1. Introduction
Breast cancer remains a significant global health concern, presenting persistent challenges despite substantial advancements in early detection techniques and the development of more effective treatment modalities [
1]. While these advancements have led to improved survival rates, breast cancer continues to account for a considerable proportion of cancer-related morbidity and mortality worldwide, underscoring the need for further refinement in patient management strategies. Accurate prognostic evaluation is particularly vital, as it provides clinicians with essential tools to tailor treatment approaches, optimize resource allocation, and offer patients a realistic understanding of their disease trajectory and survival expectations [
1]
Systemic inflammation is increasingly recognized as a driver of cancer progression, with SII reflecting complex interactions between immune and inflammatory responses. The activation of the systemic immune–inflammation index (SII) is influenced by the tumor microenvironment through the release of pro-inflammatory cytokines, chemokines, and growth factors [
2]. In the presence of breast cancer, the tumor and its microenvironment promote an inflammatory cascade characterized by elevated levels of neutrophils and platelets alongside suppressed lymphocyte activity. Neutrophils secrete matrix metalloproteinases (MMPs) and reactive oxygen species (ROS), which degrade extracellular matrices and facilitate tumor invasion [
3]. Platelets interact with tumor cells to enhance their adhesion and protect them from immune surveillance, while also promoting angiogenesis through the release of vascular endothelial growth factor (VEGF). This pro-inflammatory milieu not only supports tumor growth and metastasis but also contributes to immune evasion [
4]. Breast cancer-specific subtypes, such as HER2-enriched and triple-negative breast cancers, may exhibit heightened inflammatory responses due to their aggressive biological behavior and higher propensity to induce systemic inflammation [
2].
Among the various biomarkers of systemic inflammation, the systemic immune–inflammation index (SII) has gained increasing attention in recent years for its potential as a prognostic indicator in oncology [
5]. SII, a parameter derived from routine blood tests, is calculated using the formula (Platelet Count × Neutrophil Count)/Lymphocyte Count, reflecting the complex interplay between inflammation, coagulation, and immune activity [
5]. Elevated levels of SII have been associated with poor outcomes across a range of malignancies, including lung, colorectal, gastric, pancreatic, and gynecological cancers [
6]. In the context of breast cancer, SII is particularly noteworthy as it serves as a surrogate marker for systemic inflammation, with higher values often correlating with more aggressive disease phenotypes and poorer patient outcomes [
7,
8].
The link between elevated SII levels and adverse outcomes in breast cancer has been consistently highlighted in the literature. Higher SII levels have been associated with an increased risk of recurrence, disease progression, and mortality [
6]. This suggests that SII captures key elements of the inflammatory milieu that contribute to tumor aggressiveness and metastasis. Despite this, the precise biological mechanisms driving the association between elevated SII and poorer clinical outcomes remain incompletely understood, warranting further investigation. A deeper understanding of these mechanisms could refine SII’s clinical utility and support its integration into personalized treatment approaches, ultimately enabling clinicians to better stratify patients and optimize therapeutic strategies [
9].
Despite its potential, SII’s role in routine clinical practice is still a matter of ongoing debate. While systemic inflammation indices such as SII offer insights into tumor-host interactions, they have not demonstrated consistent predictive value for tailored treatment decisions in clinical practice. Some of the uncertainty stems from variability in cutoff values used to define high and low SII levels, as well as limited understanding of how SII interacts with other clinicopathological variables. Moreover, systemic inflammation is influenced by a multitude of factors beyond cancer biology, such as comorbidities, lifestyle factors, and concurrent infections, which could potentially confound its prognostic significance.
In light of these challenges, this study aims to contribute to the growing body of evidence on SII by comprehensively evaluating its prognostic significance in a cohort of breast cancer patients undergoing curative surgical resection. At our institution, the majority of patients in this study underwent Madden mastectomy, offering a well-defined clinical context for the investigation. By analyzing the relationship between SII and clinicopathological factors, as well as its impact on long-term outcomes such as recurrence and survival, this study seeks to clarify whether SII can serve as a reliable biomarker for risk stratification and personalized care. If validated, SII could become an accessible and cost-effective tool for guiding clinical decision-making, particularly in resource-limited settings where more advanced molecular diagnostics may not be feasible. The findings of this research aim to bridge the gap between promising preliminary data and practical clinical application, advancing the use of SII as part of a multifaceted approach to breast cancer management.
