Predictors of Cardiovascular Symptoms Among Long COVID Patients: Data from the Polish Long COVID Cardiovascular (PoLoCOV-CVD) Study
Abstract
1. Introduction
2. Materials and Methods
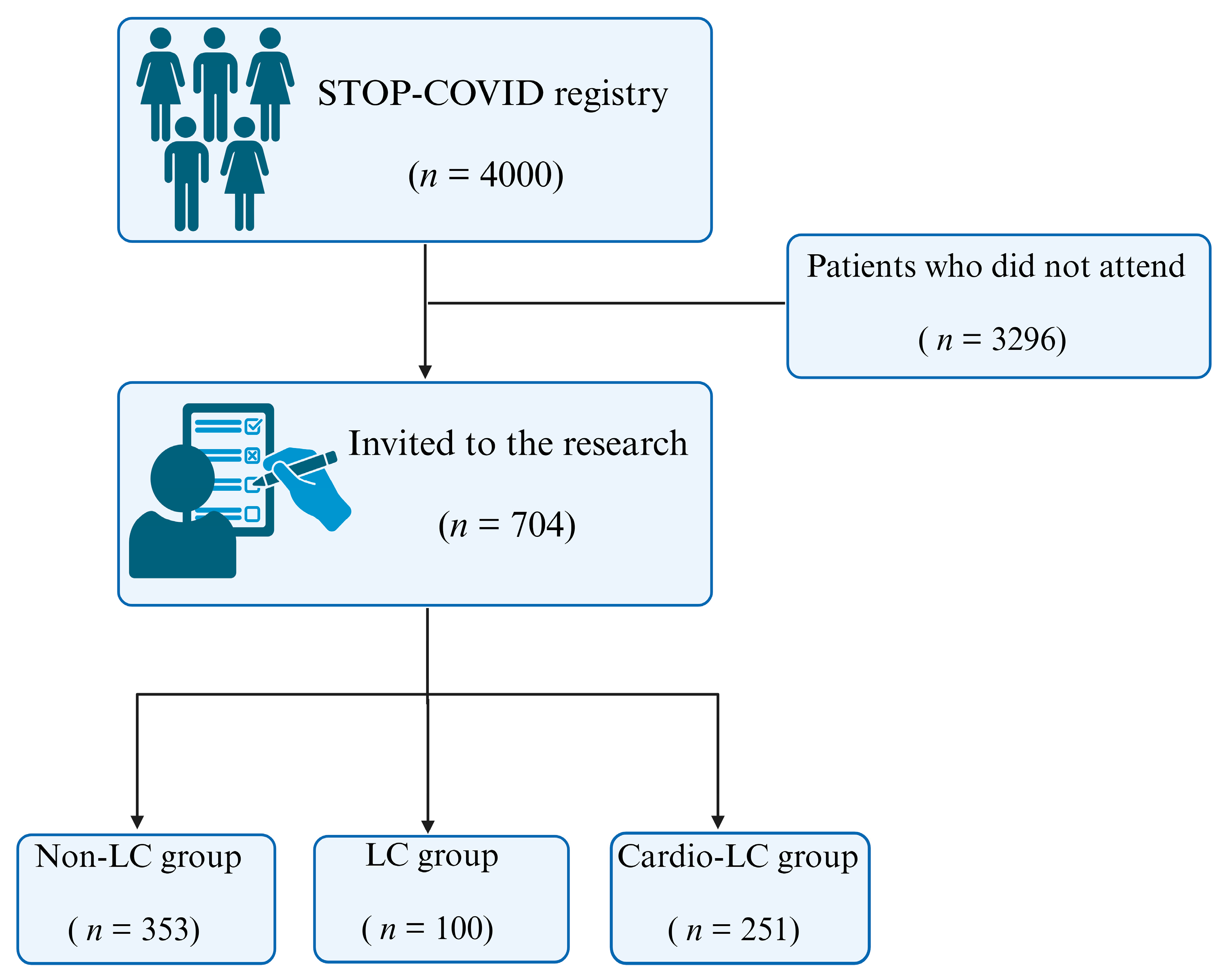
2.1. Study Population
- Non-LC group: Individuals who recovered from SARS-CoV-2 infection without experiencing any post-recovery symptoms (n = 353);
- LC group: Individuals diagnosed with Long COVID who did not exhibit cardiovascular symptoms (n = 100);
- Cardio-LC group: Individuals diagnosed with Long COVID who experienced cardiovascular symptoms (n = 251).
2.2. Study Variables
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
3.2. Comparison of Patients with and Without Cardiovascular Symptoms Among Patients with Long COVID
3.3. Comparison of Patients with Cardiovascular Symptoms and Patients Without Long-COVID
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Hekmatnia, Y.; Rahmani, F.; Feili, Z.; Ebrahimzadeh, F. A review of the effect of COVID-19 on immune responses of the body. J. Fam. Med. Prim. Care 2022, 11, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, A.; Sheikh, A.A.E.; Khan, A.M.; Khalid, S.N.; Khan, J.; Nasrullah, A.; Sagheer, S.; Sheikh, A.B. COVID-19 Vaccine and Long COVID: A Scoping Review. Life 2022, 12, 1066. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Long COVID or Post-COVID Conditions. 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 6 October 2023).
