Outcome of Facial Nerve Integrity After Parotid Gland Surgery With and Without Intraoperative Monitoring: A Ten-Year Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Intraoperative Monitoring and Operative Technique
2.2. Postoperative Management
2.3. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.1.1. Demographic and Clinical Characteristics
3.1.2. Tumor Characteristics
3.1.3. Surgical Outcomes
| NIM | No NIM | Total | ||
|---|---|---|---|---|
| Sex | Male | 63 | 72 | 155 |
| Female | 72 | 102 | 174 | |
| Mean age (Years) | 60.1 | 62.2 | 61.3 | |
| Tumor site | Superficial lobe | 95 | 143 | 238 |
| Inferior lobe | 30 | 45 | 75 | |
| Deep lobe | 10 | 6 | 16 | |
| Histopathology | Pleomorphic adenoma | 70 | 101 | 171 |
| Warthin’s tumor | 50 | 87 | 137 | |
| Lymphoepithelial cyst | 10 | 5 | 15 | |
| Oncocytomas | 5 | 0 | 5 | |
| Operative Time (Min) | 105.3 | 108.5 | 107.2 | |
| Facial nerve dysfunction | Total cases | 7 | 10 | 17 |
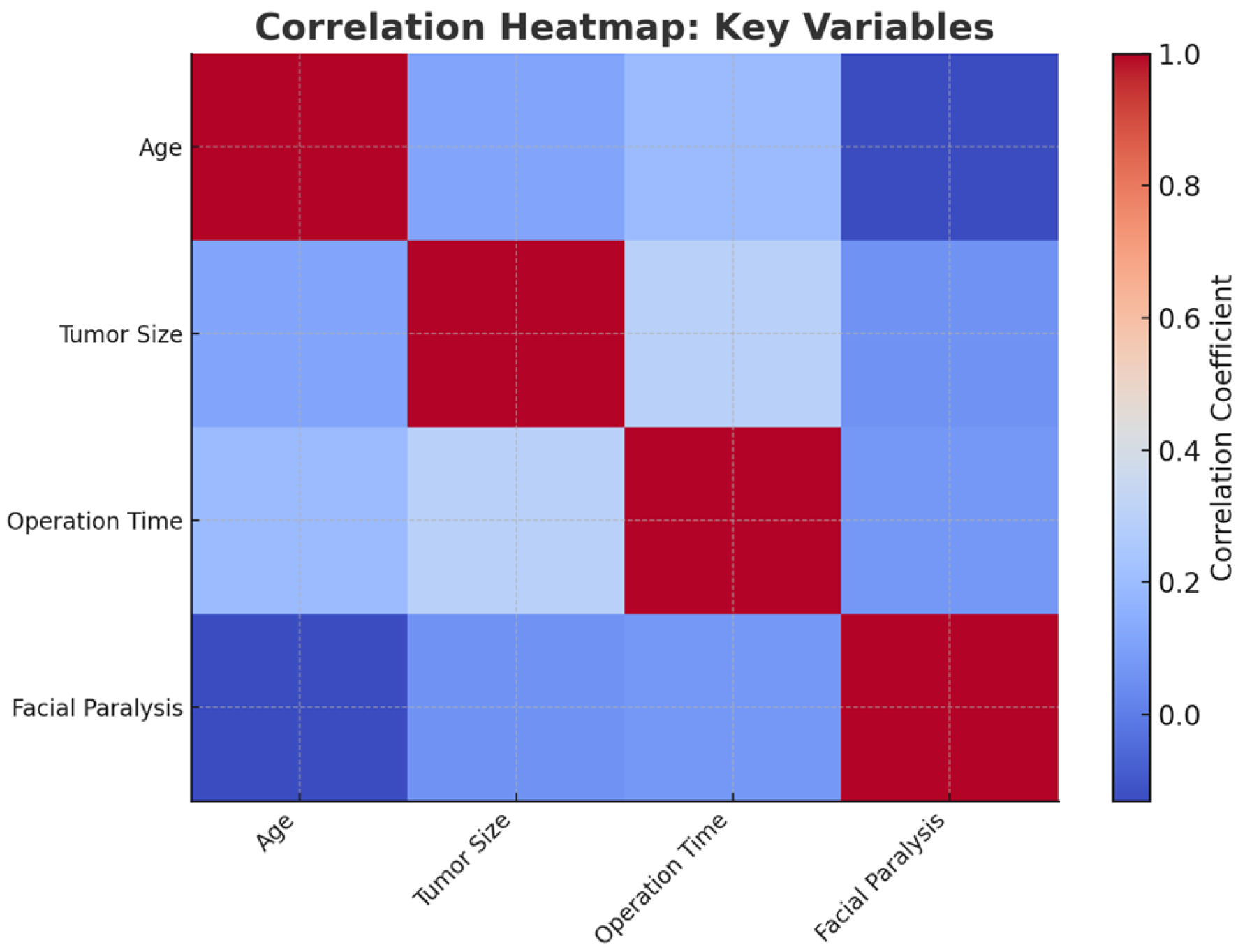
3.2. Inferential Statistical Analysis
- Sex: no significant association was observed between patient sex and the occurrence of facial paralysis or palsy (p = 0.451), indicating that likelihood is not influenced by the patient’s sex.
