1. Introduction
Hyperparathyroidism (HPT) is a pathological condition characterized by hyperactivity of the parathyroid glands, resulting in aberrantly elevated levels of parathyroid hormone (PTH) in the circulatory system. HPT can be categorized into primary, secondary, and tertiary hyperparathyroidism [
1]. Primary hyperparathyroidism (PHPT) results from a dysregulated increase in PTH secretion by the parathyroid glands, leading to concurrent hypercalcemia, as the parathyroid cells exhibit diminished sensitivity to or insensitivity to the suppressive influence of hypercalcemia. PHPT is typically acquired and is associated with autonomous glandular hyperplasia, with a prevalence of 85% for a single adenoma, 15% for multiple adenomas or parathyroid hyperplasia, and 1% for parathyroid cancer [
2]. The musculoskeletal and urinary systems are predominantly affected by PHPT, resulting in conditions such as osteoporosis, osteopenia, osteoarticular pain, pathological fractures, kidney and ureteral stones, pyonephrosis, and renal failure [
2]. Neurological manifestations may also transpire, with a wide spectrum of symptoms that range from mild (depressive mood, fatigue, and sleep disturbances) to severe (anxiety disorders, depression, cognitive impairment, hallucinations, and delusions) and, in extreme cases, coma [
3]. Additionally, PHPT may manifest with hypertension, cardiac arrhythmias, or treatment-resistant anemia. Individuals with PHPT are at an increased risk of peptic ulcer disease, acute pancreatitis, and biliary tract stones [
4,
5]. According to the Fifth International Workshop on Evaluation and Management of Primary Hyperparathyroidism, the diagnosis of PHPT relies on laboratory test results, which primarily reveal elevated levels of PTH and calcium in the blood. In cases where high PTH levels coexist with normocalcemia, diagnostic evaluation should be expanded to include measurements of calcidiol (25(OH)D3) and creatinine levels to rule out secondary hyperparathyroidism [
6]. Imaging modalities play a pivotal role in guiding surgical interventions and serve as adjunctive tools in the diagnostic process of PHPT, assisting surgeons in the preoperative planning and identification of potentially affected parathyroid glands. Standard imaging modalities for parathyroid glands include computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography (USG), and scintigraphy. On ultrasound, enlarged parathyroid glands appear as hypoechoic structures compared with the surrounding tissue. Pathologically altered parathyroid glands located around the thyroid or within its parenchyma are the easiest to identify. However, ultrasound is not very effective for identifying ectopically located parathyroid glands [
7]. CT and MRI are characterized by high, similar effectiveness and are mainly used in the diagnosis of recurrent or persistent hyperparathyroidism [
8]. In preoperative diagnostics for locating adenomas, scintigraphy with [99mTc]Tc-MIBI radioisotope is widely used. According to various authors, the sensitivity of this test ranges from 55% to 100%, but it decreases in cases of generalized hyperplasia. Parathyroid scintigraphy can be performed via two methods: the double-tracer technique or the dual-phase technique [
9]. In complex cases where the outcomes of conventional imaging tests are inconclusive or previous surgical interventions have been unsuccessful, positron emission tomography/computed tomography (PET/CT) using [11C]C-methionine or [18F]F-choline can be considered. The mechanism behind the increased accumulation of radiotracers in parathyroid adenoma cells remains unclear, but it is suspected to be due to increased cell proliferation and elevated production of preProPTH (a precursor of PTH). This approach can be helpful in determining the location of small or multiple parathyroid adenomas [
10,
11,
12]. In the management of severe hypercalcemia, the foundational tenet is the attainment of adequate hydration and loop diuretics deployment to stimulate diuresis and augment calcium excretion [
13]. A non-surgical approach for PHPT treatment is limited for patients with a high risk of postoperative complications. Bisphosphonates, agents inhibiting osteoclast-dependent bone osteolysis, may be deemed necessary in select cases. While the efficacy of cinacalcet in the context of PHPT remains less established, its application is extended to patients exhibiting secondary or tertiary hyperparathyroidism. Cinacalcet potentiates the sensitivity of the calcium-sensing receptor (CaSR), resulting in diminished PTH secretion and commensurate reductions in calcium levels [
14]. Recent studies have suggested that cinacalcet may also possess the potential to reduce the size of parathyroid adenomas; however, these findings require further investigation to confirm their validity [
15]. Parathyroidectomy represents the contemporary gold standard in the therapeutic landscape of PHPT [
16]. Nevertheless, this surgical intervention is not devoid of potential postoperative complications, including hemorrhage, postoperative wound infection, and recurrent laryngeal nerve palsy. The principal objective of this study was to conduct a retrospective analysis of the efficacy of surgical intervention in PHPT, elucidating the factors influencing the extent of the procedure. Additionally, the investigation sought to scrutinize the incidence of postoperative complications.
