Assessment of the Relationship between Pre-Existing Muscle Atrophy, Subcutaneous Fat Volume, and the Prognosis of COVID-19
Abstract
1. Introduction
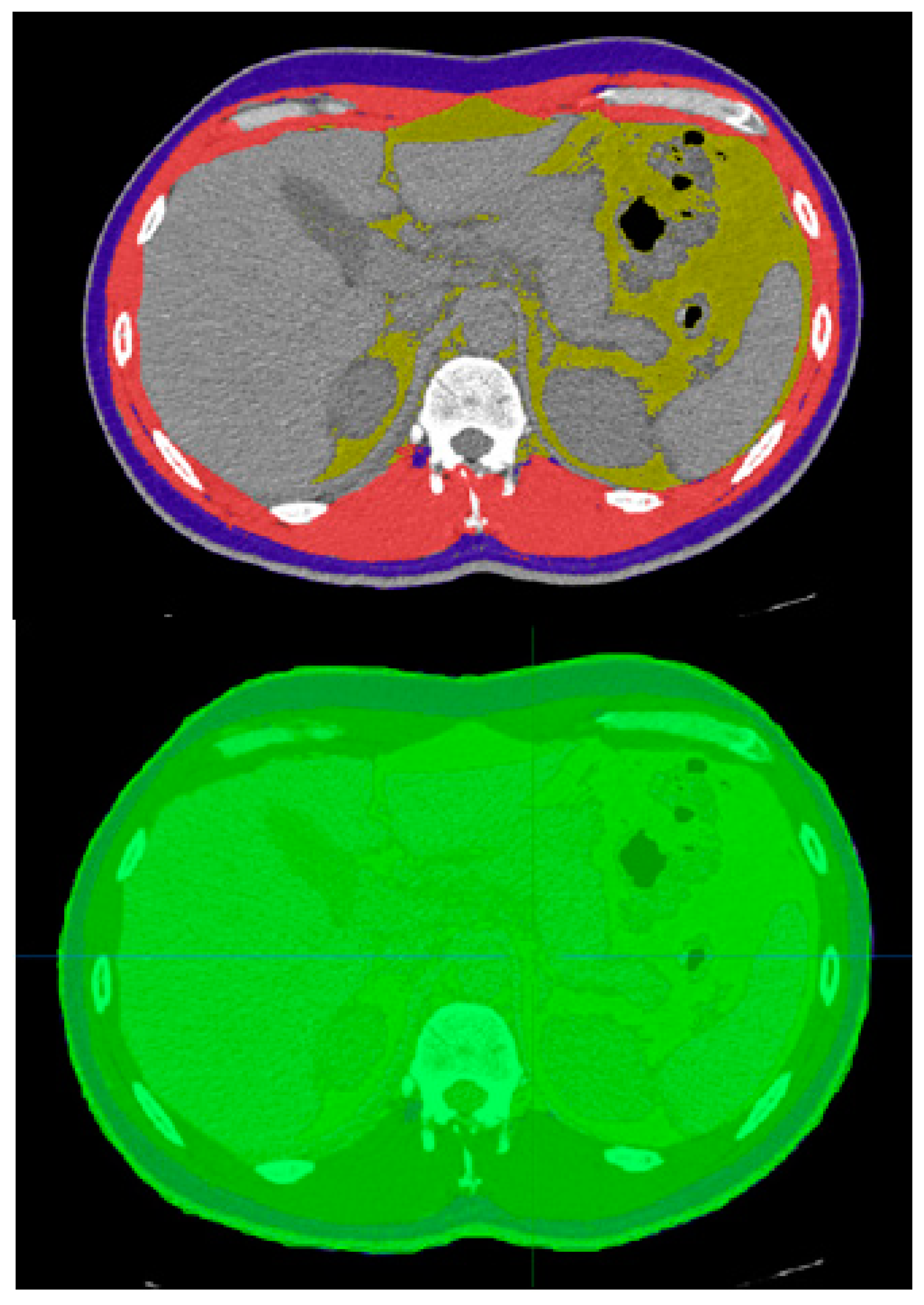
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Data Analysis
3. Results
3.1. Descriptive Results
3.2. Analytical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Estakhr, M.; Tabrizi, R.; Ghotbi, Z.; Shahabi, S.; Habibzadeh, A.; Bashi, A.; Borhani-Haghighi, A. Is facial nerve palsy an early manifestation of COVID-19? A literature review. Am. J. Med. Sci. 2022, 364, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, A.; Lankarani, K.B.; Farjam, M.; Akbari, M.; Kashani, S.M.A.; Karimimoghadam, Z.; Wang, K.; Imanieh, M.H.; Tabrizi, R.; Ahmadizar, F. Prevalence of fungal drug resistance in COVID-19 infection: A global meta-analysis. Curr. Fungal Infect. Rep. 2022, 16, 154–164. [Google Scholar] [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol.-Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.; Braun, W.; Pourhassan, M.; Schweitzer, L.; Glüer, C.-C.; Bosy-Westphal, A.; Müller, M.J. Gender-specific associations in age-related changes in resting energy expenditure (REE) and MRI measured body composition in healthy Caucasians. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 941–946. [Google Scholar] [CrossRef]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1057–1072. [Google Scholar] [CrossRef]
- Bahat, G.; Ilhan, B. Sarcopenia and the cardiometabolic syndrome: A narrative review. Eur. Geriatr. Med. 2016, 7, 220–223. [Google Scholar] [CrossRef]
- Schaap, L.A.; Van Schoor, N.M.; Lips, P.; Visser, M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: The longitudinal aging study Amsterdam. J. Gerontol. Ser. A 2018, 73, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Umegaki, H.; Makino, T.; Cheng, X.W.; Shimada, H.; Kuzuya, M. Association between sarcopenia and depressive mood in urban-dwelling older adults: A cross-sectional study. Geriatr. Gerontol. Int. 2019, 19, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Kastrinis, A.; Katsoulaki, M.; Billis, E.; Gliatis, J. Sarcopenia and its impact on quality of life. In GeNeDis 2016: Genetics and Neurodegeneration; Springer: Cham, Switzerland, 2017; pp. 213–218. [Google Scholar]
- Sipers, W.M.; de Blois, W.; Schols, J.M.; van Loon, L.J.; Verdijk, L.B. Sarcopenia is related to mortality in the acutely hospitalized geriatric patient. J. Nutr. Health Aging 2019, 23, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Fávaro-Moreira, N.C.; Krausch-Hofmann, S.; Matthys, C.; Vereecken, C.; Vanhauwaert, E.; Declercq, A.; Bekkering, G.E.; Duyck, J. Risk factors for malnutrition in older adults: A systematic review of the literature based on longitudinal data. Adv. Nutr. 2016, 7, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Vatic, M.; von Haehling, S.; Ebner, N. Inflammatory biomarkers of frailty. Exp. Gerontol. 