-Brady, R.; Hagström, J.; Syrjänen, S.; Leivo, I. Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: A nationwide study in Finland. Head Neck 2019, 41, 349–357. [Google Scholar] [CrossRef]
- Yildirim, H.C.; Kupik, G.E.; Mustafayev, T.Z.; Berber, T.; Yavuz, B.; Cetinayak, O.; Akagunduz, O.; Bıcakcı, B.C.; Arslan, S.A.; Soykut, E.D.; et al. A multicenter retrospective analysis of patients with nasopharyngeal carcinoma treated in IMRT era from a nonendemic population: Turkish Society for Radiation Oncology Head and Neck Cancer Group Study (TROD 01-001). Head Neck 2023, 45, 1194–1205. [Google Scholar] [CrossRef]
- Slevin, F.; Pan, S.; Mistry, H.; Sen, M.; Foran, B.; Slevin, N.; Dixon, L.; Thomson, D.; Prestwich, R. A Multicentre UK Study of Outcomes of Nasopharyngeal Carcinoma Treated With Intensity-Modulated Radiotherapy ± Chemotherapy. Clin. Oncol. 2020, 32, 238–249. [Google Scholar] [CrossRef]
- Au, K.; Ngan, R.K.; Ng, A.W.; Poon, D.M.; Ng, W.; Yuen, K.; Lee, V.H.; Tung, S.Y.; Chan, A.T.; Sze, H.C.; et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol. 2018, 77, 16–21. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.-S.; Chen, X.-Z.; Hu, G.-Q.; Cheng, Z.-B.; Sun, Y.; Li, W.-X.; Chen, Y.-Y.; Xie, F.-Y.; Liang, S.-B.; et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: Long-term results of a phase 3 multicentre randomised controlled trial. Eur. J. Cancer 2017, 75, 150–158. [Google Scholar] [CrossRef]
- Li, H.; Wu, Q.; Luo, H.; Wu, J.; Su, W.; Yu, L. Comparison of TPF and PF induction chemotherapy combined with cisplatin concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: A systematic review and meta-analysis. Medicine 2025, 104, e41278. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hu, G.-Q.; Zhang, N.; Zhu, X.-D.; Yang, K.-Y.; Jin, F.; Shi, M.; Chen, Y.P.; Hu, W.-H.; et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N. Engl. J. Med. 2019, 381, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cao, S.-M.; Guo, L.; Hua, Y.-J.; Huang, P.-Y.; Zhang, X.-L.; Lin, M.; You, R.; Zou, X.; Liu, Y.-P.; et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: Long-term results of a phase III multicentre randomised controlled trial. Eur. J. Cancer 2019, 119, 87–96. [Google Scholar] [CrossRef]
- Petit, C.; Ma, J.; Lacas, B.; Ng, W.T.; Chan, A.T.C.; Hong, R.-L.; Chen, M.-Y.; Chen, L.; Huang, P.-Y.; Tan, T.; et al. Role of chemotherapy in patients with nasopharynx carcinoma treated with radiotherapy (MAC-NPC): An updated individual patient data network meta-analysis. Lancet Oncol. 2023, 24, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Lee, A.W.; Carmel, A.; Tong, N.W.; Ma, J.; Chan, A.T.; Hong, R.L.; Chen, M.-Y.; Chen, L.; Huang, P.-Y.; et al. Meta-analysis of chemotherapy in nasopharynx carcinoma (MAC-NPC): An update on 26 trials and 7080 patients. Clin. Transl. Radiat. Oncol. 