4. Discussion
Despite a noticeable increase in awareness and greater interest in the field of PHPT, with a significantly growing number of publications, many authors emphasize the underdiagnosis of this disease [
17,
18,
19]. Owing to the lack of characteristic symptoms and the peak incidence of the disease in the postmenopausal period, PHPT poses a challenge to its diagnosis. Clinicians may disregard elevated serum calcium levels and ignore PHPT as a potential cause of symptoms, leading to delayed diagnosis and treatment [
20,
21]. Epidemiological studies of the European population indicate that the increased availability of tests for total calcium and PTH levels in serum has a positive effect on increased detection of the disease [
22,
23]. An analysis of epidemiological studies based on the American population highlights the importance of assessing calcium and PTH levels in asymptomatic patients [
20,
24].
Our study included patients who underwent surgery for primary PHPT between 2008 and 2017 at one center, comprising 110 (88%) women and 15 (12%) men. Research has confirmed that female sex predisposes individuals to the development of PHPT, especially at postmenopausal ages [
25]. The reduced antiproliferative effect of estrogens on parathyroid cells plays an important role in this regard [
26]. Women receiving hormone replacement therapy have a reduced risk of developing PHPT [
27]. This investigation revealed intriguing insights into the relationships between blood calcium levels and various symptoms associated with PHPT. Notably, a statistically significant association was found between elevated blood calcium levels and specific symptoms reported by patients. For example, individuals who experienced osteoarticular pain presented higher blood calcium levels than did those without this symptom (12.05 mg/dL vs. 11.03 mg/dL;
p < 0.05). Similarly, patients reporting muscle weakness or osteoporotic changes also presented elevated blood calcium levels (11.73 mg/dL vs. 11.03 mg/dL;
p < 0.05 and 12.05 mg/dL vs. 11.02 mg/dL;
p < 0.05, respectively). Furthermore, this study revealed a notable correlation between PTH levels and osteoporosis, highlighting an interplay between hormonal regulation and skeletal health (356.03 pg/mL vs. 297.64 pg/mL;
p < 0.05). However, Chan et al.’s analysis revealed contrasting findings showing no relationship between the levels of total calcium and PTH in the blood and symptoms of the musculoskeletal system [
28]. Interestingly, sex did not emerge as a significant factor in reported PHPT symptoms, underscoring the multifactorial nature of the condition. Nonetheless, age proved to be a pertinent determinant, with older individuals exhibiting a greater prevalence of muscle weakness (98.1% vs. 51.3%;
p < 0.05), osteoarticular pain (86.7% vs. 31.9%;
p < 0.05), and osteoporosis (86.7% vs. 33.3%;
p < 0.05) than their younger counterparts.
The psychiatric sequelae observed in patients with PHPT may stem from various physiological mechanisms. These include heightened neurotransmitter transmission due to diminished monoamine oxidase (MAO) activity, impaired functioning of the ATP-dependent sodium-potassium pump (Na
+/K
+ ATPase), and elevated Ca
2+ concentration within synapses [
29]. While the literature generally indicates a lack of an association between psychological symptomatology in patients with PHPT and serum calcium levels, certain studies suggest a heightened risk of severe psychotic disorders in patients whose hypercalcemia exceeds 14 g/dL [
3,
30,
31]. In our study, depressive mood was prevalent among 103 (82.4%) patients. However, our analysis revealed no significant correlation between the occurrence of depressive mood and serum calcium or PTH levels, the number of pathologically altered parathyroid glands, or the age and sex of the patients. These findings underscore the complexity of psychiatric manifestations in PHPT, suggesting that factors beyond calcium and PTH levels may contribute to the development of depressive symptoms. Further research is warranted to elucidate the underlying mechanisms and optimize therapeutic approaches for psychiatric sequelae in patients with PHPT.