2020, 133, 110858. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Royal jelly as an intelligent anti-aging agent—A focus on cognitive aging and Alzheimer’s disease: A review. Antioxidants 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Approaches to nutritional screening in patients with Coronavirus Disease 2019 (COVID-19). Int. J. Environ. Res. Public Health 2021, 18, 2772. [Google Scholar] [CrossRef]
- Tehrani, S.; Killander, A.; Åstrand, P.; Jakobsson, J.; Gille-Johnson, P. Risk factors for death in adult COVID-19 patients: Frailty predicts fatal outcome in older patients. Int. J. Infect. Dis. 2021, 102, 415–421. [Google Scholar] [CrossRef]
- Azzolino, D.; Saporiti, E.; Proietti, M.; Cesari, M. Nutritional considerations in frail older patients with COVID-19. J. Nutr. Health Aging 2020, 24, 696–698. [Google Scholar] [CrossRef]
- Sawaya, Y.; Ishizaka, M.; Kubo, A.; Shiba, T.; Hirose, T.; Onoda, K.; Maruyama, H.; Urano, T. Association between skeletal muscle mass index and lung function/respiratory muscle strength in older adults requiring long-term care or support. J. Phys. Ther. Sci. 2020, 32, 754–759. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Skeletal muscle damage in COVID-19: A call for action. Medicina 2021, 57, 372. [Google Scholar] [CrossRef]
- Haraj, N.E.; El Aziz, S.; Chadli, A.; Dafir, A.; Mjabber, A.; Aissaoui, O.; Barrou, L.; El Hamidi, C.E.K.; Nsiri, A.; Harrar, R.A. Nutritional status assessment in patients with COVID-19 after discharge from the intensive care unit. Clin. Nutr. ESPEN 2021, 41, 423–428. [Google Scholar] [CrossRef]
- Gualtieri, P.; Falcone, C.; Romano, L.; Macheda, S.; Correale, P.; Arciello, P.; Polimeni, N.; De Lorenzo, A. Body composition findings by computed tomography in SARS-CoV-2 patients: Increased risk of muscle wasting in obesity. Int. J. Mol. Sci. 2020, 21, 4670. [Google Scholar] [CrossRef] [PubMed]
- Pediconi, F.; Rizzo, V.; Schiaffino, S.; Cozzi, A.; Della Pepa, G.; Galati, F.; Catalano, C.; Sardanelli, F. Visceral adipose tissue area predicts intensive care unit admission in COVID-19 patients. Obes. Res. Clin. Pract. 2021, 15, 89–92. [Google Scholar] [CrossRef]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef]
- Rosenquist, K.J.; Pedley, A.; Massaro, J.M.; Therkelsen, K.E.; Murabito, J.M.; Hoffmann, U.; Fox, C.S. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc. Imaging 2013, 6, 762–771. [Google Scholar] [CrossRef]