- Side of the tumor: the side of the tumor (left or right) did not show a significant relationship with paralysis or palsy (p = 0.574), suggesting no dependency between tumor side and facial nerve integrity outcomes.
- Histological Type: Tumor histology was not significantly associated with the risk of paralysis or palsy (p = 0.317). This implies that the histological type of the tumor does not predict the likelihood of nerve damage.
- Tumor location (superficial or inferior) was significantly associated with paralysis or palsy (p = 0.035). Patients with tumors in the inferior lobe showed a higher incidence of facial nerve injury compared to those with tumors in the superficial lobe. This indicates that tumor location plays an important role in predicting the risk of damage, potentially due to proximity to the main facial nerve.
3.2.1. Comparison of Means (T-Tests)
- Age: Patients who developed facial paralysis or palsy had a significantly higher mean age compared to those who did not (t = −2.41, p = 0.027 95% CI for mean difference: 3.2 to 9.5 years). This result indicates that age is a significant factor, with older patients being at higher risk.
- Tumor size: There was no significant difference in tumor size between patients with and without facial paralysis or palsy (t = 1.30, p = 0.211 95% CI for mean difference: −0.2 to 0.6 cm). This suggests that tumor size alone does not influence the likelihood of nerve injury.
- Operation time: Surgical duration was not significantly associated with the occurrence of paralysis or palsy (t = 1.60, p = 0.126 95% CI for mean difference: −5.0 to 12.5 min). This implies that longer surgeries do not necessarily lead to a higher risk of injury in this dataset.
3.2.2. Multivariate Logistic Regression
3.2.3. Exploration of Interactions
3.2.4. Stratified Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECD | Extracapsular enucleation |
| SP | Superficial parotidectomy |
| TP | Total parotidectomy |
| IONM | Intraoperative nerve monitoring |
| NIM | Nerve integrity monitor |
| SD | Standard Deviation |
References
- Kawata, R.; Kinoshita, I.; Omura, S.; Higashino, M.; Nishikawa, S.; Terada, T.; Haginomori, S.; Kurisu, Y.; Hirose, Y.; Tochizawa, T. Risk Factors of Postoperative Facial Palsy for Benign Parotid Tumors: Outcome of 1,018 Patients. Laryngoscope 2021, 131, E2857–E2864. [Google Scholar] [CrossRef] [PubMed]
- Haring, C.T.; Ellsperman, S.E.; Edwards, B.M.; Kileny, P.; Kovatch, D.; Mannarelli, G.R.; Meloch, M.A.; Miller, C.; Pitts, C.; Prince, M.E.P.; et al. Assessment of Intraoperative Nerve Monitoring Parameters Associated with Facial Nerve Outcome in Parotidectomy for Benign Disease. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 1137. [Google Scholar] [CrossRef] [PubMed]
- Głuszkiewicz, E.; Sowa, P.; Zieliński, M.; Adamczyk-Sowa, M.; Misiołek, M.; Ścierski, W. Prospective Assessment of Risk Factors Influencing Facial Nerve Paresis in Patients after Surgery for Parotid Gland Tumors. Medicina 2022, 58, 1726. [Google Scholar] [CrossRef] [PubMed]
- Klintworth, N.; Zenk, J.; Koch, M.; Iro, H. Postoperative Complications after Extracapsular Dissection of Benign Parotid Lesions with Particular Reference to Facial Nerve Function. Laryngoscope 2010, 120, 484–490. [Google Scholar] [CrossRef]