3. Results
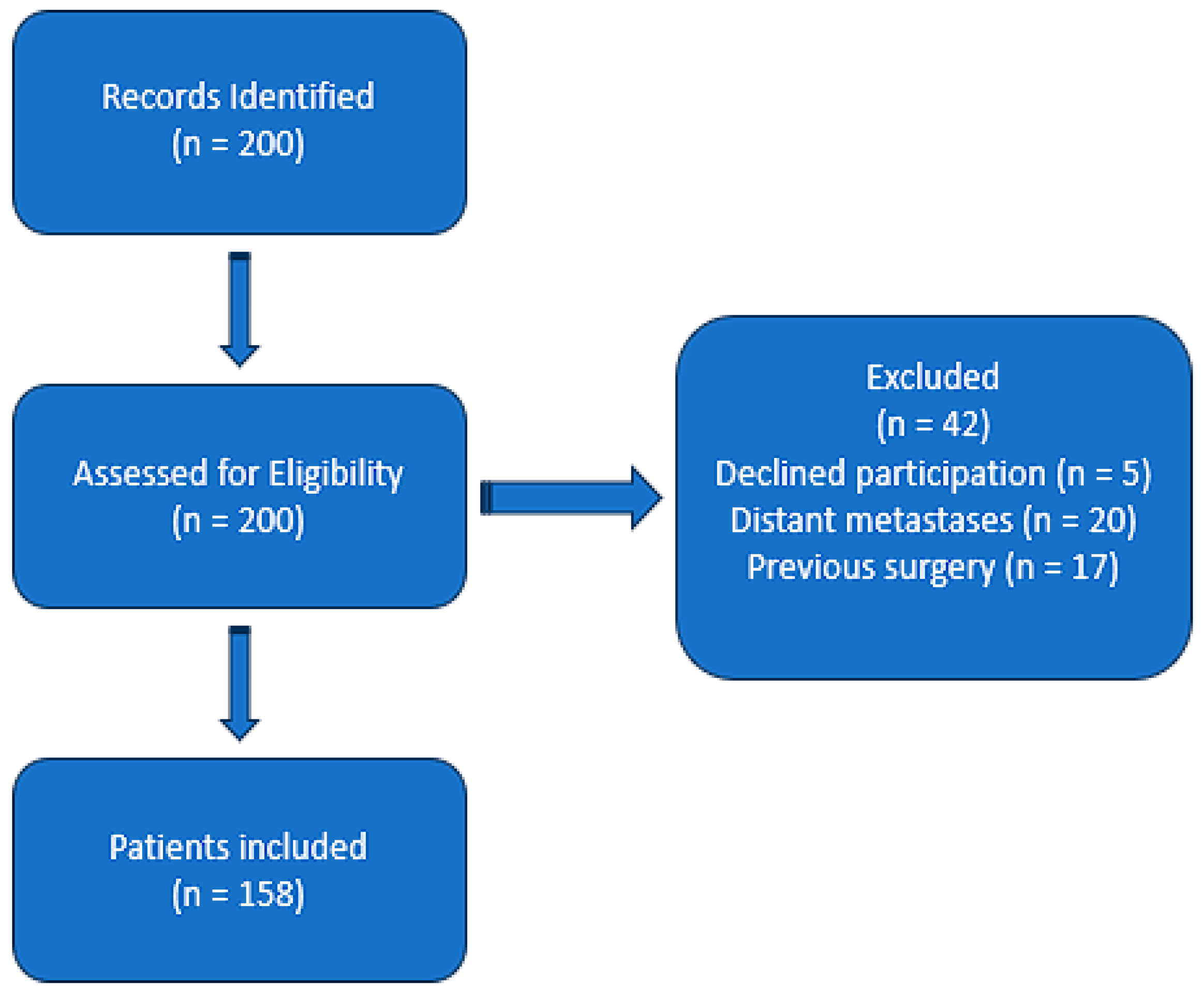
A total of 158 patients with histologically confirmed breast cancer were included in this study. As shown in
Table 1, an expanded analysis of baseline characteristics revealed that 49.6% of patients aged >50 years had diabetes, compared to 57.1% among patients aged <50 years. Similarly, hypertension was present in 48.9% of patients aged >50 years and 57.1% in those <50 years. Smoking history was noted in 49.6% of patients >50 years and 61.9% of those <50 years. BMI distributions showed that among patients aged >50 years, 31.4% were normal weight, 29.9% were classified as Obesity Class I, and 15.3% as Obesity Class II, with 23.4% categorized as overweight. Among younger patients (<50 years), 28.6% were normal weight, while 23.8% and 14.3% were classified as Obesity Class I and II, respectively, and 33.3% were overweight. The median follow-up duration was 24 months, with a range of 12–36 months, providing sufficient data for outcome analysis. All individuals had complete preoperative blood counts, enabling the systemic immune–inflammation index (SII) calculation. As shown in
Table 2, the mean patient age was 62.76 ± 11.15 years, ranging from 32 to 87 years. The mean SII value was 722.94 ± 447.88, with a minimum of 144.82 and a maximum of 3382.50, illustrating considerable heterogeneity in the inflammatory and immune-related profiles of the cohort. Baseline characteristics indicated a predominance of patients originating from urban areas, consistent with the referral patterns to our center (
Table 3). Tumor staging reflected a spectrum of disease severity, with T2 lesions being the most frequently encountered, and the majority of patients presenting with no regional lymph node involvement (N0). Regarding molecular and histopathological markers, a substantial proportion of tumors were hormone receptor-negative and lacked HER2 overexpression, though these distributions varied across the sample. These descriptive findings provided a diverse clinical substrate upon which to evaluate the prognostic significance of the SII.
