Evaluation of Dose Reduction Factors and Impact on Progression-Free Survival in Patients Treated with CDK 4/6 Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design and Data Collection
2.3. Statistical Analysis
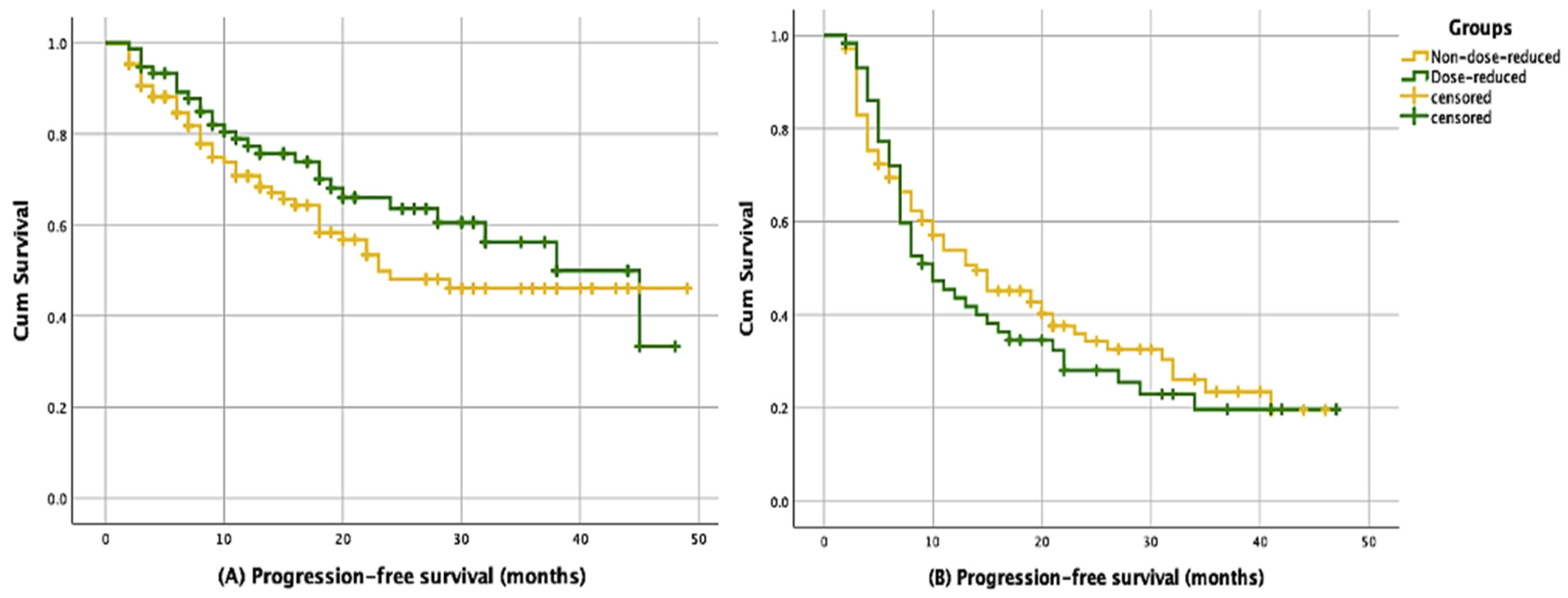
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.W.; Allred, D.C.; Anderson, B.O.; Burstein, H.J.; Carter, W.B.; Edge, S.B.; Erban, J.K.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. Invasive breast cancer. J. Natl. Compr. Cancer Netw. 2011, 9, 136–222. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Diéras, V.; Gelmon, K.A.; Finn, R.S.; Slamon, D.J.; Martin, M.; Neven, P.; Shparyk, Y.; Mori, A.; Lu, D.R.; et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: Results from the PALOMA-2 trial. Ann. Oncol. 2018, 29, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547, Erratum in Ann. Oncol. 2019, 30, 1842. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439, Erratum in Lancet Oncol. 2016, 17, e136Erratum in Lancet Oncol. 2016, 17, e270. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2− Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224, Erratum in Clin. Cancer Res. 2018, 24, 5485. [Google Scholar] [CrossRef]
- Stanciu, I.M.; Parosanu, A.I.; Nitipir, C. An Overview of the Safety Profile and Clinical Impact of CDK4/6 Inhibitors in Breast Cancer-A Systematic Review of Randomized Phase II and III Clinical Trials. Biomolecules 2023, 13, 1422. [Google Scholar] [CrossRef]
- Burris, H.A.; Chan, A.; Bardia, A.; Beck, J.T.; Sohn, J.; Neven, P.; Tripathy, D.; Im, S.-A.; Chia, S.; Esteva, F.J.; et al. Safety and impact of dose reductions on efficacy in the randomised MONALEESA-2, -3 and -7 trials in hormone receptor-positive, HER2-negative advanced breast cancer. Br. J. Cancer 2021, 125, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Ettl, J.; Im, S.-A.; Ro, J.; Masuda, N.; Colleoni, M.; Schnell, P.; Bananis, E.; Lu, D.R.; Cristofanilli, M.; Rugo, H.S.; et al. Hematologic adverse events following palbociclib dose reduction in patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer: Pooled analysis from randomized phase 2 and 3 studies. Breast Cancer Res. 2020, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Huober, J.; García-Sáenz, J.A.; Masuda, N.; Sohn, J.H.; Andre, V.A.M.; Barriga, S.; Cox, J.; Goetz, M. Management of Abemaciclib-Associated Adverse Events in Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Safety Analysis of MONARCH 2 and MONARCH 3. Oncologist 2020, 26, e53–e65, Erratum in Oncologist 2021, 26, e522. