The Evaluation of New-Generation Biomarker sCD14ST Provides New Insight into COVID-19’s Effect on Bone Remodeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Enrollment
2.2. Blood Sample Collection and Serum Preparation
2.3. Evaluation of sCD14-ST
2.4. Quantification of SuPAR and Osteoimmunological Markers
3. Results
3.1. Evaluation of sCD14ST in COVID-19-Positive and COVID-19-Negative Patients
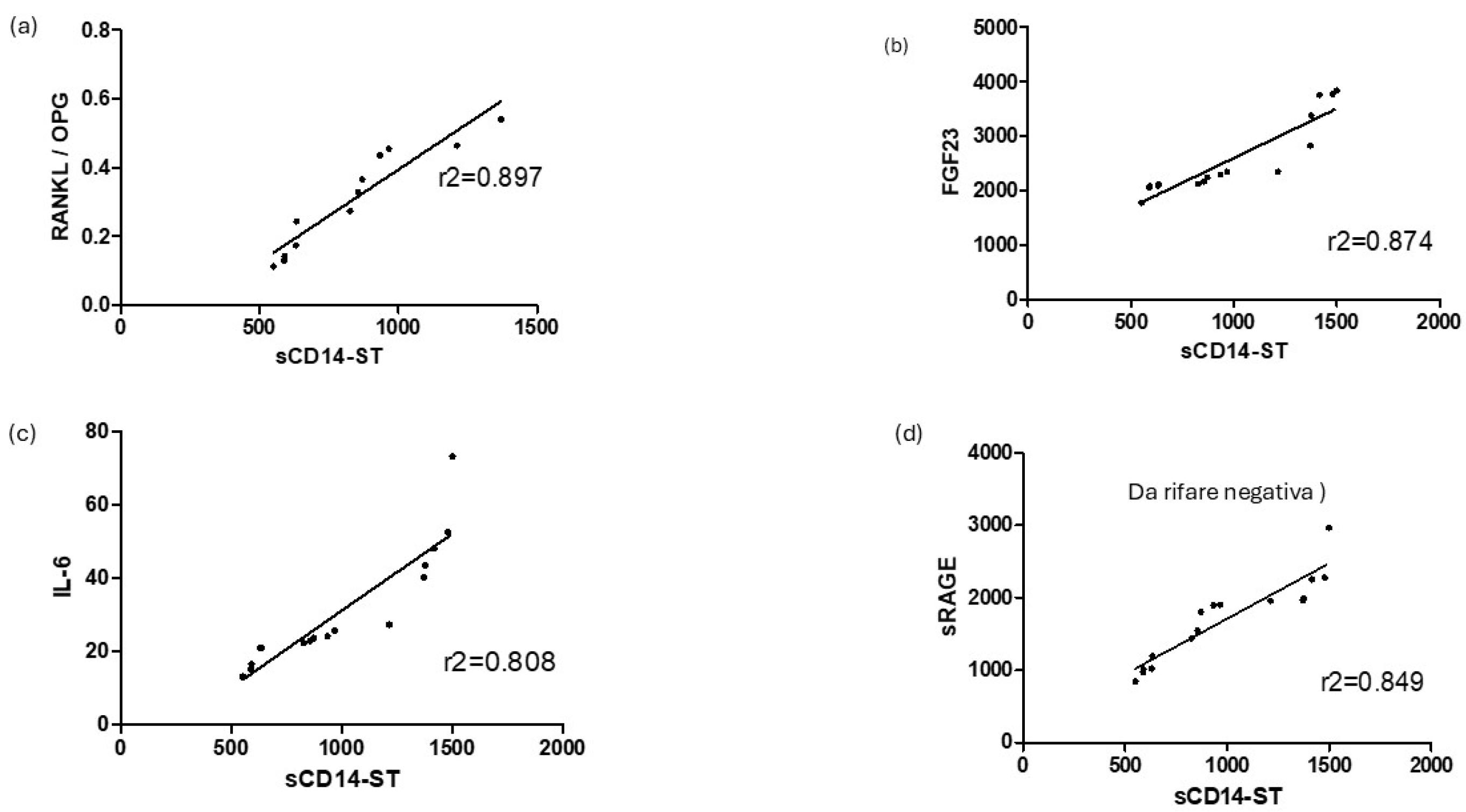
3.2. Correlation Between sCD14ST and Markers of Inflammation
3.3. Correlation Between sCD14ST and Osteoimmunological Markers
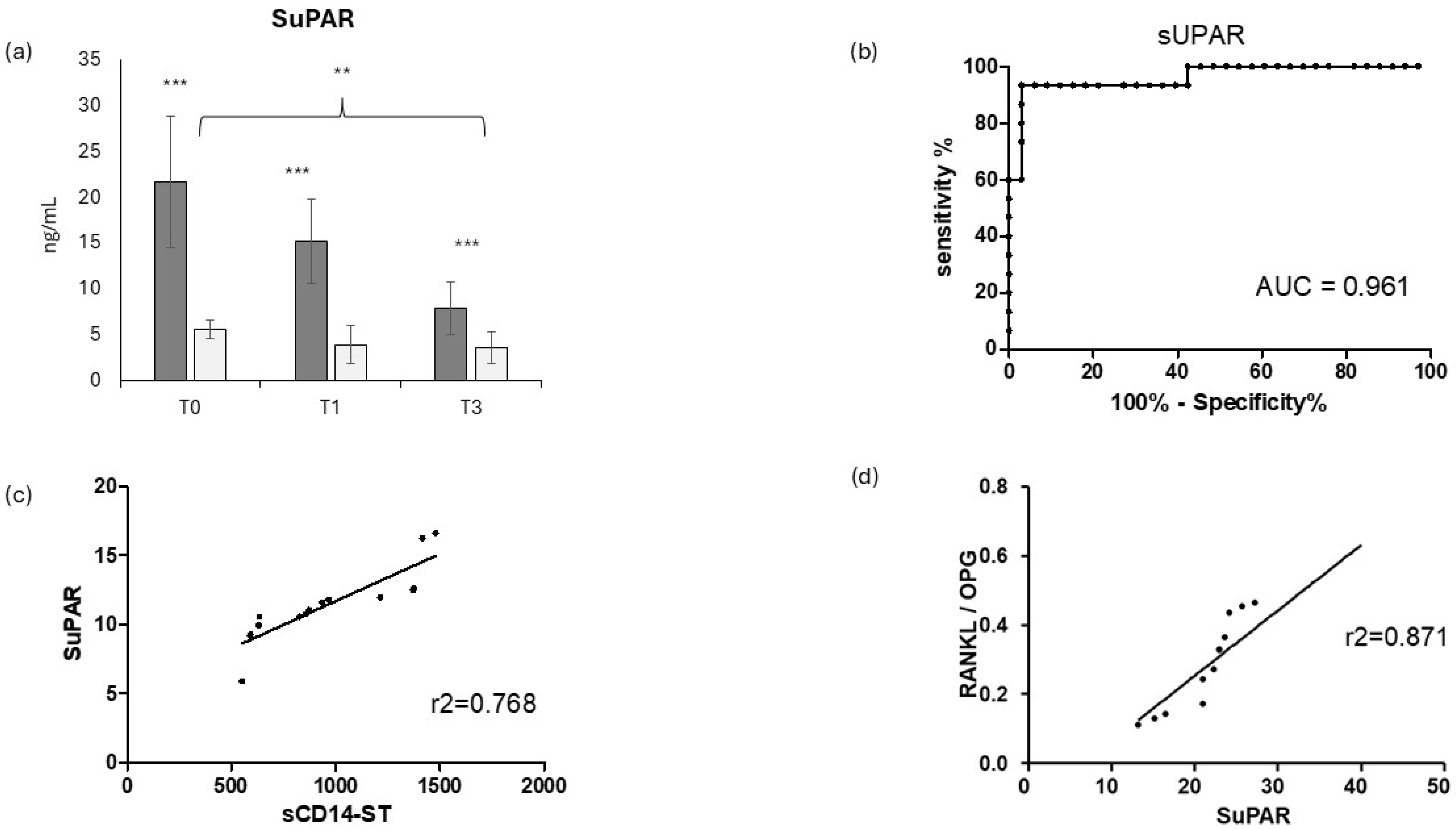
3.4. Evaluation of SuPAR in COVID-19-Positive and COVID-19-Negative Patients and Correlation with sCD14ST and Osteoimmunological Biomakers
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, T.; Liu, X.; Wei, Y.; Li, X.; Zheng, B.; Gong, Q.; Dong, L.; Zhong, J. Laboratory Predictors of COVID19 Mortality: A Retrospective Analysis from Tongji Hospital in Wuhan. Mediat. Inflamm. 2021, 2021, 6687412. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, G.; Strumia, A.; Piliego, C.; Bruno, F.; Del Buono, R.; Costa, F.; Scarlata, S.; Agrò, F.E. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef]
- Galliera, E.; Massaccesi, L.; Yu, L.; He, J.; Ranucci, M.; Romanelli, M.M.C. SCD14-ST and New Generation Inflammatory Biomarkers in the Prediction of COVID-19 Outcome. Biomolecules 2022, 12, 826. [Google Scholar] [CrossRef]
- Shirakawa, K.; Naitou, K.; Hirose, J.; Takahashi, T.; Furusako, S. Presepsin (sCD14-ST): Development and evaluation of one-step ELISA with a new standard that is similar to the form of presepsin in septic patients. Clin. Chem. Lab. Med. 2011, 49, 937–939. [Google Scholar] [CrossRef]
- Mussap, M.; Puxeddu, E.; Puddu, M.; Ottonello, G.; Coghe, F.; Comite, P.; Cibecchini, F.; Fanos, V. Soluble CD14 subtype (sCD14-ST) presepsin in premature and full term critically ill newborns with sepsis and SIRS. Clin. Chim. Acta 2015, 451, 65–70. [Google Scholar] [CrossRef]
- Okamura, Y. Usefulness of Presepsin Measurement: A New Biomarker for Sepsis. Rinsho Byori. 2015, 63, 62–71. [Google Scholar]