PHPT is commonly associated with an elevated risk of cholelithiasis, affecting approximately 22–30% of patients [
32,
33]. Saito et al. and Broulik et al. proposed a model in which the key element is the impact of PTH and hypercalcemia on gallbladder contractility impairment, leading to an increased calcium concentration in bile secretions and slowed bile flow in ducts. Furthermore, the authors highlight a significantly higher prevalence of gallstones among women. This sexual dimorphism is attributed to the influence of estrogen, which enhances cholesterol absorption from the digestive system, promotes cholesterol secretion into bile, and inhibits deoxycholic acid synthesis [
34,
35]. Broulik et al. corroborated a more frequent occurrence of cholelithiasis in patients with PHPT and reported a positive correlation between the incidence of cholelithiasis and advanced age. In our investigation, cholelithiasis was documented in 68 (54%) patients, with no discernible difference in occurrence between women and men (55.4% vs. 46.6%;
p = 0.521). However, a significantly greater prevalence of cholelithiasis was noted in older patients (86.7% vs. 30.5%;
p < 0.05). Moreover, patients with cholelithiasis presented significantly elevated levels of both calcium (12.03 mg/dL vs. 11.06 mg/dL;
p < 0.05) and PTH (357 pg/mL vs. 298.52 pg/mL;
p < 0.05).
Patients with PHPT face a substantial risk, ranging from 40% to 60%, of developing renal complications, with urinary tract stones being the most prevalent among them [
2]. The precise etiopathology underlying the formation of renal and urinary tract deposits remains elusive; however, hypercalciuria has been identified as a key risk factor [
36]. Clinical investigations commonly report a heightened incidence of urolithiasis in male patients with PHPT under the age of 50 [
36,
37]. In contrast to findings from previously published studies, our research did not reveal any correlation between patient sex or age and the risk of urolithiasis development. Nevertheless, individuals afflicted with urolithiasis presented significantly elevated concentrations of total calcium (12.03 mg/dL vs. 11.02 mg/dL;
p < 0.05) and PTH (356.90 pg/mL vs. 296.42 pg/mL;
p < 0.05), mirroring the observations reported by Corbett et al. [
38]. These findings underscore the complexity of the relationship between PHPT and renal complications, emphasizing the multifaceted nature of disease progression.
The primary cause of extended hospital stays following parathyroidectomy is often severe hypocalcemia [
39]. Lansdown et al. conducted an analysis of 17,498 patients who underwent surgery between 2014 and 2019 and reported a shorter duration of hospitalization for patients operated on by surgeons who had performed more than 60 parathyroidectomies than for those who had performed fewer than 10 procedures [
40]. Additionally, their study highlighted the influence of surgeon experience on various outcomes, including a lower percentage of readmissions within 30 days post-procedure (11.7% vs. 7%), a decreased incidence of postoperative hypoparathyroidism (16.3% vs. 10.9%), fewer instances of PHPT recurrence within a year of surgery (2.5% vs. 1%), and reduced postoperative mortality (1% vs. 0.5%). Conversely, research by Thomas et al., which included 7313 patients, identified a heightened risk of prolonged hospitalization among individuals aged over 65 years [
41]. In our study, the hospitalization period ranged from 3 to 16 days, with a median of 4.40 days. The longest hospitalization, which lasted 16 days, involved an 82-year-old patient who experienced cardiopulmonary failure in the postoperative period. Among the 11 patients who experienced postoperative complications, the duration of hospitalization was significantly longer than that in the remainder of the cohort (9.30 days vs. 3.92 days;
p < 0.05). Similarly, patients aged 65 and older experienced prolonged hospital stays (5.16 days vs. 3.90 days;
p < 0.05). However, our investigation did not identify any disparities in hospitalization duration on the basis of surgeon experience.