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, G.; Hlauschek, D.; Mayer, E.L.; Deutschmann, C.; Kacerovsky-Strobl, S.; Martin, M.; Meisel, J.L.; Zdenkowski, N.; Loibl, S.; Balic, M.; et al. Impact of BMI in Patients With Early Hormone Receptor-Positive Breast Cancer Receiving Endocrine Therapy With or Without Palbociclib in the PALLAS Trial. J. Clin. Oncol. 2023, 41, 5118–5130. [Google Scholar] [CrossRef]
- Lavery, L.; DiSogra, K.; Lea, J.; Trufan, S.J.; Symanowski, J.T.; Roberts, A.; Moore, D.C.; Heeke, A.; Pal, S. Risk factors associated with palbociclib-induced neutropenia in patients with metastatic breast cancer. Support. Care Cancer 2022, 30, 9803–9809. [Google Scholar] [CrossRef]
- Flach, J.; Bakker, S.T.; Mohrin, M.; Conroy, P.C.; Pietras, E.M.; Reynaud, D.; Alvarez, S.; Diolaiti, M.E.; Ugarte, F.; Forsberg, E.C.; et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 2014, 512, 198–202. [Google Scholar] [CrossRef]
- Mahlknecht, U.; Kaiser, S. Age-related changes in peripheral blood counts in humans. Exp. Ther. Med. 2010, 1, 1019–1025. [Google Scholar] [CrossRef]
- Rossi, V.; Berchialla, P.; Giannarelli, D.; Nisticò, C.; Ferretti, G.; Gasparro, S.; Russillo, M.; Catania, G.; Vigna, L.; Mancusi, R.L.; et al. Should All Patients With HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers 2019, 11, 1661. [Google Scholar] [CrossRef]
- Roncato, R.; Peruzzi, E.; Gerratana, L.; Posocco, B.; Nuzzo, S.; Montico, M.; Orleni, M.; Corsetti, S.; Bartoletti, M.; Gagno, S.; et al. Clinical impact of body mass index on palbociclib treatment outcomes and effect on exposure. Biomed. Pharmacother. 2023, 164, 114906. [Google Scholar] [CrossRef]
- Maltoni, R.; Roncadori, A.; Balzi, W.; Mazza, M.; Nicolini, F.; Palleschi, M.; Ulivi, P.; Bravaccini, S. An Italian Real-World Study Highlights the Importance of Some Clinicopathological Characteristics Useful in Identifying Metastatic Breast Cancer Patients Resistant to CDK4/6 Inhibitors and Hormone Therapy. Biomedicines 2024, 12, 498. [Google Scholar] [CrossRef]
- Gelmon, K.; Walshe, J.M.; Mahtani, R.; Joy, A.A.; Karuturi, M.; Neven, P.; Lu, D.R.; Kim, S.; Schnell, P.; Bananis, E.; et al. Efficacy and safety of palbociclib in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with preexisting conditions: A post hoc analysis of PALOMA-2. Breast 2021, 59, 321–326. [Google Scholar] [CrossRef]
| Total Patiens (n = 474) | |
