Increased Mortality Associated with Amiodarone Compared to Other Antiarrhythmic Drugs in New-Onset Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Diagnosis of AF
2.3. Prescription of AAD
2.4. Definitions
2.5. Primary Outcome Endpoint
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
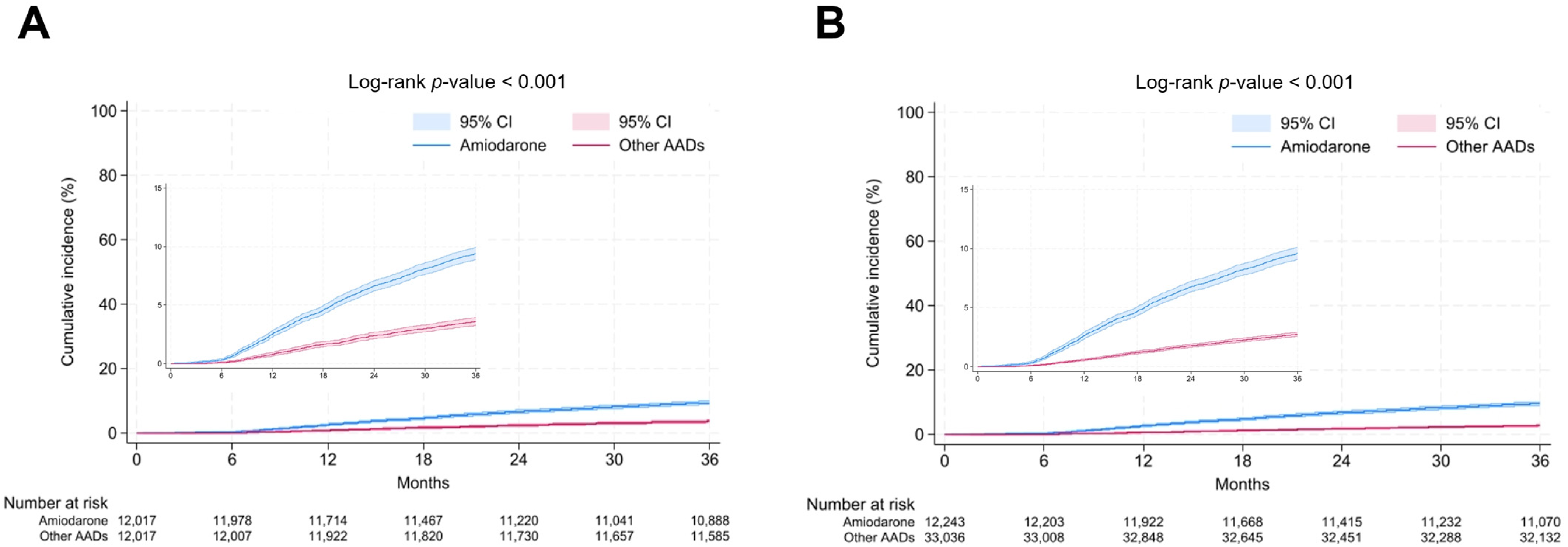
3.2. Antiarrhythmic Drugs on All-Cause Death
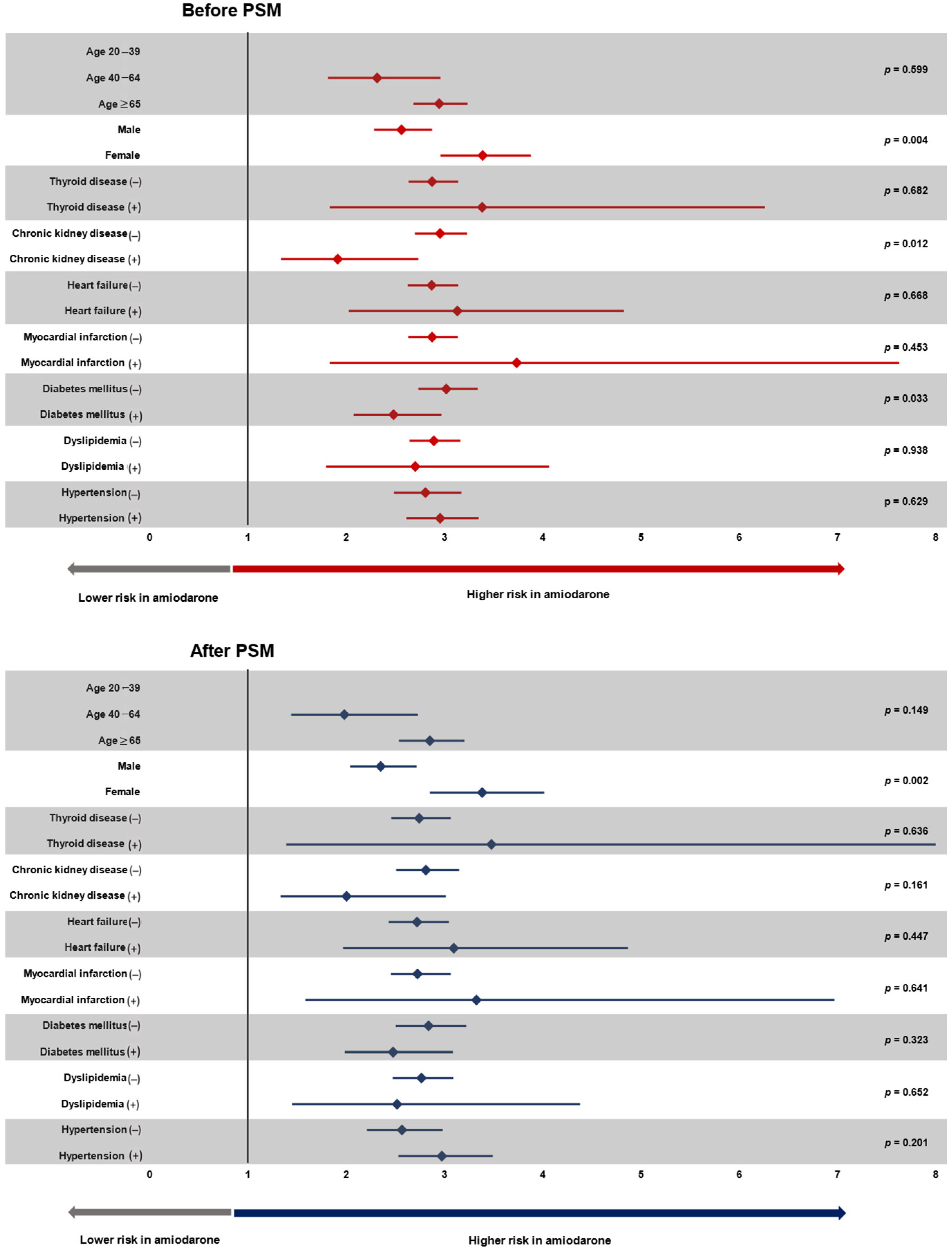
3.3. Subgroup Analysis
4. Discussion
4.1. Antiarrhythmic Drug in AF
4.2. Underlying Mechanism
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kim, Y.G.; Choi, J.-I.; Kim, H.-J.; Min, K.; Choi, Y.Y.; Shim, J.; Son, H.S.; Kim, Y.-H. A Watch-Type Electrocardiography Is a Reliable Tool for Detecting Paroxysmal Cardiac Arrhythmias. J. Clin. Med. 2022, 11, 3333. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Van Gelder, I.C.; Hagens, V.E.; Bosker, H.A.; Kingma, J.H.; Kamp, O.; Kingma, T.; Said, S.A.; Darmanata, J.I.; Timmermans, A.J.; Tijssen, J.G. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002, 347, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M. Early rhythm-control therapy in patients with atrial fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Talajic, M.; Dorian, P.; Connolly, S.; Eisenberg, M.J.; Green, M.; Kus, T.; Lambert, J.; Dubuc, M.; Gagné, P. Amiodarone to prevent recurrence of atrial fibrillation. N. Engl. J. Med. 2000, 342, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, S.N.; Reda, D.J.; Tang, X.C.; Lopez, B.; Harris, C.L.; Fletcher, R.D.; Sharma, S.C.; Atwood, J.E.; Jacobson, A.K. Amiodarone versus sotalol for atrial fibrillation. N. Engl. J. Med. 2005, 352, 1861–1872. [Google Scholar] [CrossRef]
- e1 AFADSI. Maintenance of sinus rhythm in patients with atrial fibrillation: An AFFIRM substudy of the first antiarrhythmic drug. J. Am. Coll. Cardiol. 2003, 42, 20–29. [Google Scholar] [CrossRef]
- Pearce, E.N.; Farwell, A.P.; Braverman, L.E. Thyroiditis. New Engl. J. Med. 2003, 348, 2646–2655. [Google Scholar] [CrossRef]
- Goldschlager, N.; Epstein, A.E.; Naccarelli, G.V.; Olshansky, B.; Singh, B.; Collard, H.R.; Murphy, E. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm 2007, 4, 1250–1259. [Google Scholar] [CrossRef]