Our investigation revealed that the most frequent site of adenoma occurrence was the left inferior parathyroid gland (
n = 65; 43%), with an atypical location observed in 22 (14.7%) of the excised parathyroid glands, predominantly involving intrathymic sites (
n = 10; 6.6%). No statistically significant differences were found between adenoma location and sex. Analysis of laboratory parameters revealed a notably lower calcium level on admission in patients with right inferior parathyroid adenomas than in those with other parathyroid glands, along with significantly higher calcium levels on the first postoperative day (
p < 0.05). Similarly, significantly elevated calcium levels on admission were observed in patients with left inferior parathyroid adenomas compared with those with adenomas affecting other glands, with a corresponding decrease on the first postoperative day (
p < 0.05). No statistically significant differences were noted in calcium levels in patients with adenomas affecting other parathyroid glands, and no correlation was found between PTH levels and adenoma location. However, a significant increase in the mean inorganic phosphate level was observed on the first postoperative day following parathyroid adenoma removal (2.57 mg/dL vs. 3.20 mg/dL;
p < 0.05). A negative prognostic indicator is the concurrent presence of adenomas affecting two or more parathyroid glands. According to the available literature, the prevalence of dual adenomas ranges from 1% to 10%, with women and individuals over 60 years of age being at increased risk [
42]. Surprisingly, in our study, a substantial number of patients had dual parathyroid adenomas (
n = 24; 19.2%). The most frequent scenario involved the coexistence of a right inferior parathyroid adenoma with a left inferior one (
n = 8; 33%) and a right inferior one with a left superior one (
n = 8; 33%). There was no statistically significant disparity in the occurrence of dual parathyroid adenomas between sexes; however, their incidence was notably greater in the older patient group (62.5% vs. 37.5%;
p < 0.05). With respect to calcium levels at admission, no statistically significant differences were detected between patients with adenomas affecting two parathyroid glands and those with adenomas of a single gland. Nonetheless, calcium levels were significantly lower on the first post-procedure day in patients with pathology involving two glands (8.84 mg/dL vs. 9.41 mg/dL;