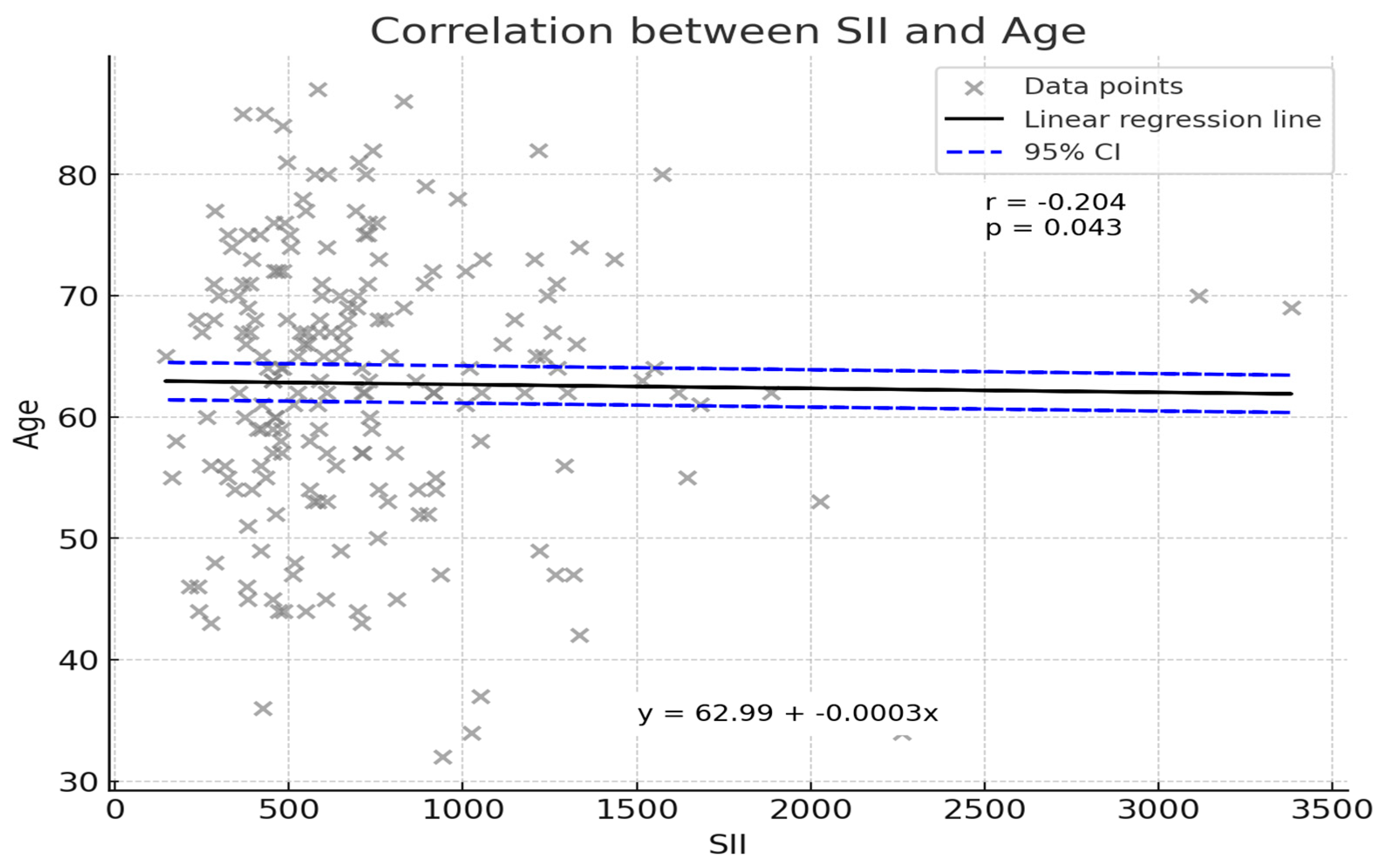
An initial correlation analysis sought to determine whether patient age was associated with SII values. As illustrated in
Figure 2, a weak but statistically significant inverse correlation was observed (Pearson’s r = −0.204,
p = 0.043). Although the strength of this relationship was modest, the finding indicates that younger patients tended to exhibit slightly higher SII values. The linear regression line (y = 62.99 − 0.0003x) suggests that for every unit increase in SII, patient age demonstrated a marginal decrease. While this association does not by itself confer clinical significance, it underscores the complexity of host–tumor interactions and the potential influence of patient demographic factors on systemic inflammatory responses.