- Hamada, M.; Varkoly, K.S.; Riyadh, O.; Beladi, R.; Munuswamy-Ramanujam, G.; Rawls, A.; Wilson-Rawls, J.; Chen, H.; McFadden, G.; Lucas, A.R. Urokinase-Type Plasminogen Activator Receptor (uPAR) in Inflammation and Disease: A Unique Inflammatory Pathway Activator. Biomedicines 2024, 12, 1167. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Petersen, J.E.V.; Eugen-Olsen, J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front. Immunol. 2021, 12, 780641. [Google Scholar] [CrossRef]
- Louka, M.M.; Tatsi, E.B.; Vassiliu, S.; Theoharis, G.; Straka, K.M.; Filippatos, F.M.; Dourdouna, M.M.M.; Siahanidou, T.; Syriopoulou, V.; Michos, A. The Soluble Urokinase Plasminogen Activator Receptor as a Severity Biomarker in Children With Acute COVID-19 or Multisystem Inflammatory Syndrome. Pediatr. Infect. Dis. J. 2024, 43, 477–482. [Google Scholar] [CrossRef]
- Christaki, M.; Samanidou, V.; Liontos, A.; Konstantopoulou, R.; Milionis, H. Post-COVID-19 Multisystem Inflammatory Syndrome in Adults (MIS-A) With Elevated Levels of Soluble Urokinase Plasminogen Activator Receptor (suPAR) Treated With Anakinra: A Case Report. Cureus 2024, 16, e70848. [Google Scholar] [CrossRef]
- Arientová, S.; Matúšková, K.; Bartoš, O.; Beran, O.; Holub, M. Evaluation of Soluble Urokinase Plasminogen Activator Receptor in COVID-19 Patients. J. Clin. Med. 2024, 13, 6340. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Zhu, Y.Z.; Yang, L.P. Biochemical indicators of coronavirus disease 2019 exacerbation and the clinical implications. Pharmacol. Res. 2020, 159, 104946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Liu, X.Y.; Xu, W.X.; Yang, Y.P. Reevaluation of prognostic and severity indicators for COVID19 patients in the emergency department. Ann. Med. 2024, 56, 2417178. [Google Scholar] [CrossRef]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. J. Bone Jt. Surg. Am. 2020, 102, 1197–1204. [Google Scholar] [CrossRef]
- Hu, C.L.; Zheng, M.J.; He, X.X.; Liu, D.C.; Jin, Z.Q.; Xu, W.H.; Lin, P.Y.; Cheng, J.W.; Wei, Q.G. COVID19 and bone health. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3191–3200. [Google Scholar] [CrossRef]
- Ahn, S.H.; Seo, S.H.; Jung, C.Y.; Yu, D.H.; Kim, Y.; Cho, Y.; Seo, D.H.; Kim, S.H.; Yoo, J.I.; Hong, S. Clinical outcomes of COVID19 infection in patients with osteoporosis: A nationwide cohort study in Korea using the common data model. Sci. Rep. 2024, 14, 17738. [Google Scholar] [CrossRef]
