Heart–Liver Interplay in Patients with Fontan Circulation
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Biochemistry Assesment
2.3. Assessment of Cardiac Performance
2.4. Liver Assessment
- -
- LSPS (liver stiffness-spleen size to platelet ratio)
- -
- APRI (AST to platelet ratio index)
- -
- FIB-4 (Fibrosis 4)
2.5. Statistical Analysis
3. Results
3.1. General Population: Clinical Data
3.1.1. Anatomical Findings
3.1.2. Functional Status
3.1.3. Laboratory Exams
3.1.4. Medication
3.1.5. Arrhythmias
3.2. Echocardiographic and Functional Data
3.2.1. Systolic Function
3.2.2. Diastolic Function
3.3. CPET Data
3.4. Liver Assessment in Fontan Circulation
3.4.1. Laboratory Exams
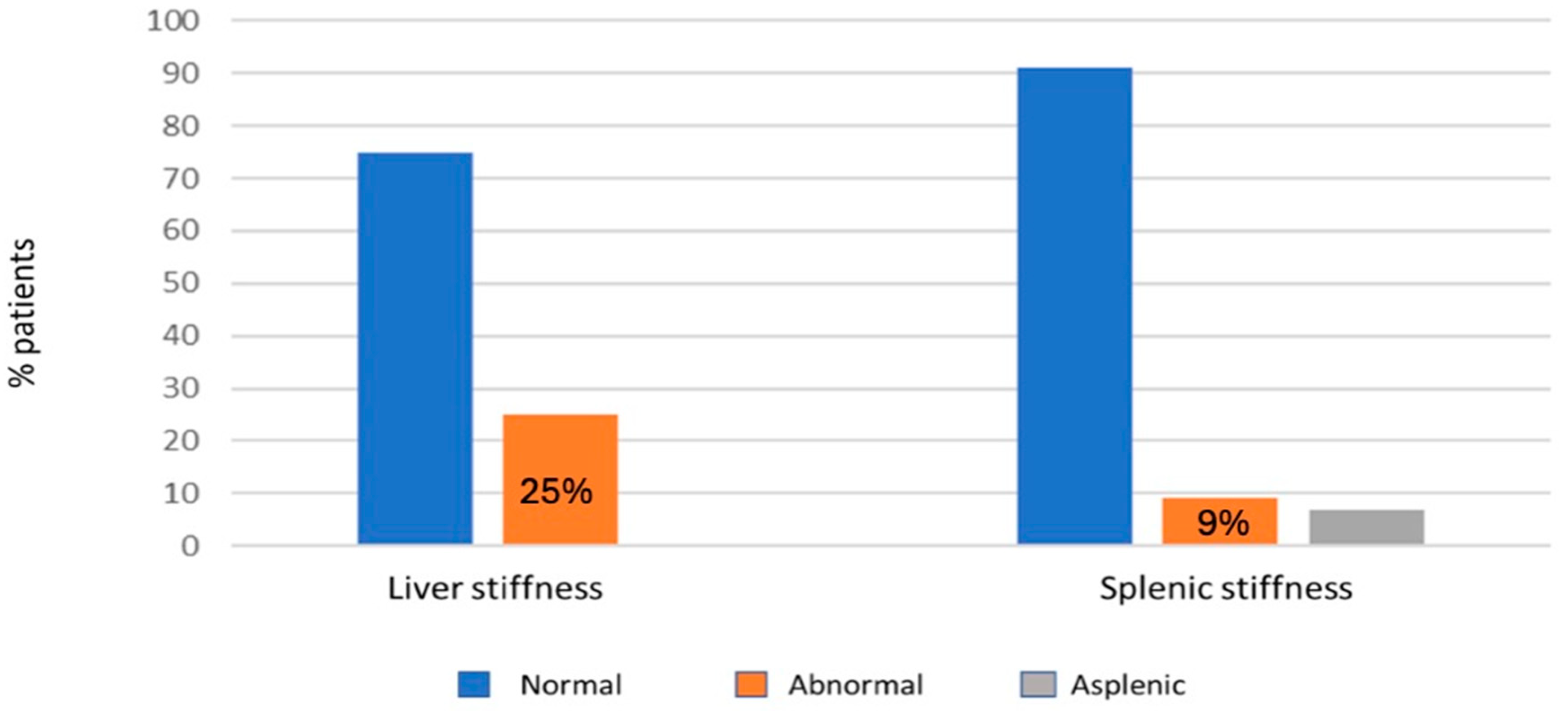
3.4.2. Fibroscan Data
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAVV | systemic atrio-ventricular valve |
| SAVVR | systemic atrio-ventricular valve regurgitation |
| TAPSE | tricuspid annular plane systolic excursion |
| MAPSE | mitral annular plane systolic excursion |
| EF | ejection fraction |
| FAC | fractional area change |
| GLS | global longitudinal strain |
| STE | speckle tracking echocardiography |
| NYHA | New York Heart Association |
| IVC | inferior vena cava |
| CPET | cardiopulmonary exercise test |
| VO2 max | maximum oxygen consumption |
| PLE | protein losing enteropathy |
| FALD | Fontan-associated liver disease |
| LSPS | liver stiffness-spleen size to platelet ratio |
| APRI | AST to platelet ratio index |
| FIB-4 | Fibrosis 4 |
References
- Khairy, P.; Poirier, N.; Mercier, L.-A. Univentricular heart. Circulation 2007, 115, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Schwedler, G.; Lindinger, A.; Lange, P.E.; Sax, U.; Olchvary, J.; Peters, B.; Bauer, U.; Hense, H.-W. Frequency and spectrum of congenital heart defects among live births in Germany: A study of the Competence Network for Congenital Heart Defects. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2011, 100, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- de Leval, M.R.; Kilner, P.; Gewillig, M.; Bull, C. Total cavopulmonary connection: A logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J. Thorac. Cardiovasc. Surg. 1988, 96, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, I.; McCracken, C.E.; Petit, C.J.; Sachdeva, R. Late outcomes after the Fontan procedure in patients with single ventricle: A meta-analysis. Heart Br. Card. Soc. 2018, 104, 1508–1514. [Google Scholar] [CrossRef]
- Poh, C.L.; d’Udekem, Y. Life After Surviving Fontan Surgery: A Meta-Analysis of the Incidence and Predictors of Late Death. Heart Lung Circ. 2018, 27, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.; Zanella, L.; Castaldi, B.; Di Salvo, G. How Will Artificial Intelligence Shape the Future of Decision-Making in Congenital Heart Disease? J. Clin. Med. 2024, 13, 2996. [Google Scholar] [CrossRef]