- Molfino, A.; Imbimbo, G.; Rizzo, V.; Muscaritoli, M.; Alampi, D. The link between nutritional status and outcomes in COVID-19 patients in ICU: Is obesity or sarcopenia the real problem? Eur. J. Intern. Med. 2021, 91, 93–95. [Google Scholar] [CrossRef]
- Ng, C.C.; Lee, Z.Y.; Chan, W.Y.; Jamaluddin, M.F.; Tan, L.J.; Sitaram, P.N.; Ruslan, S.R.; Hasan, M.S. Low muscularity as assessed by abdominal computed tomography on intensive care unit admission is associated with mortality in a critically ill Asian population. J. Parenter. Enter. Nutr. 2020, 44, 425–433. [Google Scholar] [CrossRef]
- Toledo, D.O.; Carvalho, A.M.; Oliveira, A.M.; Toloi, J.M.; Silva, A.C.; de Mattos Farah, J.F.; Prado, C.M.; Silva, J.M., Jr. The use of computed tomography images as a prognostic marker in critically ill cancer patients. Clin. Nutr. ESPEN 2018, 25, 114–120. [Google Scholar] [CrossRef]
- Moisey, L.L.; Mourtzakis, M.; Cotton, B.A.; Premji, T.; Heyland, D.K.; Wade, C.E.; Bulger, E.; Kozar, R.A. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit. Care 2013, 17, R206. [Google Scholar] [CrossRef]
- Heyland, D.K.; Stapleton, R.D.; Mourtzakis, M.; Hough, C.L.; Morris, P.; Deutz, N.E.; Colantuoni, E.; Day, A.; Prado, C.M.; Needham, D.M. Combining nutrition and exercise to optimize survival and recovery from critical illness: Conceptual and methodological issues. Clin. Nutr. 2016, 35, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.; Bressem, K.; Albrecht, J.; Thieß, H.-M.; Vahldiek, J.; Hamm, B.; Makowski, M.R.; Niehues, A.; Niehues, S.M.; Adams, L.C. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism 2020, 110, 154317. [Google Scholar] [CrossRef]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, N.; Zeng, Y.; Xiao, B.; Wang, P.; Zhou, C.; Xia, Y.; Zhao, Z.; Xiao, T.; Li, H. COVID-19 and sarcopenia-related traits: A bidirectional Mendelian randomization study. Front. Endocrinol. 2023, 14, 1162936. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Boirie, Y.; Bensid, L.; Pereira, B.; Ghelis, N.; Dupuis, C.; Tournadre, A.; Boyer, L.; Cassagnes, L. Thoracic sarcopenia as a predictive factor of SARS-CoV-2 evolution. Clin. Nutr. 2022, 41, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Demerath, E.W.; Sun, S.S.; Rogers, N.; Lee, M.; Reed, D.; Choh, A.C.; Couch, W.; Czerwinski, S.A.; Chumlea, W.C.; Siervogel, R.M. Anatomical patterning of visceral adipose tissue: Race, sex, and age variation. Obesity 2007, 15, 2984–2993. [Google Scholar] [CrossRef]
- Després, J.-P.; Couillard, C.; Gagnon, J.; Bergeron, J.; Leon, A.S.; Rao, D.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: The Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1932–1938. [Google Scholar] [CrossRef]
| Subgroups | Outpatient | Ward | ICU | Dead | Total | p-Value | |
|---|---|---|---|---|---|---|---|