- Haudenschild, A.K.; Christiansen, B.A.; Orr, S.; Ball, E.E.; Weiss, C.M.; Liu, H.; Fyhrie, D.P.; Yik, J.H.; Coffey, L.L.; Haudenschild, D.R. Acute bone loss following SARS-CoV-2 infection in mice. J. Orthop. Res. 2023, 41, 1945–1952. [Google Scholar] [CrossRef]
- Harris, A.; Creecy, A.; Awosanya, O.D.; McCune, T.; Ozanne, M.V.; Toepp, A.J.; Kacena, M.A.; Qiao, X. SARS-CoV-2 and its Multifaceted Impact on Bone Health: Mechanisms and Clinical Evidence. Curr. Osteoporos. Rep. 2024, 22, 135–145. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245. [Google Scholar] [CrossRef]
- Nakashima, T.; Takayanagi, H. Osteoimmunology: Crosstalk between the immune and bone SYSTEMS. J. Clin. Immunol. 2009, 29, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Terashima, A.; Takayanagi, H. Overview of Osteoimmunology. Calcif. Tissue Int. 2018, 102, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Wu, B.; Dong, Z.; Wang, Y.; Lu, J.; Shi, P.; Bai, W.; Wang, Z. Potential role of the OPG/RANK/RANKL axis in prostate cancer invasion and bone metastasis. Oncol. Rep. 2014, 32, 2605–2611. [Google Scholar] [CrossRef]
- Spelling, P.; Bonfa, E.; Caparbo, V.F.; Pereira, R.M.R. Osteoprotegerin/RANKL system imbalance in active polyarticular-onset juvenile idiopathic arthritis: A bone damage biomarker? Scand. J. Rheumatol. 2008, 37, 439–444. [Google Scholar] [CrossRef]
- Vega, D.; Maalouf, N.M.; Sakhaee, K. Clinical review: The role of receptor activator of nuclear factor-κB [RANK]/RANK ligand/osteoprotegerin: Clinical implications. J. Clin. Endocrinol. Metab. 2007, 92, 4514–4521. [Google Scholar] [CrossRef]
- Colombini, A.; Lombardi, G.; Galliera, E.; Dogliotti, G.; Randelli, P.; Meerssemann, A.; Mineo, G.; Cabitza, P.; Corsi, M.M. Plasma and drainage fluid levels of soluble receptor activator of nuclear factor-kB (sRANK), soluble receptor activator of nuclear factor-kB ligand (sRANKL) and osteoprotegerin (OPG) during proximal humerus fracture healing. Int. Orthop. 2011, 35, 777–782. [Google Scholar] [CrossRef]
- Canciani, E.; Dellavia, C.; Marazzi, M.G.; Augusti, D.; Carmagnola, D.; Vianello, E.; Canullo, L.; Galliera, E. RNA isolation from alveolar bone and gene expression analysis of RANK, RANKL and OPG: A new tool to monitor bone remodeling and healing in different bone substitutes used for prosthetic rehabilitation. Arch. Oral. Biol. 2017, 80, 56–61. [Google Scholar] [CrossRef]