- Gewillig, M.; Goldberg, D.J. Failure of the fontan circulation. Heart Fail. Clin. 2014, 10, 105–116. [Google Scholar] [CrossRef]
- Gnanappa, G.K.; Celermajer, D.S.; Sholler, G.F.; Gentles, T.; Winlaw, D.; d’Udekem, Y.; Ayer, J. The Long-Term Management of Children and Adults with a Fontan Circulation: A Systematic Review and Survey of Current Practice in Australia and New Zealand. Pediatr. Cardiol. 2017, 38, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, L.; Zhou, Z.; Tu, J.; Ma, J.; Chen, J. Effect of liver abnormalities on mortality in Fontan patients: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2024, 24, 385. [Google Scholar] [CrossRef] [PubMed]
- Padalino, M.A.; Chemello, L.; Cavalletto, L.; Angelini, A.; Fedrigo, M. Prognostic Value of Liver and Spleen Stiffness in Patients with Fontan Associated Liver Disease (FALD): A Case Series with Histopathologic Comparison. J. Cardiovasc. Dev. Dis. 2021, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Walker, T.T.; Bove, K.; Veldtman, G. Fontan-associated liver disease: A review. J. Cardiol. 2019, 74, 223–232. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Surrey, L.F.; Glatz, A.C.; Dodds, K.; O’Byrne, M.L.; Lin, H.C.; Fogel, M.; Rome, J.J.; Rand, E.B.; Russo, P.; et al. Hepatic Fibrosis Is Universal Following Fontan Operation, and Severity is Associated With Time From Surgery: A Liver Biopsy and Hemodynamic Study. J. Am. Heart Assoc. 2017, 6, e004809. [Google Scholar] [CrossRef]
- Chemello, L.; Padalino, M.; Zanon, C.; Benvegnu’, L.; Biffanti, R.; Mancuso, D.; Cavalletto, L. Role of Transient Elastography to Stage Fontan-Associated Liver Disease (FALD) in Adults with Single Ventricle Congenital Heart Disease Correction. J. Cardiovasc. Dev. Dis. 2021, 8, 117. [Google Scholar] [CrossRef]
- Gewillig, M.; Brown, S.C. The Fontan circulation after 45 years: Update in physiology. Heart Br. Card. Soc. 2016, 102, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Margossian, R.; Sleeper, L.A.; Pearson, G.D.; Barker, P.C.; Mertens, L.; Quartermain, M.D.; Su, J.T.; Shirali, G.; Chen, S.; Colan, S.D.; et al. Assessment of Diastolic Function in Single-Ventricle Patients After the Fontan Procedure. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016, 29, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Martin Talavera, M.; Manso, B.; Cejudo Ramos, P.; Rodriguez Puras, M.J.; Wals Rodriguez, A.J.; Garcia de Vinuesa, P.G. Determinants of oxygen uptake and prognostic factors in cardiopulmonary exercise test in patients with Fontan surgery. Cardiol. Young 2022, 32, 1285–1288. [Google Scholar] [CrossRef]
- Arvanitaki, A.; Frigiola, A.; Iannaccone, G.; Montanaro, C. Benefit of Exercise in Patients with a Fontan Circulation. Int. J. Cardiol. 2023, 392, 131288. [Google Scholar] [CrossRef]