- Olshansky, B.; Sami, M.; Rubin, A.; Kostis, J.; Shorofsky, S.; Slee, A.; Greene, H.L.; NHLBI AFFIRM Investigators. Use of amiodarone for atrial fibrillation in patients with preexisting pulmonary disease in the AFFIRM study. Am. J. Cardiol. 2005, 95, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 1705–1725. [Google Scholar] [CrossRef] [PubMed]
- Zimetbaum, P. Amiodarone for Atrial Fibrillation. N. Engl. J. Med. 2007, 356, 935–941. [Google Scholar] [CrossRef]
- Køber, L.; Torp-Pedersen, C.; McMurray, J.J.; Gøtzsche, O.; Lévy, S.; Crijns, H.; Amlie, J.; Carlsen, J. Increased mortality after dronedarone therapy for severe heart failure. N. Engl. J. Med. 2008, 358, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Echt, D.S.; Liebson, P.R.; Mitchell, L.B.; Peters, R.W.; Obias-Manno, D.; Barker, A.H.; Arensberg, D.; Baker, A.; Friedman, L.; Greene, H.L. Mortality and morbidity in patients receiving encainide, flecainide, or placebo: The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991, 324, 781–788. [Google Scholar] [CrossRef]
- Kim, Y.G.; Han, K.-D.; Kim, D.Y.; Choi, Y.Y.; Choi, H.Y.; Roh, S.-Y.; Shim, J.; Kim, J.S.; Choi, J.-I.; Kim, Y.-H. Different Influence of Blood Pressure on New-Onset Atrial Fibrillation in Pre-and Postmenopausal Women: A Nationwide Population-Based Study. Hypertension 2021, 77, 1500–1509. [Google Scholar] [CrossRef]
- Kim, Y.G.; Han, K.-D.; Choi, J.-I.; Choi, Y.Y.; Choi, H.Y.; Boo, K.Y.; Kim, D.Y.; Lee, K.-N.; Shim, J.; Kim, J.-S. Non-genetic risk factors for atrial fibrillation are equally important in both young and old age: A nationwide population-based study. Eur. J. Prev. Cardiol. 2021, 28, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Crijns, H.J.; Van Eickels, M.; Gaudin, C.; Page, R.L.; Torp-Pedersen, C.; Connolly, S.J. Effect of dronedarone on cardiovascular events in atrial fibrillation. N. Engl. J. Med. 2009, 360, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Pierucci, N.; Piro, A.; Trivigno, S.; Chimenti, C.; Galardo, G.; Miraldi, F.; Vizza, C.D. Incidence and determinants of spontaneous cardioversion of early onset symptomatic atrial fibrillation. Medicina 2022, 58, 1513. [Google Scholar] [CrossRef] [PubMed]