2022, 32, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, G.; Ciuleanu, E.; Bobos, M.; Kalogera-Fountzila, A.; Eleftheraki, A.; Karayannopoulou, G.; Zaramboukas, T.; Nikolaou, A.; Markou, K.; Resiga, L.; et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: A randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann. Oncol. 2012, 23, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Ou, D.; Blanchard, P.; El Khoury, C.; De Felice, F.; Even, C.; Levy, A.; Nguyen, F.; Janot, F.; Gorphe, P.; Deutsch, E.; et al. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy or chemoradiotherapy alone in locally advanced non-endemic nasopharyngeal carcinoma. Oral Oncol. 2016, 62, 114–121. [Google Scholar] [CrossRef]
- Blanchard, P.; Lee, A.; Marguet, S.; Leclercq, J.; Ng, W.T.; Ma, J.; Chan, A.T.C.; Huang, P.-Y.; Benhamou, E.; Zhu, G.; et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015, 16, 645–655. [Google Scholar] [CrossRef]
- Tang, L.-L.; Chen, L.; Hu, C.-S.; Yi, J.-L.; Li, J.-G.; He, X.; Jin, F.; Zhu, X.-D.; Chen, X.-Z.; Sun, Y.; et al. CACA guidelines for holistic integrative management of nasopharyngeal carcinoma. Holist. Integr. Oncol. 2023, 2, 24. [Google Scholar] [CrossRef]
- Ou, X.; Xu, T.; He, X.; Ying, H.; Hu, C. Who benefited most from higher cumulative dose of cisplatin among patients with locally advanced nasopharyngeal carcinoma treated by intensity-modulated radiation therapy? A retrospective study of 527 cases. J. Cancer 2017, 8, 2836–2845. [Google Scholar] [CrossRef][Green Version]
- Peng, H.; Chen, L.; Li, W.-F.; Guo, R.; Mao, Y.-P.; Zhang, Y.; Zhang, F.; Liu, L.-Z.; Tian, L.; Lin, A.-H.; et al. The Cumulative Cisplatin Dose Affects the Long-Term Survival Outcomes of Patients with Nasopharyngeal Carcinoma Receiving Concurrent Chemoradiotherapy. Sci. Rep. 2016, 6, 24332. [Google Scholar] [CrossRef]
- Zhang, B.; Mo, Z.; Du, W.; Wang, Y.; Liu, L.; Wei, Y. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol. 2015, 51, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tian, Y.-M.; Sun, X.-M.; Chen, C.-Y.; Han, F.; Xiao, W.-W.; Deng, X.-W.; Lu, T.-X. Late toxicities after intensity-modulated radiotherapy for nasopharyngeal carcinoma: Patient and treatment-related risk factors. Br. J. Cancer 2014, 110, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.; Gill, G.; Lane, C.; Lane, C.; Myers, C.; Myers, C.; Kerr, E.D.; Kerr, E.D.; Lambert, P.; Lambert, P.; et al. Longitudinal functional outcomes and late effects of radiation following treatment of nasopharyngeal carcinoma: Secondary analysis of a prospective cohort study. J. Otolaryngol. Head Neck Surg. 2022, 51, 41. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Number (%) |
|---|---|
| Age, years | |
| Median | 50 (19.80) |
| <45 | 52 (32.3) |
| >45 | 109 (67.7) |
| Gender | |
| Female | 53 (32.9) |
| Male | 108 (67.1) |
| Pathological type | |
| WHO type I | 2 (1.2) |
| WHO type II | 17 (10.6) |
| WHO type III | 142 (88.2) |
| T stage | |
| T1 | 45 (28) |
| T2 | 50 (31.1) |
| T3 | 20 (12.4) |
| T4 | 46 (28.6) |
| N stage | |
| N0 | 12 (7.5) |
| N1 | 20 (12.4) |