- de Franchis, R. Baveno VI Faculty Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- Liu, C.; Ji, D.; Huang, F.; Huang, Y.; Du, X.; Liu, C.; Mao, X.; Zhang, Q.; Fang, C.; Ju, S.; et al. Accuracy of liver stiffness-based model by different imaging modalities in compensated advanced chronic liver disease. Eur. J. Gastroenterol. Hepatol. 2020, 32, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.S.; Hoque, M.N.; Azam, M.G.; Kabir, M.A.; Islam, M.S.; Mamoon, M.A.; Ahmed, S.; Siddiqui, N.I. Prediction of Esophageal Varices in Chronic Liver Disease by Liver Stiffness-Spleen Size-to-Plalelet Ratio Risk Score. Mymensingh Med. J. 2021, 30, 115–122. [Google Scholar] [PubMed]
- Jarasvaraparn, C.; Thoe, J.; Rodenbarger, A.; Masuoka, H.; Payne, R.M.; Markham, L.W.; Molleston, J.P. Biomarkers of fibrosis and portal hypertension in Fontan-associated liver disease in children and adults. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2024, 56, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Martin de Miguel, I.; Kamath, P.S.; Egbe, A.C.; Jain, C.C.; Cetta, F.; Connolly, H.M.; Miranda, W.R. Haemodynamic and prognostic associations of liver fibrosis scores in Fontan-associated liver disease. Heart Br. Card. Soc. 2023, 109, 619–625. [Google Scholar] [CrossRef] [PubMed]
- d’Udekem, Y.; Iyengar, A.J.; Galati, J.C.; Forsdick, V.; Weintraub, R.G.; Wheaton, G.R.; Bullock, A.; Justo, R.N.; Grigg, L.E.; Sholler, G.F.; et al. Redefining expectations of long-term survival after the Fontan procedure: Twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 2014, 130, S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Laubham, M.; Blais, B.; Kamp, A.N. Atrial Arrhythmias in Adults with Fontan Palliation. Cardiol. Ther. 2023, 12, 473–487. [Google Scholar] [CrossRef]
- Stephenson, E.A.; Lu, M.; Berul, C.I.; Etheridge, S.P.; Idriss, S.F.; Margossian, R.; Reed, J.H.; Prakash, A.; Sleeper, L.A.; Vetter, V.L.; et al. Arrhythmias in a contemporary fontan cohort: Prevalence and clinical associations in a multicenter cross-sectional study. J. Am. Coll. Cardiol. 2010, 56, 890–896. [Google Scholar] [CrossRef]
- Menon, S.C.; Gray, R.; Tani, L.Y. Evaluation of ventricular filling pressures and ventricular function by Doppler echocardiography in patients with functional single ventricle: Correlation with simultaneous cardiac catheterization. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2011, 24, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Pomiato, E.; Palmieri, R.; Di Già, G.; Piemonte, F.; Porzio, O.; Gagliardi, M.G. The Effects of Exercise Training on Cardiopulmonary Exercise Testing and Cardiac Biomarkers in Adult Patients with Hypoplastic Left Heart Syndrome and Fontan Circulation. J. Cardiovasc. Dev. Dis. 2022, 9, 171. [Google Scholar] [CrossRef]