- Brien, J.F.; Jimmo, S.; Brennan, F.J.; Ford, S.E.; Armstrong, P.W. Distribution of amiodarone and its metabolite, desethylamiodarone, in human tissues. Can. J. Physiol. Pharmacol. 1987, 65, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-L.; Seo, J.-B.; Chung, W.-Y.; Kim, S.-H.; Kim, M.-A.; Zo, J.-H. The incidence and predictors of overall adverse effects caused by low dose amiodarone in real-world clinical practice. Korean J. Intern. Med. 2014, 29, 588. [Google Scholar] [CrossRef]
- Adams, P.; Holt, D.; Storey, G.; Morley, A.; Callaghan, J.; Campbell, R. Amiodarone and its desethyl metabolite: Tissue distribution and morphologic changes during long-term therapy. Circulation 1985, 72, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Lafuente, C.; Alvarez, J.C.; Leenhardt, A.; Mouly, S.; Extramiana, F.; Caulin, C.; Funck-Brentano, C.; Bergmann, J.F. Amiodarone concentrations in plasma and fat tissue during chronic treatment and related toxicity. Br. J. Clin. Pharmacol. 2009, 67, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.D.; Somberg, J.C. Pharmacology and pharmacokinetics of amiodarone. J. Clin. Pharmacol. 1991, 31, 1061–1069. [Google Scholar] [CrossRef]
- Freemantle, N.; Lafuente-Lafuente, C.; Mitchell, S.; Eckert, L.; Reynolds, M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 2011, 13, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Gnoth, M.J.; Buetehorn, U.; Muenster, U.; Schwarz, T.; Sandmann, S. In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. J. Pharmacol. Exp. Ther. 2011, 338, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Leef, G.; Alam, M.B.; Rattan, R.; Munir, M.B.; Patel, D.; Khattak, F.; Adelstein, E.; Jain, S.K.; Saba, S. Mortality risk of long-term amiodarone therapy for atrial fibrillation patients without structural heart disease. Cardiol. J. 2015, 22, 622–629. [Google Scholar] [CrossRef]

| Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| AAD (+) | Amiodarone | Other AADs | p Value | Amiodarone | Other AADs | p Value | ASD | |
| n | 45,279 | 12,243 | 33,036 | 12,017 | 12,017 | |||