p < 0.05). Furthermore, patients with dual adenomas presented significantly greater PTH levels at admission and on the first postoperative day.
The current gold standard for treating PHPT is surgery [
8,
9]. Depending on the center’s expertise and technical capabilities, surgical interventions are conducted in a minimally invasive manner or via traditional unilateral or bilateral neck exploration. Surgical management of PHPT, irrespective of the chosen approach, has a high efficacy rate ranging from 97% to 99% [
43]. In our investigation, 93 (74.4%) patients underwent OMIP procedures, 11 (8.8%) underwent unilateral neck exploration, and 21 (16.8%) underwent bilateral neck exploration. The mean operation duration was 53 min. Our findings revealed a significantly shorter duration for OMIP procedures than for both unilateral and bilateral neck explorations (45 min vs. 65.80 min;
p < 0.05), with no notable disparity between the two neck exploration techniques. Consistent with findings from other studies [
44,
45,
46], our research demonstrated a significantly shorter procedure duration with the minimally invasive approach and no significant time discrepancies between unilateral and bilateral neck explorations. Additionally, in our study, procedure duration was significantly correlated with operator experience (45 min vs. 57 min;
p < 0.05).
The incidence of complications varies significantly in the available literature (1–59.7%) and largely depends on whether the study investigators included transient hypocalcemia as a postoperative complication [
47,
48,
49]. In our study, postoperative hypocalcemia was observed in 47 (37.6%) patients, with calcium levels ranging from 5.20 to 8.90 mg/dL. The patient with extremely low serum calcium values (5.20 mg/dL) presented symptoms of tetany. Additionally, two (1.6%) patients reported symptoms of paresthesia in the form of numbness in the fingers of the upper limbs and tingling around the mouth. In each case, intravenous infusions of calcium chloride (CaCl
2) and magnesium sulfate (MgSO
4) were administered, resulting in the disappearance of symptoms. Our observations were consistent with the findings of other authors, demonstrating no correlation between patient age and the occurrence of postoperative complications [