- Egbe, A.C.; Driscoll, D.J.; Khan, A.R.; Said, S.S.; Akintoye, E.; Berganza, F.M.; Connolly, H.M. Cardiopulmonary exercise test in adults with prior Fontan operation: The prognostic value of serial testing. Int. J. Cardiol. 2017, 235, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kresoja, K.-P. Exercise haemodynamics in post-Fontan patients: Diastolic dysfunction, again and again? Eur. J. Heart Fail. 2024, 26, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Paridon, S.M.; Mitchell, P.D.; Colan, S.D.; Williams, R.V.; Blaufox, A.; Li, J.S.; Margossian, R.; Mital, S.; Russell, J.; Rhodes, J.; et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J. Am. Coll. Cardiol. 2008, 52, 99–107. [Google Scholar] [CrossRef]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.-Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e234–e284. [Google Scholar] [CrossRef]
- Schleiger, A.; Kramer, P.; Salzmann, M.; Danne, F.; Schubert, S.; Bassir, C.; Müller, T.; Tacke, F.; Müller, H.-P.; Berger, F.; et al. Evaluation of Fontan failure by classifying the severity of Fontan-associated liver disease: A single-centre cross-sectional study. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Colecchia, A.; Colli, A.; Casazza, G.; Mandolesi, D.; Schiumerini, R.; Reggiani, L.B.; Marasco, G.; Taddia, M.; Lisotti, A.; Mazzella, G.; et al. Spleen stiffness measurement can predict clinical complications in compensated HCV-related cirrhosis: A prospective study. J. Hepatol. 2014, 60, 1158–1164. [Google Scholar] [CrossRef]
- Simmons, M.A.; Revzin, M.; To, U.; Liapakis, A.; Fahey, J.; Elder, R.W. A window into portal hemodynamics in adult fontan patients? Int. J. Cardiol. 2021, 323, 61–64. [Google Scholar] [CrossRef]
- Aliyev, B.; Bayramoglu, Z.; Nişli, K.; Omeroğlu, R.E.; Dindar, A. Quantification of Hepatic and Splenic Stiffness After Fontan Procedure in Children and Clinical Implications. Ultrasound Q. 2020, 36, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Egbe, A.C.; Miranda, W.R.; Anderson, J.H.; Borlaug, B.A. Hemodynamic and Clinical Implications of Impaired Pulmonary Vascular Reserve in the Fontan Circulation. J. Am. Coll. Cardiol. 2020, 76, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Bano, M.; Hussain, T.; Samels, M.R.; Butts, R.J.; Kirk, R.; Levine, B.D. Cardiovascular remodelling in response to exercise training in patients after the Fontan procedure: A pilot study. Cardiol. Young 2024, 34, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Dipchand, A.I.; Honjo, O.; Alonso-Gonzalez, R.; McDonald, M.; Roche, S.L. Heart Transplant Indications, Considerations, and Outcomes in Fontan Patients: Age-Related Nuances, Transplant Listing, and Disease-Specific Indications. Can. J. Cardiol. 