| Age group | <0.001 | 0.377 | 0.017 | |||||
| −39 | 1299 | 220 (1.8%) | 1079 (3.3%) | 220 (1.8%) | 205 (1.7%) | |||
| 40–64 | 20,659 | 4555 (37.2%) | 16,104 (48.7%) | 4528 (37.7%) | 4445 (37.0%) | |||
| 65– | 23,321 | 7468 (61.0%) | 15,853 (48.0%) | 7269 (60.5%) | 7367 (61.3%) | |||
| Sex | <0.001 | 0.555 | 0.008 | |||||
| Male | 27,606 | 7281 (59.5%) | 20,325 (61.5%) | 7158 (59.6%) | 7113 (59.2%) | |||
| Female | 7673 | 4962 (40.5%) | 12,711 (38.8%) | 4859 (40.4%) | 4904 (40.8%) | |||
| Current smoker * | <0.001 | <0.001 | < 0.001 | |||||
| Non-smoker | 15,564 | 3963 (32.4%) | 11,601 (35.1%) | 3931 (32.7%) | 3936 (32.8%) | |||
| Former-smoker | 7395 | 1698 (13.9%) | 5697 (17.2%) | 1681 (14.0%) | 1863 (15.5%) | |||
| Current-smoker | 4422 | 1216 (9.9%) | 3206 (9.7%) | 1208 (10.1%) | 987 (8.2%) | |||
| Missing value | 17,898 | 5366 (43.8%) | 12,532 (37.9%) | 5197 (43.2%) | 5231 (43.5%) | |||
| Alcohol consumption * | <0.001 | 0.888 | 0.005 | |||||
| Non | 15,778 | 4160 (34.0%) | 11,618 (35.2%) | 4115 (34.2%) | 4087 (34.0%) | |||
| Mild to moderate | 9252 | 2105 (17.2%) | 7147 (21.6%) | 2096 (17.4%) | 2112 (17.6%) | |||
| Heavy | 2319 | 603 (4.9%) | 1716 (5.2%) | 600 (5.0%) | 579 (4.8%) | |||
| Missing value | 17,930 | 5375 (43.9%) | 12,555 (38.0%) | 5206 (43.3%) | 5239 (43.6%) | |||
| Regular exercise | <0.001 | 0.834 | 0.006 | |||||
| Yes | 9884 | 2301 (18.8%) | 7583 (23.0%) | 4529 (37.7%) | 4484 (37.3%) | |||
| No | 18,283 | 5366 (43.8%) | 12,917 (39.1%) | 2291 (19.1%) | 2301 (19.1%) | |||
| Missing value | 17,112 | 4576 (37.4%) | 12,536 (37.9%) | 5197 (43.2%) | 5232 (43.5%) | |||
| Income quartile | <0.001 | 0.990 | 0.003 | |||||
| Q1 (lowest income) | 9145 | 2800 (22.9%) | 6345 (19.2%) | 2734 (22.8%) | 2741 (22.8%) | |||
| Q2 | 6899 | 1904 (15.6%) | 4995 (15.1%) | 1869 (15.6%) | 1872 (15.6%) | |||
| Q3 | 9989 | 2707 (22.1%) | 7282 (22.0%) | 2648 (22.0%) | 2626 (21.9%) | |||
| Q4 (highest income) | 18,541 | 4617 (37.7%) | 13,924 (42.1%) | 4551 (37.9%) | 4571 (38.0%) | |||
| Missing value | 705 | 215 (1.8%) | 490 (1.5%) | 215 (1.8%) | 207 (1.7%) | |||
| Diabetes mellitus | 7033 | 2234 (18.2%) | 4799 (14.5%) | <0.001 | 2197 (18.3%) | 2131 (17.7%) | 0.268 | 0.015 |