- Galliera, E.; Massaccesi, L.; Mangiavini, L.; De Vecchi, E.; Villa, F.; Romanelli, M.M.C.; Peretti, G. Effects of COVID-19 on bone fragility: A new perspective from osteoimmunological biomarkers. Front. Immunol. 2024, 15, 1493643. [Google Scholar] [CrossRef]
- Kazemi-Sufi, S.; Alipour, S.; Rabieepour, M.; Roshan-Milani, S.; Naderi, R. Serum proinflammatory cytokines, receptor activator of nuclear factor kappa-Β ligand (RANKL), osteoprotegerin (OPG) and RANKL/OPG ratio in mild and severe COVID-19. BMC Infect. Dis. 2024, 24, 1047. [Google Scholar] [CrossRef]
- Creecy, A.; Awosanya, O.D.; Harris, A.; Qiao, X.; Ozanne, M.; Toepp, A.J.; Kacena, M.A.; McCune, T. COVID19 and Bone Loss: A Review of Risk Factors, Mechanisms, and Future Directions. Curr. Osteoporos. Rep. 2024, 22, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Galliera, E.; Massaccesi, L.; Suardi, V.; de Vecchi, E.; Villa, F.; Yi, Z.; Suo, G.; Lovati, A.B.; Logoluso, N.; Romanelli, M.M.C.; et al. sCD14-ST and Related Osteoimmunological Biomarkers: A New Diagnostic Approach to Osteomyelitis. Diagnostics 2024, 14, 1588. [Google Scholar] [CrossRef] [PubMed]
- Kindle, L.; Rothe, L.; Kriss, M.; Osdoby, P.; Collin-Osdoby, P. Human microvascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J. Bone Miner. Res. 2006, 21, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Zacarias, J.M.V.; de Alencar, J.B.; Tsuneto, P.Y.; de Souza, V.H.; Silva, C.O.; Visentainer, J.E.L.; Sell, A.M. The Influence of TLR4, CD14, OPG, and RANKL Polymorphisms in Periodontitis: A Case-Control Study. Mediat. Inflamm. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Auréal, M.; Machuca-Gayet, I.; Coury, F. Rheumatoid arthritis in the view of osteoimmunology. Biomolecules 2021, 11, 48. [Google Scholar] [CrossRef]
- Alves, C.H.; Farrell, E.; Vis, M.; Colin, E.M.; Lubberts, E. Animal Models of Bone Loss in Inflammatory Arthritis: From Cytokines in the Bench to Novel Treatments for Bone Loss in the Bedside—A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 27–47. [Google Scholar] [CrossRef]