- Ruas, J.J.; Rodrigues, J.; Ribeiro, M.; Pinto Moura, C. Facial Nerve Dysfunction Following Parotidectomy: Role of Intraoperative Facial Nerve Monitoring. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 1479–1484. [Google Scholar] [CrossRef]
- Toulgoat, F.; Sarrazin, J.L.; Benoudiba, F.; Pereon, Y.; Auffray-Calvier, E.; Daumas-Duport, B.; Lintia-Gaultier, A.; Desal, H.A. Facial Nerve: From Anatomy to Pathology. Diagn. Interv. Imaging 2013, 94, 1033–1042. [Google Scholar] [CrossRef]
- Higashino, M.; Kinoshita, I.; Jinnin, T.; Terada, T.; Kawata, R. Predicting Postoperative Facial Nerve Paralysis by Using Intraoperative Nerve Monitoring during Parotid Surgery. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 3855–3860. [Google Scholar] [CrossRef]
- House, J.W.; Brackmann, D.E. Facial Nerve Grading System. Otolaryngol.–Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef]
- Savvas, E.; Hillmann, S.; Weiss, D.; Koopmann, M.; Rudack, C.; Alberty, J. Association Between Facial Nerve Monitoring With Postoperative Facial Paralysis in Parotidectomy. JAMA Otolaryngol.–Head Neck Surg. 2016, 142, 828. [Google Scholar] [CrossRef]
- Verma, R.; Reddy, G.G.S.S.; Kumar, B.P.; Bakshi, J. Outcome of Postoperative Facial Nerve Function Following Electromyographic Facial Nerve Monitoring During Parotidectomy for Benign Lesions: A Retrospective Study. Indian J. Surg. Oncol. 2022, 13, 495–499. [Google Scholar] [CrossRef]
- Consorti, G.; Monarchi, G.; Paglianiti, M.; Betti, E.; Balercia, P. Reduction of Post-Surgical Facial Edema Following Bromelain and Coumarin Intake in Traumatology: A Prospective Study with 100 Patients. J. Clin. Med. 2024, 13, 922. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, I.; Kawata, R.; Higashino, M.; Nishikawa, S.; Terada, T.; Haginomori, S.-I. Effectiveness of Intraoperative Facial Nerve Monitoring and Risk Factors Related to Postoperative Facial Nerve Paralysis in Patients with Benign Parotid Tumors: A 20-Year Study with 902 Patients. Auris Nasus Larynx 2021, 48, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Grosheva, M.; Klussmann, J.P.; Grimminger, C.; Wittekindt, C.; Beutner, D.; Pantel, M.; Volk, G.F.; Guntinas-Lichius, O. Electromyographic Facial Nerve Monitoring during Parotidectomy for Benign Lesions Does Not Improve the Outcome of Postoperative Facial Nerve Function: A Prospective Two-center Trial. Laryngoscope 2009, 119, 2299–2305. [Google Scholar] [CrossRef]
- Ikoma, R.; Ishitoya, J.; Sakuma, Y.; Hirama, M.; Shiono, O.; Komatsu, M.; Oridate, N. Temporary Facial Nerve Dysfunction after Parotidectomy Correlates with Tumor Location. Auris Nasus Larynx 2014, 41, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Ruohoalho, J.; Mäkitie, A.A.; Aro, K.; Atula, T.; Haapaniemi, A.; Keski–Säntti, H.; Takala, A.; Bäck, L.J. Complications after Surgery for Benign Parotid Gland Neoplasms: A Prospective Cohort Study. Head Neck 2017, 39, 170–176. [Google Scholar] [CrossRef]