| Hypertension | 19,479 | 5265 (43.0%) | 14,214 (43.0%) | 0.967 | 5182 (43.1%) | 5194 (43.2%) | 0.876 | 0.002 |
| Dyslipidemia | 3555 | 681 (5.6%) | 2874 (8.7%) | <0.001 | 678 (5.6%) | 674 (5.6%) | 0.911 | 0.001 |
| Heart failure | 1037 | 603 (4.9%) | 434 (1.3%) | <0.001 | 433 (3.6%) | 406 (3.4%) | 0.343 | 0.013 |
| Myocardial infarction | 547 | 316 (2.6%) | 231 (0.7%) | <0.001 | 241 (2.0%) | 205 (1.7%) | 0.085 | 0.024 |
| Chronic kidney disease | 888 | 369 (3.0%) | 519 (1.6%) | <0.001 | 305 (2.5%) | 355 (3.0%) | 0.848 | 0.003 |
| Hypo- or hyper-thyroidism | 1340 | 256 (2.1%) | 1084 (3.3%) | <0.001 | 254 (2.1%) | 244 (2.0%) | 0.651 | 0.005 |
| Stroke | 2312 | 702 (5.7%) | 1610 (4.9%) | <0.001 | 692 (5.8%) | 680 (5.7%) | 0.739 | 0.004 |
| Age | 64.5 ± 12.1 | 67.5 ± 12.1 | 63.4 ± 11.9 | <0.001 | 67.3 ± 12.2 | 66.2 ± 11.5 | <0.001 | 0.096 |
| Fasting glucose (mg/dL) * | 105 ± 26 | 107.7 ± 30.3 | 104.1 ± 24.3 | <0.001 | 107.7 ± 30.2 | 105.4 ± 25.8 | <0.001 | |
| Body mass index (kg/m2) * | 24.9 ± 3.3 | 25.1 ± 3.5 | 24.9 ± 3.3 | <0.001 | 25.1 ± 3.6 | 24.8 ± 3.3 | <0.001 | |
| Waist circumference (cm) * | 85.4 ± 9 | 86.2 ± 9.3 | 85.1 ± 8.8 | <0.001 | 86.2 ± 9.3 | 85.2 ± 8.9 | <0.001 | |
| Systolic blood pressure (mmHg) * | 127.4 ± 15.3 | 128.4 ± 16 | 127 ± 15.1 | <0.001 | 128.4 ± 16 | 128.1 ± 15.3 | 0.346 | |
| Diastolic blood pressure (mmHg) * | 77.8 ± 10.2 | 78 ± 10.6 | 77.7 ± 10.1 | 0.014 | 78 ± 10.6 | 77.7 ± 10.1 | 0.029 | |
| eGFR * | 83 ± 26 | 80.1 ± 25.8 | 84 ± 26 | <0.001 | 80.2 ± 25.8 | 81.9 ± 23.8 | <0.001 | |
| Total cholesterol (mg/dL) * | 188.3 ± 43.5 | 185.6 ± 46.9 | 189.2 ± 42.3 | <0.001 | 185.7 ± 46.9 | 187.1 ± 49.5 | 0.090 | |
| Catheter ablation | 5053 (11.2%) | 885 (7.2%) | 4168 (12.6%) | <0.001 | 879 (7.3%) | 1333 (11.1%) | <0.001 | |
| MPR (mean) | 0.90 ± 0.15 | 0.87 ± 0.16 | 0.91 ± 0.14 | <0.001 | 0.87 ± 0.16 | 0.91 ± 0.14 | <0.001 | |
| n | Event Number (All-Cause Death) | Duration (Person × Year) | Incidence | Non-Adjusted | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Before PSM | ||||||||||