- Geusens, P.; Lems, W.F. Osteoimmunology and osteoporosis. Arthritis Res. Ther. 2011, 13, 242. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef]
- Fukada, A.; Kitagawa, Y.; Matsuoka, M.; Sakai, J.; Imai, K.; Tarumoto, N.; Orihara, Y.; Kawamura, R.; Takeuchi, S.; Maesaki, S.; et al. Presepsin as a predictive biomarker of severity in COVID-19: A case series. J. Med. Virol. 2021, 93, 99–101. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. Presepsin value predicts the risk of developing severe/critical COVID-19 illness: Results of a pooled analysis. Clin. Chem. Lab. Med. 2021, 60, e1–e3. [Google Scholar] [CrossRef]
- Guarino, M.; Perna, B.; Maritati, M.; Remelli, F.; Trevisan, C.; Spampinato, M.D.; Costanzini, A.; Volpato, S.; Contini, C.; De Giorgio, R. Presepsin levels and COVID-19 severity: A systematic review and meta-analysis. Clin. Exp. Med. 2023, 23, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mansoor, M.; Shaikh, M.S.; Siddiqui, I. Presepsin as a Predictive Biomarker of Severity in COVID-19: A Systematic Review. Indian J. Crit. Care Med. 2021, 25, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Nica, S.; Nica, R.I.; Nica, H.A.; Miricescu, D.; Abdelfatah, M.A.A.K.; Schiopu, O.M.; Nedelcu, I.C.; Cimponeriu, D.G.; Stefani, C.; Stanescu-Spinu, I.-I.; et al. Characteristics of Patients with Persistent COVID-19 Symptoms and Unscheduled Return Visits to a Centre for COVID-19 Evaluation. Diseases 2024, 12, 199. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, A.; Gheibi Hayat, S.M.; Taghizadeh, H.; Akbari, A.; Inabadi, M.; Savardashtaki, A.; Johnston, T.P.; Sahebkar, A. COVID19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert. Rev. Anti Infect. Ther. 2021, 19, 345–357. [Google Scholar] [CrossRef]
- Younis, N.K.; Zareef, R.O.; Maktabi, M.A.N.; Mahfouz, R. The Era of the Coronavirus Disease 2019 Pandemic: A Review on Dynamics, Clinical Symptoms and Complications, Diagnosis, and Treatment. Genet. Test. Mol. Biomark. 2021, 25, 85–101. [Google Scholar] [CrossRef]
- da Silva, L.N.M.; Filho, A.G.O.; Guimarães, J.B. Musculoskeletal manifestations of COVID-19. Skelet. Radiol. 2024, 53, 2009–2022. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Shen, Y.; Qian, S.; Tang, M.; He, J.; Lu, H.; Zhang, N. Long-term effects of COVID19 infection on bone mineral density. J. Glob. Health 2024, 14, 05029. [Google Scholar] [CrossRef]