50]. However, hypocalcemia occurred more frequently in the group of patients who underwent surgery by a surgeon with less experience (50% vs. 30.3%;
p < 0.05). Differences between treatment techniques were also significant. Hypocalcemia occurred least frequently after OMIP procedures (21.5% vs. 84.3%;
p < 0.05) and most frequently after bilateral neck exploration (90.4% vs. 26.9%;
p < 0.05). Among the remaining complications, four (3.2%) cases of postoperative wound infection and three (2.4%) cases of postoperative bleeding were observed. Symptoms of cardiorespiratory failure were noted in one (0.8%) patient. This patient required treatment in the intensive care unit. In our study, no relationship between the patient’s sex or age and the risk of complications was observed. There was no relationship between the operator’s experience or the location of the pathologically changed parathyroid gland and the occurrence of complications during the postoperative period. However, the influence of comorbidities on the increased risk of complications (20.9% vs. 2.4%;
p < 0.05) included a greater risk of bleeding in the postoperative period (6.9% vs. 0%;
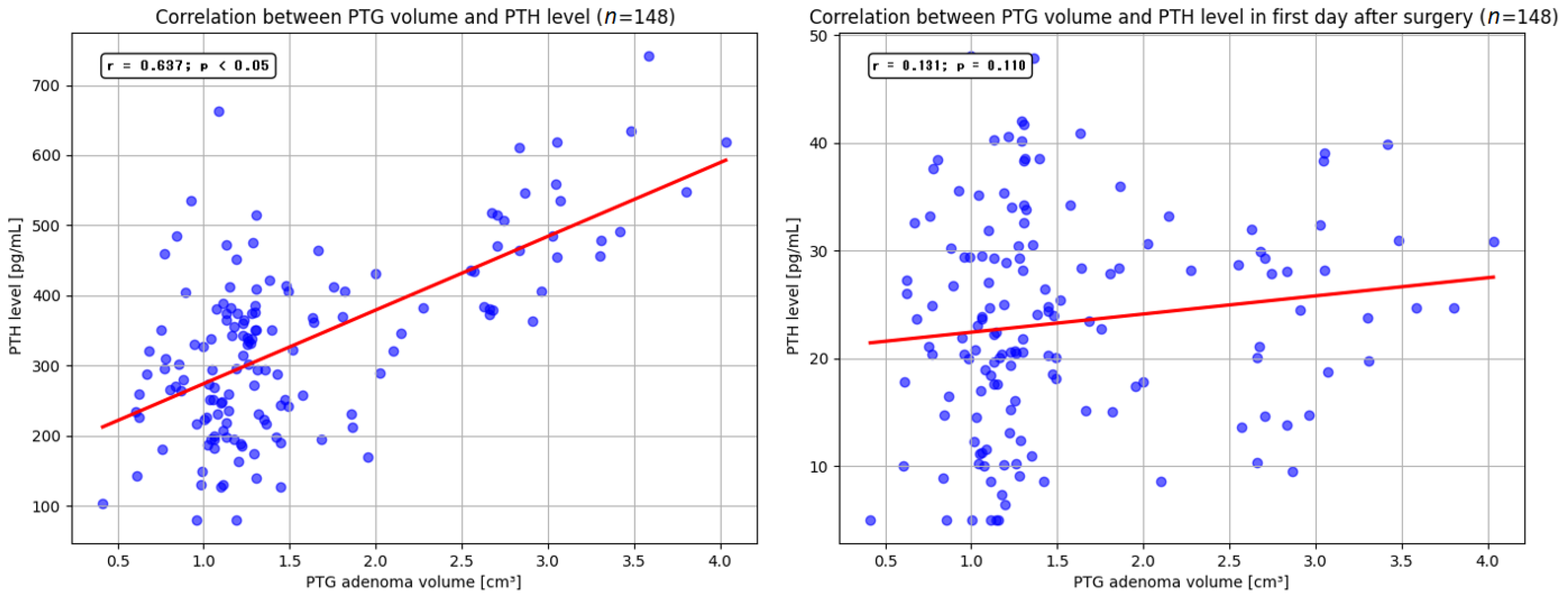
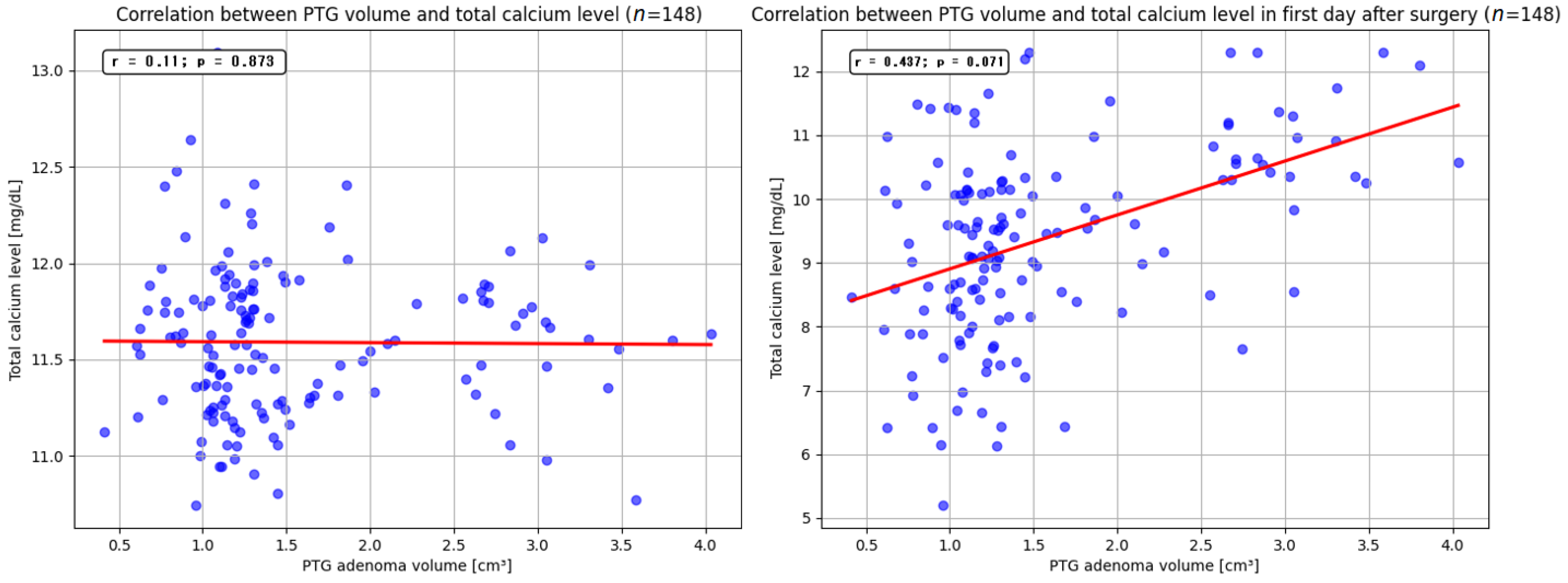
p < 0.05). The mean volume of the resected parathyroid gland was 1.9 cm
3 (range, 0.4–4.05 cm
3). Our findings indicate that larger adenoma size may be associated with higher preoperative serum PTH levels. Similar observations were reported by Ramas et al. and Rezkallah et al., who additionally noted that this correlation could enable a more accurate estimation of gland volume, potentially minimizing unnecessary tissue dissection [
51,
52]. In contrast to other studies, our analysis did not demonstrate a significant relationship between parathyroid adenoma volume and total serum calcium levels. Furthermore, we did not detect a statistically significant influence of resected adenoma volume on the size of PTH and total calcium levels reduction on the first day after surgery. Nevertheless, several published reports have highlighted that removal of larger parathyroid lesions may precipitate a marked decrease in PTH and total calcium levels, leading to clinical manifestations of hypocalcemia [
53,
54].
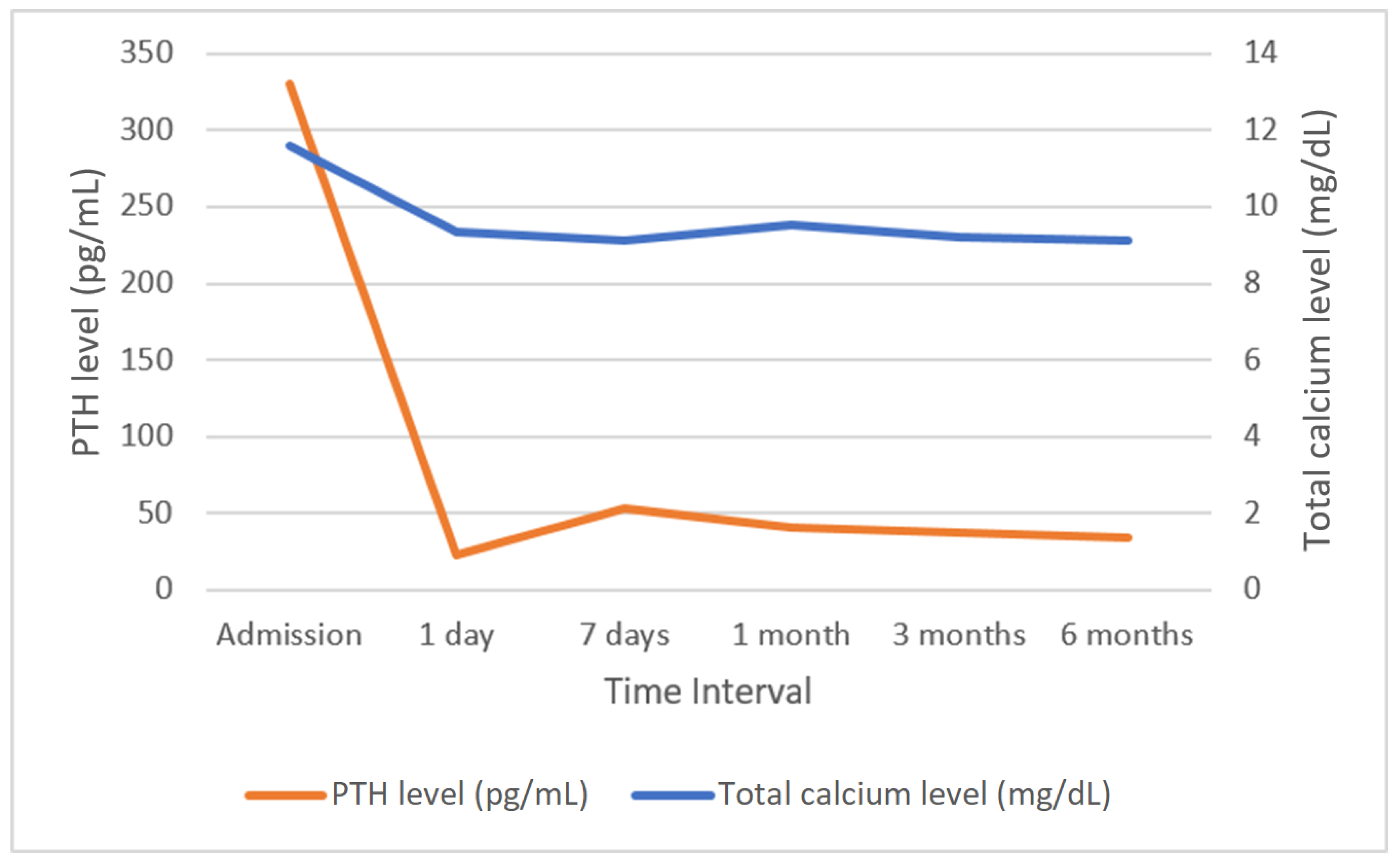
Successful parathyroidectomy is a procedure after which hypercalcemia does not develop within a period of six months [