|---|---|
| Age (min–max) | 56 (27–87) |
| Menopausal status (%) | |
| Pre-menopausal | 148 (31.2) |
| Post-menopasual | 326 (68.8) |
| BMI (%) | |
| <25 | 142 (31.1) |
| ≥25 | 312 (68.9) |
| Unknown | 20 |
| BSA (%) | |
| <1.7 | 126 (27.8) |
| ≥1.7 | 328 (72.2) |
| Unknown | 20 |
| ECOG PS (%) | |
| PS 0–1 | 452 (95.4) |
| PS ≥ 2 | 22 (4.6) |
| Charlson CI (%) | |
| 0–1 | 369 (77.8) |
| ≥2 | 105 (22.2) |
| De novo metastatic (%) | |
| Yes | 271 (57.2) |
| No | 203 (42.8) |
| Histological Subtype (%) | |
| Invasive ductal carcinoma | 334 (76.9) |
| Invasive lobular carcinoma | 47 (10.8) |
| Others | 53 (12.2) |
| Unknown | 40 |
| Progesterone receptor status (%) | |
| Positive (≥%1) | 387 (81.6) |
| Negative (<%1) | 87 (18.4) |
| HER-2 receptor status (%) | |
| Positive (1 or 2 + and fısh negative) | 91 (19.2) |
| Negative | 383 (80.8) |
| Number of metastatic sites (%) | |
| <3 | 299 (69.2) |
| ≥3 | 133 (30.8) |
| Bone metastases only (%) | |
| Yes | 139 (32.1) |
| No | 293 (67.9) |
| Liver or lung metastases (%) | |
| Yes | 199 (46.1) |
| No | 233 (53.9) |
| Brain metastases (%) | |
| Yes | 409 (94.6) |
| No | 23 (5.4) |
| Total Patiens (n = 474) | |
|---|---|
| CDK 4/6 inhibitors (%) | |
| Ribociclib | 343 (72.4) |
| Palbociclib | 131 (27.6) |
| Combination endocrine therapy (%) | |
| Aromatase inhibitors | 314 (66.2) |
| Fulvestrant | 160 (33.8) |
| Treatment Line (%) | |
| First line | 307 (64.8) |
| Second and subsequent lines | 167 (35.2) |
| Dose reduction (%) (any cause) | |
| No | 305 (64.3) |
| Yes | 169 (35.7) |
| One time | 140 (29.5) |
| Two times | 29 (6.1) |
| Neutropenia (%) (any grade) | 431 (90.9) |
| Neutropenia (%) (grade 3–4) | 231 (48.7) |
| Prolonged QT interval (%) (grade 3–4) | 12 (2.5) |
| Thrombocytopenia (%) (grade 3–4) | 9 (1.8) |
| Anemia (%) (grade 3–4) | 7 (1.4) |
| Hepatobiliary toxicity (%) (grade 3–4) | 6 (1.2) |
| Diarrhea (%) (grade 3–4) | 3 (0.6) |
| Stomatitis (%) (grade 3–4) | 1 (0.2) |
| Pulmonary embolism (%) (grade 3–4) | 1 (0.2) |
| n (%) | Median PFS (%95 CI) Months | p | |
|---|---|---|---|
| First-line treatment | |||
| Non-dose-reduced | 197 (64.1) | 24.8 (17.1–29.9) | |
| Dose-reduced | 110 (35.8) | 37.1 (25.4–50.5) | 0.114 |
| Overall | 307 | 29.4 (19.1–38.8) | |
| Second- and subsequent-line treatment | |||
| Non-dose-reduced | 108 (64.6) | 14.4 (7.8–20.1) | |
| Dose-reduced | 59 (35.3) | 10.6 (5.9–14.2) | 0.528 |
| Overall | 167 | 13.1 (9.6–16.3) | |
| B | OR | OR %95CI | p | |