| N2 | 94 (58.4) |
| N3 | 35 (21.7) |
| AJCC/UICC 8th ed Stage, all Mo | |
| I | 4 (2.5) |
| II | 15 (9.3) |
| III | 68 (42.2) |
| IVA | 74 (46) |
| Mucositis (%) | Dermatitis (%) | Neutropenia (%) | Anemia (%) | Thrombocytopenia (%) | |
|---|---|---|---|---|---|
| G0 | 0 | 0 | 118 (73.3%) | 73 (45.3) | 116 (72) |
| G1 | 43 (26.7) | 120 (74.5) | 29 (18) | 74 (46) | 30 (18.6) |
| G2 | 90 (55.9) | 36 (22.4) | 12 (7.5) | 14 (8.7) | 15 (9.3) |
| G3 | 28 (17.4) | 5 (3.1) | 2 (1.2) | 0 | 0 |
| G4 | 0 | 0 | 0 | 0 | 0 |
| p Value | HR | 95% CI | ||
|---|---|---|---|---|
| Overall survival Age T1,T2 vs. T3,T4 N0,N1 vs. N2,N3 Induction chemo Concomitant chemo Cisplatin 40 mg/ m2 vs. 100 mg/ m2 | 0.001 0.028 0.690 0.700 0.001 0.287 | 4.029 0.918 1.213 1.179 0.232 0.510 | 1.914 0.430 0.461 0.501 0.102 0.147 | 8.479 1.960 3.191 2.774 0.528 1.762 |
| Disease free survival Age T1,T2 vs. T3,T4 N0,N1 vs. N2,N3 Induction chemo Concomitant chemo Cisplatin 40 mg/m2 vs. 100 mg/m2 | 0.011 0.634 0.251 0.305 0.082 0.825 | 2.289 1.157 1.657 1.469 0.487 0.914 | 1.209 0.634 0.699 0.705 0.216 0.410 | 4.334 2.113 3.927 3.064 1.095 2.034 |
| Locoregional relapse free survival Age T1,T2 vs. T3,T4 N0,N1 vs. N2,N3 Induction chemo Concomitant chemo Cisplatin 40 mg/m2 vs. 100 mg/m2 | 0.033 0.676 0.701 0.780 0.234 0.349 | 2.387 1.176 1.210 1.130 0.234 0.349 | 1.071 0.550 0.458 0.478 0.181 0.202 | 5.317 2.512 3.197 2.675 1.519 1.761 |
| Distant metastases free survival Age T1,T2 vs. T3,T4 N0,N1 vs. N2,N3 Induction chemo Concomitant chemo Cisplatin 40 mg/m2 vs. 100 mg/m2 | 0.098 0.994 0.187 0.304 0.029 0.435 | 2.133 0.997 2.661 1.766 0.329 1.545 | 0.868 0.426 0.622 0.597 0.121 0.518 | 5.240 2.332 11.385 5.218 0.895 4.611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahu, R.; Urian, D.; Manolescu, V.; Ungureanu, A.; Bodale, C.; Iacob, A.; Vesa, S.C.; Tiple, C.; Kacso, G. Clinical Outcomes for Nasopharyngeal Cancer in Non-Asian Patients: A Single-Center Experience. J. Clin. Med. 2025, 14, 1177. https://doi.org/10.3390/jcm14041177
Zahu R, Urian D, Manolescu V, Ungureanu A, Bodale C, Iacob A, Vesa SC, Tiple C, Kacso G. Clinical Outcomes for Nasopharyngeal Cancer in Non-Asian Patients: A Single-Center Experience. Journal of Clinical Medicine. 2025; 14(4):1177. https://doi.org/10.3390/jcm14041177
Chicago/Turabian StyleZahu, Renata, Daniela Urian, Vlad Manolescu, Andrei Ungureanu, Carmen Bodale, Alexandru Iacob, Stefan Cristian Vesa, Cristina Tiple, and Gabriel Kacso. 2025. "Clinical Outcomes for Nasopharyngeal Cancer in Non-Asian Patients: A Single-Center Experience" Journal of Clinical Medicine 14, no. 4: 1177. https://doi.org/10.3390/jcm14041177
APA StyleZahu, R., Urian, D., Manolescu, V., Ungureanu, A., Bodale, C., Iacob, A., Vesa, S. C., Tiple, C., & Kacso, G. (2025). Clinical Outcomes for Nasopharyngeal Cancer in Non-Asian Patients: A Single-Center Experience. Journal of Clinical Medicine, 14(4), 1177. https://doi.org/10.3390/jcm14041177