2022, 38, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J.; Reardon, L.C.; Aboulhosn, J.; Haeffele, C.; Chen, S.; Kim, Y.; Fuller, S.; Forbess, L.; Alshawabkeh, L.; Urey, M.A.; et al. Morbidity and Mortality in Adult Fontan Patients After Heart or Combined Heart-Liver Transplantation. J. Am. Coll. Cardiol. 2023, 81, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Fontan, F.; Kirklin, J.W.; Fernandez, G.; Costa, F.; Naftel, D.C.; Tritto, F.; Blackstone, E.H. Outcome after a “perfect” Fontan operation. Circulation 1990, 81, 1520–1536. [Google Scholar] [CrossRef] [PubMed]
| Demographics | Fontan Population (n70) |
| Age (years) on hospital admission (media ± SD) | 21 ± 9 |
| Male (n; %) | 37 (52.9%) |
| Years after surgery (media ± SD) | 16.4 ± 8.5 |
| Type of CHD (n; %) | |
| TA | 15 (21) |
| DORV | 7 (10) |
| PA/IVS | 8 (11) |
| HLHS | 13 (19) |
| MA | 4 (6) |
| DORV | 3 (6.7) |
| CAVC unbalanced | 5 (7) |
| DILV | 11 (16) |
| Criss-cross heart | 2 (3%) |
| Other | 5 (7) |
| Systemic ventricle physiology (n; %) | |
| SLV | 38 (54) |
| SRV | 25 (36) |
| Indeterminate | 7 (10) |
| Type of surgery (n; %) | |
| TCPC, intracardiac conduit | 15 (21) |
| TCPC, external conduit | 21 (49) |
| Bjork’s atrio-infundibular anastomoses | 6 (9) |
| Sp02 after surgery (media ± SD) | 92 ± 12.24 |
| NYHA class (n; %) | |
| I | 48 (72) |
| II-III | 19 (28) |
| IV | 0 |
| Biochemical data on admission (n;%); (media ± SD) | |
| NT-pro-BNP > 125 ng/L | 37 (55) |
| Hb (male population) | 19.2 ± 10.7 |
| Hb (female population) | 14.4 ± 1.63 |
| Lymphocytes (109/L) | 1.63 ± 1.4 |
| Treatment (n; %) | |
| Warfarin | 18 (26) |
| Cardioaspirin | 42 (61) |
| Beta-Blockers | 9 (13) |
| Vasodilators (n; %) | 22 (32) |
| Parameter | Value | Patients, n (%) |
|---|---|---|
| Functional single left ventricle | ||
| Mean EF | 58 ± 7% | |
| EF < 50% | 3 (8%) | |
| Functional single right ventricle | ||
| Mean FAC | 40 ± 8% | |
| FAC < 35% | 7 (23%) | |
| Diastolic function | 63 (90%) | |
| Correlation FAC- NT-proBNP value | Pearson’s r = −0.353 (p 0.071) | |
| Pathological E/A ratio (E/A < 1) | 4 (6%) | |
| Pathological E/E’ ratio (E/E’ > 12) | 11 (17%) | |
| Pathological E/E’ ratio (Margossian’s criteria) | 43% | |
| Pathological E/E’ ratio (after leg lifting test) | 60% | |
| Atrioventricular valve regurgitation | ||
| Competent or mild regurgitation | 29 (83%) | |
| Moderate or more regurgitation | 6 (17%) | |
| Functional single right ventricle with more than moderate regurgitation | 5 (20%) |
| Parameter | Mean ± SD |
|---|---|
| VO2 peak | 26.4 ± 7.7 |
| VO2 % predicted | 64.9 ± 13.9 |
| O2 pulse rest | 3.6 ± 1.1 |
| O2 pulse | 10.4 ± 2.7 |
| Delta O2 pulse | 6.9 ± 2.2 |
| O2 pulse % | 89.9 ± 15.8 |
| VE/VCO2 slope | 33.7 ± 6 |
| SpO2 rest | 95.5 ± 3.2 |
| SpO2 peak | 90.9 ± 4.2 |