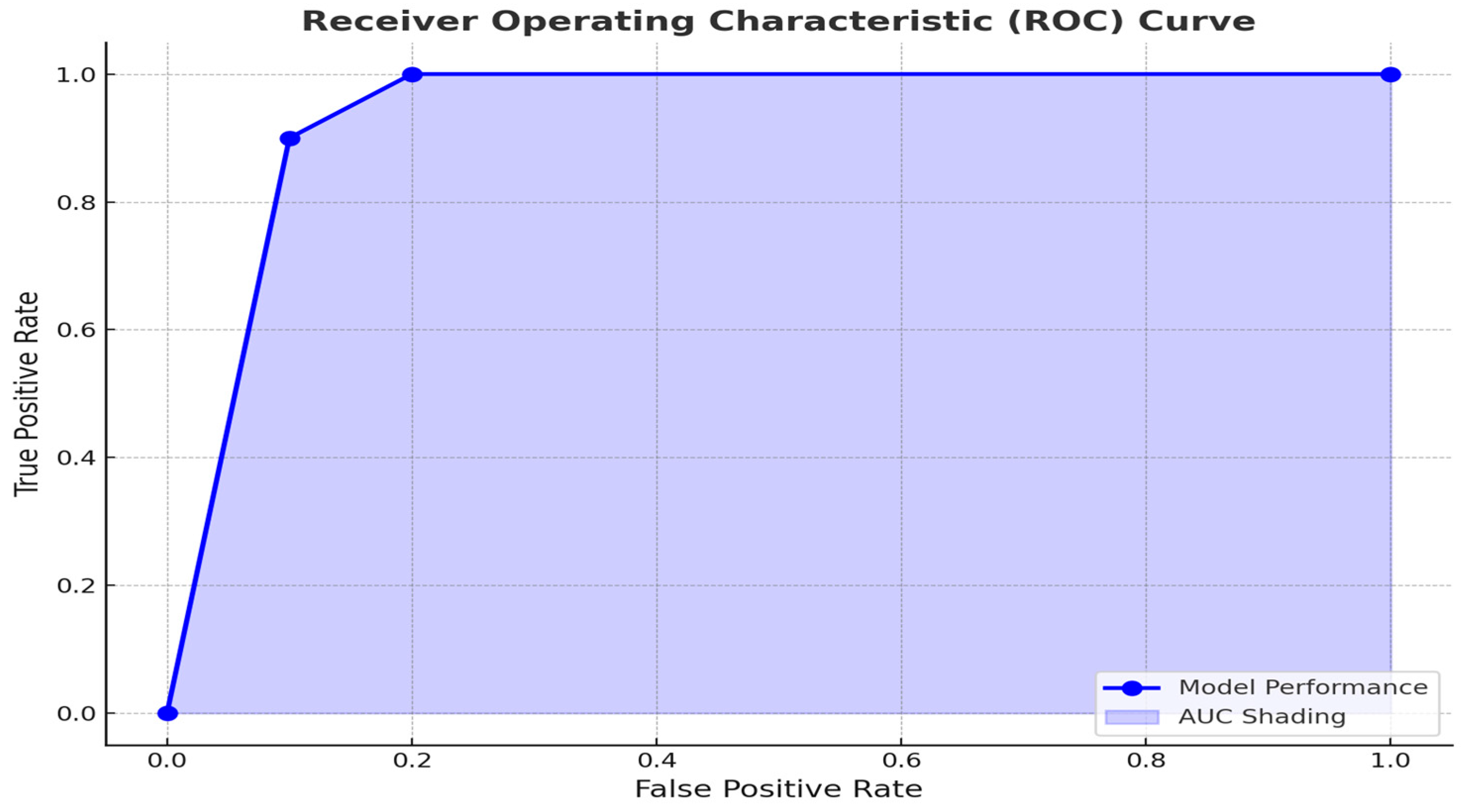
To explore the prognostic utility of the SII in predicting patient outcomes, we performed a receiver operating characteristic (ROC) curve analysis (
Figure 3). The ROC analysis revealed an optimal SII cutoff of 800, determined using the Youden index, which achieved a sensitivity of 75% and specificity of 68%. This threshold effectively stratified patients into distinct prognostic groups, with higher SII levels associated with advanced T stage and poorer outcomes. In comparison to other systemic inflammatory markers such as the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), SII offers a more integrated measure by incorporating platelet counts, which reflect coagulation pathways often activated in malignancy. This enhanced integration may explain its superior prognostic performance in some studies.