- Salvio, G.; Gianfelice, C.; Firmani, F.; Lunetti, S.; Balercia, G.; Giacchetti, G. Bone Metabolism in SARS-CoV-2 Disease: Possible Osteoimmunology and Gender Implications. Clin. Rev. Bone Miner. Metab. 2020, 18, 51–57. [Google Scholar] [CrossRef]
- Ghosh, R.; Dey, R.; Sawoo, R.; Haque, W.; Bishayi, B. Endogenous neutralization of TGF-β and IL-6 ameliorates septic arthritis by altering RANKL/OPG interaction in lymphocytes. Mol. Immunol. 2022, 152, 183–206. [Google Scholar] [CrossRef]
- Palmqvist, P.; Persson, E.; Conaway, H.H.; Lerner, U.H. IL-6, Leukemia Inhibitory Factor, and Oncostatin M Stimulate Bone Resorption and Regulate the Expression of Receptor Activator of NF-κB Ligand, Osteoprotegerin, and Receptor Activator of NF-κB in Mouse Calvariae. J. Immunol. 2002, 169, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Steeve, K.T.; Marc, P.; Sandrine, T.; Dominique, H.; Yannick, F. IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [CrossRef] [PubMed]
- López, A.G.-M. FGF23-Related Hypophosphataemic Bone Disease. Adv. Ther. 2020, 37, 25–28. [Google Scholar] [CrossRef]
- Mazess, R.B.; Bischoff-Ferrari, H.A.; Dawson-Hughes, B. Vitamin D: Bolus Is Bogus—A Narrative Review. JBMR Plus 2021, 5, e10567. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Wei, H.; Xu, X. Cytokine storm and translating IL-6 biology into effective treatments for COVID-19. Front. Med. 2023, 17, 1080–1095. [Google Scholar] [CrossRef]
- Paranga, T.G.; Mitu, I.; Pavel-Tanasa, M.; Rosu, M.F.; Miftode, I.-L.; Constantinescu, D.; Obreja, M.; Plesca, C.E.; Miftode, E. Cytokine Storm in COVID-19: Exploring IL-6 Signaling and Cytokine-Microbiome Interactions as Emerging Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 11411. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, Y.; Shi, L.; Li, L.; Zhang, D.; Gong, Z.; Wu, Q. Activation and modulation of the AGEs-RAGE axis: Implications for inflammatory pathologies and therapeutic interventions—A review. Pharmacol. Res. 2024, 206, 107282. [Google Scholar] [CrossRef]
- Saputra, G.N.R.; Yudhawati, R.; Fitriah, M. Association of soluble receptor for advanced glycation end-products (sRAGE) serum on COVID-19 severity: A cross-sectional study. Ann. Med. Surg. 2022, 74, 103303. [Google Scholar] [CrossRef]