|---|---|---|---|---|
| Age (<65 vs. ≥65) | 0.645 | 1.906 | 1.132–3.209 | 0.015 |
| Menopausal status | 0.070 | 1.072 | 0.627–1.833 | 0.798 |
| BMI (<25 vs. ≥25) | −0.554 | 0.575 | 0.332–0.995 | 0.048 |
| BSA (<1.7 vs. ≥1.7) | 0.148 | 1.159 | 0.711–1.889 | 0.553 |
| Charlson CI (0–1 vs. 2+) | 0.665 | 1.944 | 1.128–3.350 | 0.017 |
| De novo metastasis | −0.351 | 0.115 | 0.455–1.090 | 0.115 |
| Treatment lines (1. vs. 2 and subsequent line) | −0.640 | 0.938 | 0.603–1.459 | 0.877 |
| Progesterone receptor status (<%1 vs. ≥%1) | −0.092 | 0.912 | 0.521–1.596 | 0.746 |
| HER-2 receptor status (0 vs. 1 or 2+) | 0.247 | 1.281 | 0.753–2.178 | 0.361 |
| Number of metastatic sites (<3 vs. ≥3) | 0.844 | 2.325 | 1.380–3.917 | 0.002 |
| Bone metastases only | 0.222 | 1.248 | 0.656–2.374 | 0.499 |
| Liver or lung metastases | −0.343 | 0.710 | 0.406–1.242 | 0.230 |
| Brain metastases | −1.006 | 0.366 | 0.125–1.070 | 0.066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güren, A.K.; Guliyev, M.; Alan, Ö.; Çadırci, K.; Belevi, İ.N.; Gültürk, İ.; Özge, E.; Kocaaslan, E.; Ağyol, Y.; Erel, P.; et al. Evaluation of Dose Reduction Factors and Impact on Progression-Free Survival in Patients Treated with CDK 4/6 Inhibitors. J. Clin. Med. 2025, 14, 1071. https://doi.org/10.3390/jcm14041071
Güren AK, Guliyev M, Alan Ö, Çadırci K, Belevi İN, Gültürk İ, Özge E, Kocaaslan E, Ağyol Y, Erel P, et al. Evaluation of Dose Reduction Factors and Impact on Progression-Free Survival in Patients Treated with CDK 4/6 Inhibitors. Journal of Clinical Medicine. 2025; 14(4):1071. https://doi.org/10.3390/jcm14041071
Chicago/Turabian StyleGüren, Ali Kaan, Murad Guliyev, Özkan Alan, Kıvanç Çadırci, İpek Naz Belevi, İlkay Gültürk, Emre Özge, Erkam Kocaaslan, Yeşim Ağyol, Pınar Erel, and et al. 2025. "Evaluation of Dose Reduction Factors and Impact on Progression-Free Survival in Patients Treated with CDK 4/6 Inhibitors" Journal of Clinical Medicine 14, no. 4: 1071. https://doi.org/10.3390/jcm14041071
APA StyleGüren, A. K., Guliyev, M., Alan, Ö., Çadırci, K., Belevi, İ. N., Gültürk, İ., Özge, E., Kocaaslan, E., Ağyol, Y., Erel, P., Paçacı, B., Tunç, M. A., Majidova, N., Sever, N., Çelebi, A., Arıkan Erdoğan, R., Işık, S., Demirci, N. S., Sarı, M., ... Bayoğlu, İ. V. (2025). Evaluation of Dose Reduction Factors and Impact on Progression-Free Survival in Patients Treated with CDK 4/6 Inhibitors. Journal of Clinical Medicine, 14(4), 1071. https://doi.org/10.3390/jcm14041071