| other AADs | 33,036 | 904 | 97,920 | 9.2 | reference | reference | reference | reference | reference | reference |
| Amiodarone | 12,243 | 1173 | 35,051 | 33.5 | 3.63 (3.33–3.95) | 3.09 (2.84–3.37) | 2.88 (2.64–3.15) | 2.53 (2.23–2.87) | 3.18 (2.42–4.18) | 3.81 (3.30–4.40) |
| After PSM | ||||||||||
| other AADs | 12,017 | 432 | 35,473 | 12.2 | reference | reference | reference | reference | reference | reference |
| Amiodarone | 12,017 | 1127 | 34,442 | 32.7 | 2.68 (2.40–2.99) | 2.74 (2.46–3.06) | 2.75 (2.47–3.07) | 2.38 (2.03–2.79) | 3.56 (2.47–5.12) | 3.60 (3.00–4.32) |
| n | Event Number | Event Rate | Absolute Risk Reduction | Number Needed to Treat | |
|---|---|---|---|---|---|
| Before PSM | |||||
| other AADs | 33,036 | 904 | 0.027 | 0.069 | 14.61 |
| Amiodarone | 12,243 | 1173 | 0.096 | ||
| After PSM | |||||
| other AADs | 12,017 | 432 | 0.036 | 0.058 | 17.29 |
| Amiodarone | 12,017 | 1127 | 0.094 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.G.; Lee, H.S.; Kim, H.; Kim, M.; Jeong, J.H.; Choi, Y.Y.; Shim, J.; Choi, J.-I.; Kim, Y.-H. Increased Mortality Associated with Amiodarone Compared to Other Antiarrhythmic Drugs in New-Onset Atrial Fibrillation. J. Clin. Med. 2025, 14, 1168. https://doi.org/10.3390/jcm14041168
Kim YG, Lee HS, Kim H, Kim M, Jeong JH, Choi YY, Shim J, Choi J-I, Kim Y-H. Increased Mortality Associated with Amiodarone Compared to Other Antiarrhythmic Drugs in New-Onset Atrial Fibrillation. Journal of Clinical Medicine. 2025; 14(4):1168. https://doi.org/10.3390/jcm14041168
Chicago/Turabian StyleKim, Yun Gi, Hyoung Seok Lee, Hoseob Kim, Mina Kim, Joo Hee Jeong, Yun Young Choi, Jaemin Shim, Jong-Il Choi, and Young-Hoon Kim. 2025. "Increased Mortality Associated with Amiodarone Compared to Other Antiarrhythmic Drugs in New-Onset Atrial Fibrillation" Journal of Clinical Medicine 14, no. 4: 1168. https://doi.org/10.3390/jcm14041168
APA StyleKim, Y. G., Lee, H. S., Kim, H., Kim, M., Jeong, J. H., Choi, Y. Y., Shim, J., Choi, J.-I., & Kim, Y.-H. (2025). Increased Mortality Associated with Amiodarone Compared to Other Antiarrhythmic Drugs in New-Onset Atrial Fibrillation. Journal of Clinical Medicine, 14(4), 1168. https://doi.org/10.3390/jcm14041168