- Lim, A.; Radujkovic, A.; Weigand, M.A.; Merle, U. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann. Intensiv. Care 2021, 11, 50. [Google Scholar] [CrossRef]
- Wang, B.; Vashishth, D. Advanced glycation and glycoxidation end products in bone. Bone 2023, 176, 116880. [Google Scholar] [CrossRef]
- Marazzi, M.G.; Randelli, F.; Brioschi, M.; Drago, L.; Romanò, C.L.; Banfi, G.; Massaccesi, L.; Crapanzano, C.; Morelli, F.; Romanelli, M.M.C.; et al. Presepsin: A potential biomarker of PJI? A comparative analysis with known and new infection biomarkers. Int. J. Immunopathol. Pharmacol. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Galliera, E.; Drago, L.; Marazzi, M.G.; Romanò, C.; Vassena, C.; Romanelli, M.M.C. Soluble urokinase-type plasminogen activator receptor (suPAR) as new biomarker of the prosthetic joint infection: Correlation with inflammatory cytokines. Clin. Chim. Acta 2015, 441, 23–28. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galliera, E.; Massaccesi, L.; Mangiavini, L.; De Vecchi, E.; Villa, F.; Corsi Romanelli, M.M.; Peretti, G.M. The Evaluation of New-Generation Biomarker sCD14ST Provides New Insight into COVID-19’s Effect on Bone Remodeling. J. Clin. Med. 2025, 14, 979. https://doi.org/10.3390/jcm14030979
Galliera E, Massaccesi L, Mangiavini L, De Vecchi E, Villa F, Corsi Romanelli MM, Peretti GM. The Evaluation of New-Generation Biomarker sCD14ST Provides New Insight into COVID-19’s Effect on Bone Remodeling. Journal of Clinical Medicine. 2025; 14(3):979. https://doi.org/10.3390/jcm14030979
Chicago/Turabian StyleGalliera, Emanuela, Luca Massaccesi, Laura Mangiavini, Elena De Vecchi, Francesca Villa, Massimiliano Marco Corsi Romanelli, and Giuseppe Maria Peretti. 2025. "The Evaluation of New-Generation Biomarker sCD14ST Provides New Insight into COVID-19’s Effect on Bone Remodeling" Journal of Clinical Medicine 14, no. 3: 979. https://doi.org/10.3390/jcm14030979
APA StyleGalliera, E., Massaccesi, L., Mangiavini, L., De Vecchi, E., Villa, F., Corsi Romanelli, M. M., & Peretti, G. M. (2025). The Evaluation of New-Generation Biomarker sCD14ST Provides New Insight into COVID-19’s Effect on Bone Remodeling. Journal of Clinical Medicine, 14(3), 979. https://doi.org/10.3390/jcm14030979