| Variables | |||||||
| Age (Years) | 20–40 | 11 (27.5) | 16 (40) | 5 (12.5) | 8 (20) | 40 | 0.42 |
| 40–60 | 9 (20.5) | 16 (36.4) | 13 (29.5) | 6 (13.6) | 44 | ||
| ≥60 | 7 (16.3) | 17 (39.5) | 5 (11.6) | 14 (32.6) | 43 | ||
| Gender | Male | 13 (20.6) | 24 (38.1) | 11 (17.5) | 15 (23.8) | 63 | 0.97 |
| Female | 14 (21.9) | 25 (39.1) | 12 (18.8) | 13 (20.3) | 64 |
| Variables | Mean ± Standard Deviation (Median) | Minimum | Maximum | |
|---|---|---|---|---|
| Quantities | ||||
| Total muscle/body % | 0.14 ± 0.028 (0.14) | 0.075 | 0.212 | |
| SQ fat/body % | 0.19 ± 0.095 (0.17) | 0.006 | 0.45 | |
| Variables | Outpatient | Ward | ICU | Dead | p-Value | |
|---|---|---|---|---|---|---|
| Subgroups | ||||||
| Total muscle/body % | 0.02 ± 0.157 (0.16) | 0.02 ± 0.139 (0.14) | 0.03 ± 0.143 (0.14) | 0.02 ± 0.135 (0.14) | 0.01 | |
| SQ fat/body % | 0.09 ± 0.18 (0.17) | 0.09 ± 0.199 (0.17) | 0.08 ± 0.169 (0.15) | 0.10 ± 0.203 (0.2) | 0.51 | |
| Variables | Sex | Mean ± SD | p | |
|---|---|---|---|---|
| Subgroups | ||||
| Total muscle/body % | Female | 0.130 ± 0.022 | <0.001 | |
| Male | 0.157 ± 0.028 | |||
| SQ fat % | Female | 0.251 ± 0.087 | <0.001 | |
| Male | 0.131 ± 0.061 | |||
| Variables | Mean ± SD | p | |
|---|---|---|---|
| Subgroups | |||
| SQ fat% | 20–40 | 0.193 ± 0.095 | 0.79 |
| 40–60 | 0.197 ± 0.104 | ||
| >60 | 0.184 ± 0.090 | ||
| Total | 0.191 ± 0.096 | ||
| Total muscle/body % | 20–40 | 0.152 ± 0.028 | 0.01 |
| 40–60 | 0.144 ± 0.027 | ||
| >60 | 0.134 ± 0.0274 | ||
| Total | 0.143 ± 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarei, F.; Sepahdar, A.; Saeedi-Moghadam, M.; Zeinali-Rafsanjani, B. Assessment of the Relationship between Pre-Existing Muscle Atrophy, Subcutaneous Fat Volume, and the Prognosis of COVID-19. J. Clin. Med. 2025, 14, 1154. https://doi.org/10.3390/jcm14041154
Zarei F, Sepahdar A, Saeedi-Moghadam M, Zeinali-Rafsanjani B. Assessment of the Relationship between Pre-Existing Muscle Atrophy, Subcutaneous Fat Volume, and the Prognosis of COVID-19. Journal of Clinical Medicine. 2025; 14(4):1154. https://doi.org/10.3390/jcm14041154
Chicago/Turabian StyleZarei, Fariba, Afrooz Sepahdar, Mahdi Saeedi-Moghadam, and Banafsheh Zeinali-Rafsanjani. 2025. "Assessment of the Relationship between Pre-Existing Muscle Atrophy, Subcutaneous Fat Volume, and the Prognosis of COVID-19" Journal of Clinical Medicine 14, no. 4: 1154. https://doi.org/10.3390/jcm14041154
APA StyleZarei, F., Sepahdar, A., Saeedi-Moghadam, M., & Zeinali-Rafsanjani, B. (2025). Assessment of the Relationship between Pre-Existing Muscle Atrophy, Subcutaneous Fat Volume, and the Prognosis of COVID-19. Journal of Clinical Medicine, 14(4), 1154. https://doi.org/10.3390/jcm14041154