- Jin, H.; Kim, B.Y.; Kim, H.; Lee, E.; Park, W.; Choi, S.; Chung, M.K.; Son, Y.-I.; Baek, C.-H.; Jeong, H.-S. Incidence of Postoperative Facial Weakness in Parotid Tumor Surgery: A Tumor Subsite Analysis of 794 Parotidectomies. BMC Surg. 2019, 19, 199. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Gabriel, B.; Peter Klussmann, J. Risk of Facial Palsy and Severe Frey’s Syndrome after Conservative Parotidectomy for Benign Disease: Analysis of 610 Operations. Acta Otolaryngol. 2006, 126, 1104–1109. [Google Scholar] [CrossRef]
- Duque, C.S.; Londoño, A.F.; Duque, A.M.; Zuleta, J.J.; Marulanda, M.; Otálvaro, L.M.; Agudelo, M.; Dueñas, J.P.; Palacio, M.F.; Dionigi, G. Facial Nerve Monitoring in Parotid Gland Surgery: Design and Feasibility Assessment of a Potential Standardized Technique. World J. Otorhinolaryngol. Head Neck Surg. 2023, 9, 280–287. [Google Scholar] [CrossRef]
- Deneuve, S.; Quesnel, S.; Depondt, J.; Albert, S.; Panajotopoulos, A.; Gehanno, P.; Barry, B. Management of Parotid Gland Surgery in a University Teaching Hospital. Eur. Arch. Oto-Rhino-Laryngol. 2010, 267, 601–605. [Google Scholar] [CrossRef]
- Meier, J.D.; Wenig, B.L.; Manders, E.C.; Nenonene, E.K. Continuous Intraoperative Facial Nerve Monitoring in Predicting Postoperative Injury During Parotidectomy. Laryngoscope 2006, 116, 1569–1572. [Google Scholar] [CrossRef]
- Sood, A.J.; Houlton, J.J.; Nguyen, S.A.; Gillespie, M.B. Facial Nerve Monitoring during Parotidectomy. Otolaryngol.–Head Neck Surg. 2015, 152, 631–637. [Google Scholar] [CrossRef] [PubMed]
- de Campora, L.; Atturo, F.; De Luca, P.; Muller, M.; Radici, M.; Camaioni, A.; de Campora, E. Impact of Surgeon’s Experience and Tumor’s Nature in the Use of Intraoperative Facial Nerve Monitoring in Superficial Parotidectomy. Preliminary Results from a Single-Center Retrospective Analysis. Indian J. Otolaryngol. Head Neck Surg. 2024, 76, 2577–2582. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirignaco, G.; Monarchi, G.; Betti, E.; Paglianiti, M.; Catarzi, L.; Tel, A.; Vaira, L.A.; Balercia, P.; Consorti, G. Outcome of Facial Nerve Integrity After Parotid Gland Surgery With and Without Intraoperative Monitoring: A Ten-Year Retrospective Study. J. Clin. Med. 2025, 14, 1156. https://doi.org/10.3390/jcm14041156
Cirignaco G, Monarchi G, Betti E, Paglianiti M, Catarzi L, Tel A, Vaira LA, Balercia P, Consorti G. Outcome of Facial Nerve Integrity After Parotid Gland Surgery With and Without Intraoperative Monitoring: A Ten-Year Retrospective Study. Journal of Clinical Medicine. 2025; 14(4):1156. https://doi.org/10.3390/jcm14041156
Chicago/Turabian StyleCirignaco, Giulio, Gabriele Monarchi, Enrico Betti, Mariagrazia Paglianiti, Lisa Catarzi, Alessandro Tel, Luigi Angelo Vaira, Paolo Balercia, and Giuseppe Consorti. 2025. "Outcome of Facial Nerve Integrity After Parotid Gland Surgery With and Without Intraoperative Monitoring: A Ten-Year Retrospective Study" Journal of Clinical Medicine 14, no. 4: 1156. https://doi.org/10.3390/jcm14041156
APA StyleCirignaco, G., Monarchi, G., Betti, E., Paglianiti, M., Catarzi, L., Tel, A., Vaira, L. A., Balercia, P., & Consorti, G. (2025). Outcome of Facial Nerve Integrity After Parotid Gland Surgery With and Without Intraoperative Monitoring: A Ten-Year Retrospective Study. Journal of Clinical Medicine, 14(4), 1156. https://doi.org/10.3390/jcm14041156