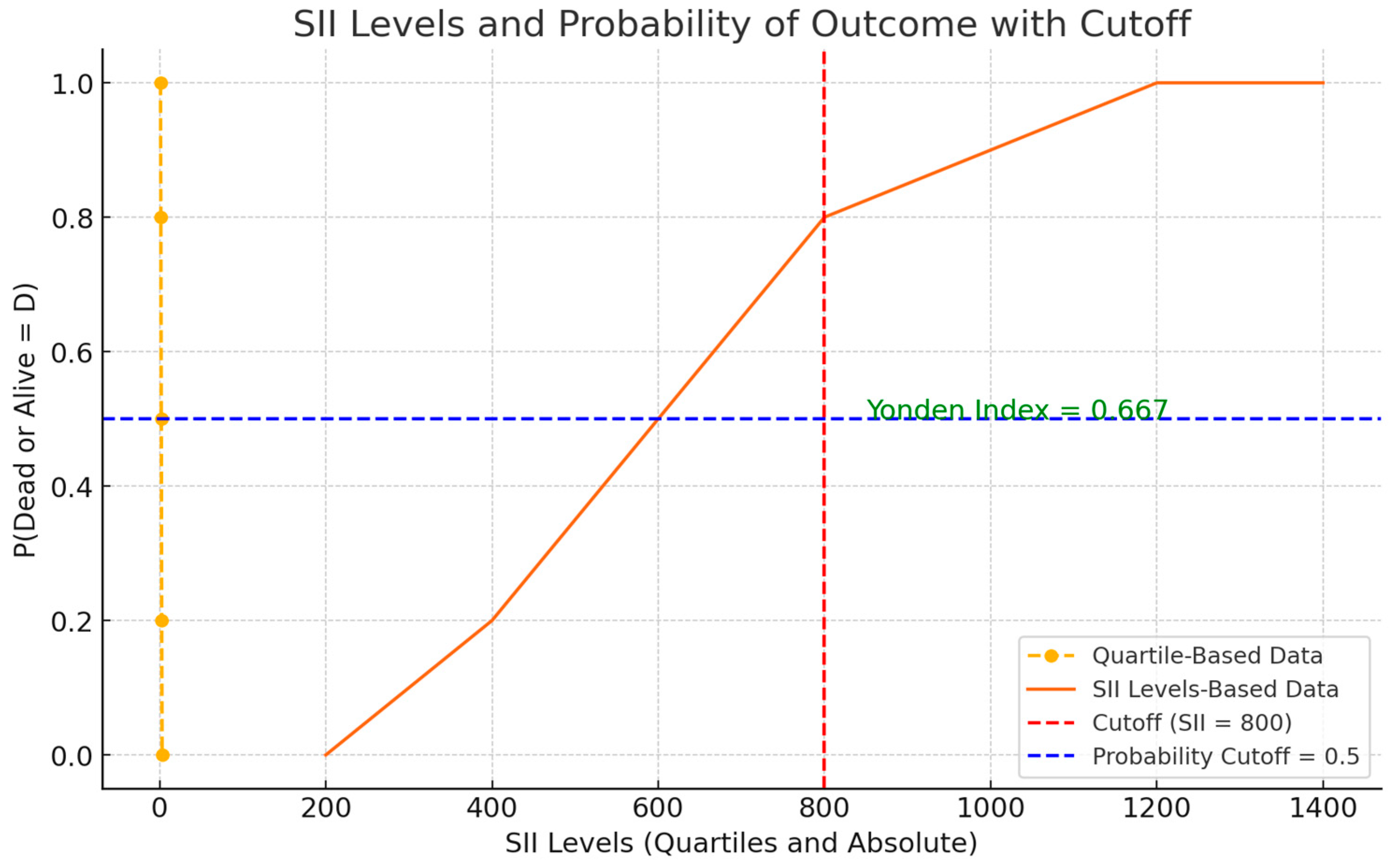
This analysis aimed to identify a threshold capable of stratifying patients into distinct prognostic categories, thereby enhancing the interpretability and clinical applicability of SII measurements. The chosen SII cutoff, determined by the maximal Youden index, provided an optimal balance between sensitivity and specificity. As depicted in
Figure 4, patients with SII values above the threshold of 800 were at a noticeably higher probability of a worse outcome over the observed follow-up period compared to those below it. Although this cutoff will require external validation, its derivation represents a crucial step toward integrating SII into routine prognostic assessments. These findings align with prior research demonstrating that systemic inflammatory markers, including SII, stratify patients into prognostic categories effectively [
11,
12].
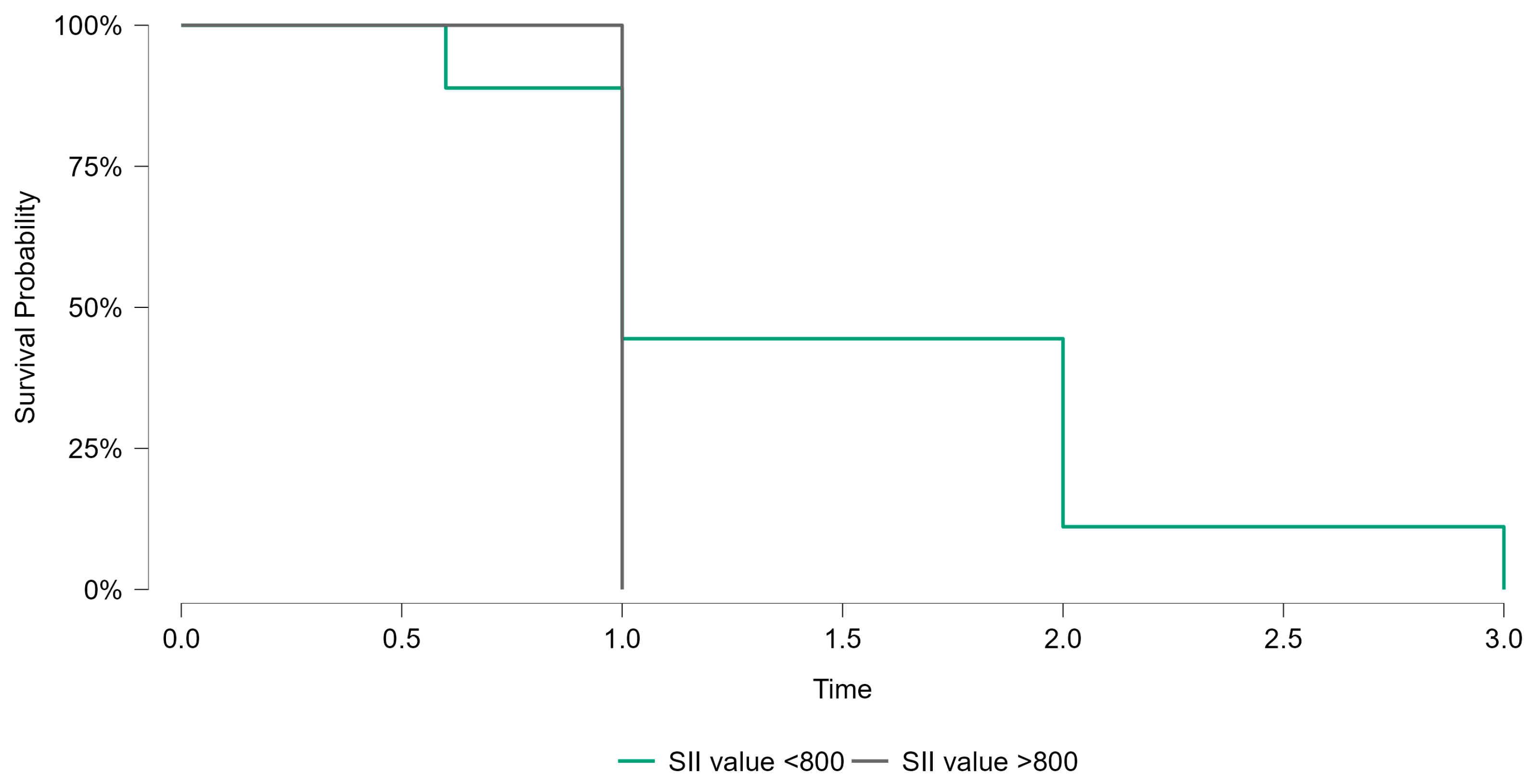
Kaplan–Meier survival analysis was performed to evaluate the prognostic significance of the systemic immune–inflammation index (SII) in breast cancer patients. Patients were stratified into two groups based on the identified cutoff value of SII (800). The survival probabilities over the follow-up period were compared between patients with SII values <800 and those with SII values >800. As shown in
Figure 5, the survival curves demonstrated no statistically significant difference between the two groups (
p = 0.415). While the trend suggested poorer survival outcomes in the high-SII group, the lack of statistical significance highlights the need for further studies with larger sample sizes to confirm these findings.
Having established an SII cutoff, the next step was to investigate potential associations between SII and key clinicopathological variables. Pearson’s correlation analysis, summarized in
Table 4, indicated a significant positive correlation between SII and T stage (r = 0.218,
p = 0.01), suggesting that patients with larger primary tumors tend to manifest higher SII values. Conversely, no statistically significant correlations emerged between SII and N stage (r = 0.004,
p = 0.96), HER2 status (r = 0.031,
p = 0.826), PR status (r = −0.073,
p = 0.302), or ER status (r = −0.075,
p = 0.29). Although a mild trend toward significance was observed in the relationship between SII and ki-67 (r = 0.246,