55,
56]. In this study, normalization of calcium levels in the sixth month after surgery was achieved in 119 (95.2%) patients. Among the six patients whose calcium levels remained above 10.50 g/dL, five underwent surgery via the OMIP technique, whereas one patient underwent unilateral neck exploration. The normalization of PTH levels after parathyroidectomy represents an additional concern. In our study, the vast majority of patients had normalized PTH levels up to a maximum of day 6 after the procedure, but in seven patients (5.6%), elevated PTH levels were maintained after hospitalization. This condition may result from advanced patient age, impaired renal function, or exceptionally large parathyroid adenomas and may affect up to one-third of patients undergoing surgical treatment for parathyroid adenomas. However, this is generally not associated with hypercalcemia and is considered a successful parathyroidectomy, but these cases require long-term postoperative monitoring and extended diagnostic imaging to detect potential adenoma recurrence [
57,
58]. The association of calcium and PTH levels with alkaline phosphatase is also an important issue. Alkaline phosphatase is a bone formation marker secreted by osteoblasts, with usually elevated levels in PHPT. High levels of alkaline phosphatase are identified as a predictor of calcium and PTH decline that correlates with postoperative hypocalcemia. After parathyroidectomy, there is a significant disconnect between the processes of bone formation and resorption, which is affected by a rapid decrease in PTH and high levels of alkaline phosphatase, which increases the risk of hypocalcemia. The decrease in calcium levels may also be lower in patients with lower alkaline phosphatase levels, but such a correlation can be found with high preoperative calcium levels. Patients with different preoperative serum calcium levels vary in factors affecting hypocalcemia, with alkaline phosphatase possibly representing one of these factors [
59,
60,
61]. However, such analysis is beyond the scope of this study. No relationship between the effectiveness of the procedure and patient sex or age was found. There were no significant differences in effectiveness between individual parathyroidectomy techniques or in the location of the surgically removed adenoma.