| Delta SpO2 | −4.6 ± 2.5 |
| RER peak | 1.4 ± 1.2 |
| HR max | 151.7 ± 29.1 |
| HR % predicted | 78.7 ± 13.7 |
| PETCO2 rest | 26.6 ± 2.9 |
| PETCO2 peak | 32.1 ± 4.6 |
| Delta PETCO2 | 5.4 ± 3.4 |
| Parameter | Correlation Value | p-Value |
|---|---|---|
| Age | ||
| VO2 max | Pearson’s ρ −0.374 | 0.035 |
| Oxygen saturation | Pearson’s ρ −0.401 | 0.001 |
| Systolic function (EF, FAC) | - | - |
| Diastolic function (E/E’, E/A) | - | - |
| GLS | Pearson’s ρ 0.371 | 0.005 |
| IVC diameter | Pearson’s ρ 0.493 | <0.001 |
| IVC diameter | ||
| EF | Pearson’s ρ −0.378 | 0.018 |
| TAPSE | Pearson’s ρ −0.808 | 0.0019 |
| Time between birth and Fontan Palliation | ||
| IVC diameter | Pearson’s ρ 0.423 | <0.01 |
| GLS | Pearson’s ρ 0.430 | 0.001 |
| Functional VO2 max | ||
| NTproBNP | Pearson’s ρ −0.301 | 0.025 |
| Right systolic function (FAC%) | ||
| NTproBNP | Pearson’s ρ −0.353 | 0.071 |
| GLS | ||
| VO2 values | Pearson’s ρ −0.475 | 0.011 |
| Time since Fontan palliation | Pearson’s ρ −0.430 | 0.001 |
| Liver Stiffness (KPa) | Normal AV Valve or Mild Insuffiency (n,%) | ≥ Moderate AV Valve Insufficiency (n,%) | Total Patients (n) |
|---|---|---|---|
| >22 | 14 (20%) | 4 (6%) | 18 |
| <22 | 50 (71%) | 2 (3%) | 51 |
| Parameter | Correlation Value (Pearson’s ρ) | p-Value |
|---|---|---|
| GGT | ||
| Time since Fontan palliation | 0.503 | <0.001 |
| EF% | −0.403 | 0.011 |
| VO2 max | −0.355 | 0.046 |
| Age at admission | 0.396 | 0.001 |
| AST | ||
| VO2 max | −0.460 | 0.008 |
| Splenic stiffness | ||
| Hepatic stiffness | 0.673 | <0.001 |
| Platelet count | −0.397 | <0.001 |
| FIB-4 | ||
| IVC diameter | 0.369 | 0.002 |
| Time since Fontan palliation | 0.508 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biffanti, R.; Sabatino, J.; Pozza, A.; Chemello, L.; Cavalletto, L.; Gasperetti, A.; Padalino, M.; Di Salvo, G. Heart–Liver Interplay in Patients with Fontan Circulation. J. Clin. Med. 2025, 14, 1114. https://doi.org/10.3390/jcm14041114
Biffanti R, Sabatino J, Pozza A, Chemello L, Cavalletto L, Gasperetti A, Padalino M, Di Salvo G. Heart–Liver Interplay in Patients with Fontan Circulation. Journal of Clinical Medicine. 2025; 14(4):1114. https://doi.org/10.3390/jcm14041114
Chicago/Turabian StyleBiffanti, Roberta, Jolanda Sabatino, Alice Pozza, Liliana Chemello, Luisa Cavalletto, Andrea Gasperetti, Massimo Padalino, and Giovanni Di Salvo. 2025. "Heart–Liver Interplay in Patients with Fontan Circulation" Journal of Clinical Medicine 14, no. 4: 1114. https://doi.org/10.3390/jcm14041114
APA StyleBiffanti, R., Sabatino, J., Pozza, A., Chemello, L., Cavalletto, L., Gasperetti, A., Padalino, M., & Di Salvo, G. (2025). Heart–Liver Interplay in Patients with Fontan Circulation. Journal of Clinical Medicine, 14(4), 1114. https://doi.org/10.3390/jcm14041114