p = 0.088), it did not reach the conventional threshold for statistical significance. These findings highlight the specificity of the SII–tumor relationship and underscore T stage as a particularly relevant variable in the context of systemic inflammatory and immune responses.
A one-way ANOVA was conducted to further elucidate the relationship between SII and categorical predictors, including T stage, N stage, histological stage, HER2 status, PR status, ER status, proliferative index (ki-67), and patient age group (
Table 5). Of these parameters, only T stage exhibited a statistically significant effect on SII (F = 3.049,
p = 0.019), corroborating our correlation results and reinforcing the notion that tumor burden at the time of diagnosis is closely aligned with systemic immune–inflammatory conditions.
To assess the prognostic value of SII in the broader clinical context, we constructed logistic regression models evaluating the likelihood of poor outcomes (i.e., death) based on SII levels and other covariates. As depicted in
Table 6, the fitted model (H
1) demonstrated a significantly better fit compared to the null model (H
0) (Χ
2 = 32.906, df = 81,
p = 0.001). Improvements in deviance, AIC, and BIC, as well as elevated pseudo-R
2 values (e.g., McFadden R
2 = 0.737; Nagelkerke R
2 = 0.781), indicate that the inclusion of SII and related clinicopathological factors substantially enhanced the model’s explanatory power. These results suggest that incorporating SII into a composite prognostic framework can refine risk stratification and improve outcome prediction for patients with breast cancer.
Collectively, our results demonstrate that SII, a readily available parameter derived from routine preoperative blood counts, correlates meaningfully with tumor size and exhibits prognostic utility when evaluated through ROC-based thresholds. While the association between SII and age is subtle, the significant relationship with T stage and the improved fit of logistic regression models that include SII underscore its potential value as a complement to established prognostic markers. By integrating SII into a multifaceted assessment, clinicians may gain deeper insights into the host–tumor interplay, ultimately informing more nuanced patient management strategies and individualized treatment plans.
4. Discussion
This study adds to the growing body of evidence supporting SII as a prognostic marker in oncology. While SII’s role has been explored in various cancers, its application within a regional Romanian cohort of breast cancer patients provides a novel perspective. By integrating SII with clinicopathological factors, the findings underscore its potential to complement existing prognostic tools and enhance risk stratification in clinical practice [
13].
A recent meta-analysis reinforced the prognostic value of inflammatory indices, including SII, across multiple cancer types [
14]. Elevated SII levels were found to correlate significantly with advanced tumor (T) stage, suggesting a link between systemic inflammation and tumor burden. These findings align with the growing body of evidence that systemic inflammation plays a pivotal role in cancer progression, contributing to tumor growth, immune evasion, angiogenesis, and metastasis [
1,
8,
15].
The significant association between SII and T stage highlights the importance of tumor size in driving systemic inflammatory responses. The observed correlations between tumor size and systemic inflammatory markers emphasize the dynamic interplay between tumor biology and the host immune response as larger tumors likely generate a higher inflammatory burden, which is reflected in elevated SII levels [
16]. This relationship supports the hypothesis that SII serves as a surrogate marker for the tumor’s impact on the host’s immune-inflammatory milieu [
17]. While no significant associations were observed between SII and other clinicopathological factors, such as N stage or hormonal receptor status, the strong relationship with tumor size suggests that SII captures specific aspects of tumor biology not necessarily linked to these parameters. This specificity may enhance its utility as an adjunctive prognostic tool, particularly in clinical scenarios where traditional markers are less informative.
Despite the significant associations observed between SII and advanced tumor stage, the lack of correlation between SII and certain clinicopathological factors, such as hormone receptor status, deserves further consideration. One possible explanation for these non-significant findings could be the inherent variability in tumor biology. Hormone receptor-positive breast cancers, particularly those with lower-grade or luminal A phenotypes, often exhibit less aggressive inflammatory profiles compared to triple-negative or HER2-enriched subtypes, which may dilute the overall relationship between SII and receptor status [
7,
9]. Additionally, the sample size in this study, while adequate for broader comparisons, may have been insufficient to detect subtle differences within smaller subgroups, such as HER2-positive or ki-67-high tumors [
15,
18]. Another plausible factor is the multifactorial nature of systemic inflammation, which may be driven by processes beyond tumor biology, such as co-morbidities, lifestyle factors, or the presence of subclinical infections [
6]. These findings emphasize the need for further studies that explore the interaction between SII and breast cancer subtypes in larger, more diverse populations to uncover potential subgroup-specific patterns [
19]. By addressing these nuances, future research can refine the understanding of SII’s prognostic value in breast cancer.
Interestingly, the observed weak inverse correlation between SII and age (r = −0.204,
p = 0.043) provides additional context for the complex interplay between patient demographics and immune response. Younger patients demonstrated marginally higher SII levels, potentially reflecting more robust immune and inflammatory activity in younger individuals compared to older patients. This observation, while modest, warrants further exploration to elucidate whether age-specific variations in immune function influence systemic inflammation and cancer prognosis [
5].
The receiver operating characteristic (ROC) curve analysis identified an optimal SII cutoff of 800, effectively stratifying patients into low- and high-risk groups. Patients with SII values above this threshold exhibited poorer prognostic features, such as advanced T stage, and were more likely to experience adverse outcomes. These results are consistent with previous studies demonstrating that elevated SII levels are associated with increased risks of recurrence, metastasis, and mortality [
6,
19]. By using a simple and accessible biomarker to stratify patients, clinicians may be better equipped to identify high-risk individuals who could benefit from closer monitoring, more aggressive treatment strategies, or participation in clinical trials.
The prognostic utility of SII was further validated through logistic regression models, which demonstrated that SII provides additional predictive value beyond standard clinicopathological markers. This reinforces the notion that SII captures unique aspects of systemic inflammation not fully accounted for by traditional variables. Importantly, SII is cost-effective and easily implementable in clinical settings, making it particularly valuable in resource-limited environments where advanced molecular diagnostics may be unavailable [
9]
Systemic inflammation is increasingly recognized as a critical driver of cancer progression. Chronic inflammation can create a pro-tumorigenic microenvironment by promoting angiogenesis, increasing vascular permeability, and facilitating immune evasion [
20]. This connection between inflammation and cancer was first proposed by Rudolf Virchow in the 19th century, who observed leukocytes in tumor tissues and hypothesized a link between inflammation and malignancy [
21]. Modern research has confirmed and expanded on this hypothesis, illustrating how inflammatory mediators such as cytokines and chemokines contribute to tumor growth, metastasis, and immune suppression [
21]. Furthermore, inflammatory cytokines released by tumors can stimulate the recruitment of neutrophils and platelets while suppressing lymphocyte activity, leading to elevated SII values. The observed correlation between SII and tumor size in this study aligns with the understanding that larger tumors exert greater systemic inflammatory effects, exacerbating the host’s immune dysregulation [
9]
Despite the significant association of SII with advanced tumor stages, its lack of correlation with HER2-enriched and triple-negative subtypes warrants caution. These subtypes often exhibit aggressive behaviors but may not always trigger proportional systemic inflammatory responses, as evidenced by the current findings. This limitation may stem from sample heterogeneity or insufficient statistical power to detect subtle differences within subgroups. Stratifying larger cohorts by molecular subtype in future studies could uncover nuanced relationships, enhancing the clinical applicability of SII in personalized oncology. While SII captures certain inflammatory processes, it may not comprehensively reflect all aspects of the tumor’s interaction with the immune system. For example, the non-significant relationship between SII and ki-67, a marker of proliferation, suggests that SII may be more indicative of systemic effects than direct tumor cell activity [
22]. The absence of BRCA mutation and neoadjuvant chemotherapy data limits the generalizability of these findings. Future studies incorporating these variables would provide more comprehensive insights into SII’s role across diverse patient subgroups. Further research is needed to elucidate the specific pathways linking SII to breast cancer outcomes [
23].
This study’s limitations include its retrospective design, single-center setting, and limited sample size, which may affect generalizability. Additionally, unmeasured confounders such as lifestyle factors or subclinical inflammation could have influenced the findings. Prospective, multicenter studies are warranted.
Furthermore, the single-center setting may restrict the generalizability of the findings. Systemic inflammatory markers like SII can also be influenced by non-cancer-related factors, including comorbidities and lifestyle variables, which were not comprehensively accounted for in this analysis. To address these limitations, future studies should employ prospective, multicenter designs and incorporate broader patient populations to validate the findings. Additionally, exploring the impact of interventions targeting systemic inflammation, such as anti-inflammatory therapies, could provide insights into SII’s role in treatment response.
The generalizability of SII findings requires further validation in diverse populations, as systemic biomarkers are influenced by multi-level factors such as comorbidities and ethnicity [
24]. Additionally, while the SII cutoff of 800 was derived through robust ROC analysis, its external validity requires confirmation in larger, prospective cohorts with diverse demographic and clinical characteristics.
Future studies should also investigate the biological mechanisms underlying the observed associations between elevated SII and poorer outcomes. For instance, it remains unclear whether interventions targeting systemic inflammation, such as anti-inflammatory agents or lifestyle modifications, could mitigate the adverse prognostic impact of high SII. Furthermore, integrating SII with other biomarkers, such as circulating tumor cells or genomic signatures, may provide a more comprehensive understanding of cancer prognosis and lead to the development of personalized therapeutic approaches [
25].
The findings of this study have important implications for clinical practice. SII’s simplicity and cost-effectiveness make it a promising adjunct in resource-limited settings. Further studies should assess its role in guiding treatment decisions, including its integration into existing systems like the TNM classification. However, as a simple and cost-effective biomarker, SII has the potential to complement existing prognostic tools and inform treatment decisions in breast cancer. By identifying high-risk patients through SII stratification, clinicians can prioritize intensive surveillance and optimize resource allocation. Additionally, the accessibility of SII makes it particularly attractive for use in low-resource settings, where advanced diagnostic technologies may not be feasible. However, translating these findings into routine practice requires robust validation and standardization of SII cutoff values across different populations and healthcare systems. Future studies should integrate SII with other established biomarkers to enhance prognostic accuracy and provide a comprehensive assessment of patient risk [
26].
5. Conclusions
This study demonstrates that the systemic immune–inflammation index (SII) shows potential as a prognostic marker in breast cancer, particularly for stratifying patients by risk and identifying associations with clinical outcomes [
8]. However, the identification of an optimal SII cutoff (800) requires external validation, and its use as a predictive tool for guiding treatment decisions cannot be established based on the current data. Multivariate analysis highlighted its utility in understanding tumor-inflammation dynamics, but further research is needed to define its clinical applicability.
Importantly, SII offers several advantages. It is derived from routine blood tests, making it both cost-effective and widely applicable, particularly in resource-limited settings where advanced molecular diagnostics may not be feasible. By incorporating SII into existing prognostic frameworks, clinicians can improve the precision of patient counseling, prioritize high-risk individuals for intensive monitoring, and tailor therapeutic strategies to individual risk profiles.
Despite these promising findings, limitations such as the study’s retrospective design and single-center setting necessitate caution in generalizing the results. Prospective, multicenter studies are essential to validate the prognostic utility of SII across diverse populations and healthcare settings. While the study highlights SII’s association with prognostic outcomes, it does not establish predictive value. Further mechanistic and clinical investigations are necessary to validate its role in guiding treatment strategies. Furthermore, investigating the biological mechanisms linking systemic inflammation and cancer progression could enhance the understanding of SII’s role in breast cancer management [
27].
Future research should also explore the integration of SII with other biomarkers, such as genomic signatures or tumor microenvironment profiles, to refine prognostic models further. As the landscape of breast cancer treatment evolves, SII has the potential to become a pivotal tool in advancing personalized oncology care.