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.; Camici, G.G.; Martins, P.D.C.; Ferdinandy, P.; Fontana, M.; Girao, H.; Gnecchi, M.; et al. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: A joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc. Res. 2023, 119, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Babicki, M.; Kolat, D.; Kapusta, J.; Kaluzinska-Kolat, Z.; Jankowski, P.; Mastalerz-Migas, A.; Banach, M.; Mordaka, R.; Chudzik, M. Prevalence and assessment of risk factors among Polish adults with post-COVID syndrome: 12-month follow-up study. Pol. Arch. Intern. Med. 2023, 133, 16512. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Lewek, J.; Kapusta, J.; Banach, M.; Jankowski, P.; Bielecka-Dabrowa, A. Predictors of Long COVID in Patients without Comorbidities: Data from the Polish Long-COVID Cardiovascular (PoLoCOV-CVD) Study. J. Clin. Med. 2022, 11, 4980. [Google Scholar] [CrossRef] [PubMed]
- Plywaczewska-Jakubowska, M.; Chudzik, M.; Babicki, M.; Kapusta, J.; Jankowski, P. Lifestyle, course of COVID-19, and risk of Long-COVID in non-hospitalized patients. Front. Med. 2022, 9, 1036556. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Post COVID-19 Condition (Long COVID). 2022. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 6 October 2023).
- Parodi, J.B.; Indavere, A.; Bobadilla Jacob, P.; Toledo, G.C.; Micali, R.G.; Waisman, G.; Masson, W.; Epstein, E.D.; Huerin, M.S. Impact of COVID-19 vaccination in post-COVID cardiac complications. Vaccine 2023, 41, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Iwahashi, H.; Kozawa, J.; Okauchi, Y.; Funahashi, T.; Imagawa, A.; Shimomura, I. Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr. J. 2013, 60, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Son, D.H.; Ha, H.S.; Park, H.M.; Kim, H.Y.; Lee, Y.J. New markers in metabolic syndrome. Adv. Clin. Chem. 2022, 110, 37–71. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- SCORE2 Working Group; ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Post-COVID Conditions: Information for Healthcare Providers. 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html (accessed on 6 October 2023).
- Wang, W.; Wang, C.Y.; Wang, S.I.; Wei, J.C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine 2022, 53, 101619. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Xie, Y.; Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2023, 11, 120–128. [Google Scholar] [CrossRef]
- Alacevich, C.; Thalmann, I.; Nicodemo, C.; de Lusignan, S.; Petrou, S. Depression and anxiety during and after episodes of COVID-19 in the community. Sci. Rep. 2023, 13, 8257. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Casini, A.; Macchi, C.; Abbate, R.; Gensini, G.F. Insomnia and risk of cardiovascular disease: A meta-analysis. Eur. J. Prev. Cardiol. 2014, 21, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.; Kuper, H.; Hemingway, H. Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur. Heart J. 2006, 27, 2763–2774. [Google Scholar] [CrossRef] [PubMed]
- Allgulander, C. Anxiety as a risk factor in cardiovascular disease. Curr. Opin. Psychiatry 2016, 29, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.R.; Zaheer, A.; Fatima, S.; Saleem, T.; Sohail, A. Mental health status of COVID-19 survivors: A cross sectional study. Virol. J. 2022, 19, 3. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.G.; Chung, S.J.; Park, D.W.; Park, T.S.; Moon, J.Y.; Kim, T.H.; Sohn, J.W.; Yoon, H.J.; Kim, S.H. New-onset asthma following COVID-19 in adults. J. Allergy Clin. Immunol. Pr. 2023, 11, 2228–2231. [Google Scholar] [CrossRef] [PubMed]
- Kwok, W.C.; Tam, T.C.C.; Lam, D.C.L.; Leung, J.K.C.; Chan, K.P.F.; Chan, S.K.S.; Chiang, K.Y.; Ip, M.S.M.; Ho, J.C.M. Worsening of asthma control after recovery from mild to moderate COVID-19 in patients from Hong Kong. Respir. Res. 2023, 24, 53. [Google Scholar] [CrossRef]
- Dolby, T.; Nafilyan, V.; Morgan, A.; Kallis, C.; Sheikh, A.; Quint, J.K. Relationship between asthma and severe COVID-19: A national cohort study. Thorax 2023, 78, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Philip, K.E.J.; Buttery, S.; Williams, P.; Vijayakumar, B.; Tonkin, J.; Cumella, A.; Renwick, L.; Ogden, L.; Quint, J.K.; Johnston, S.L.; et al. Impact of COVID-19 on people with asthma: A mixed methods analysis from a UK wide survey. BMJ Open Respir. Res. 2022, 9, e001056. [Google Scholar] [CrossRef] [PubMed]
- Tedjasukmana, R.; Budikayanti, A.; Islamiyah, W.R.; Witjaksono, A.; Hakim, M. Sleep disturbance in post COVID-19 conditions: Prevalence and quality of life. Front. Neurol. 2022, 13, 1095606. [Google Scholar] [CrossRef]
- Pena-Orbea, C.; Lapin, B.; Li, Y.; Englund, K.; Heinzinger, C.; Foldvary-Schaefer, N.; Mehra, R. Sleep Disturbance Severity and Correlates in Post-acute Sequelae of COVID-19 (PASC). J. Gen. Intern. Med. 2023, 38, 2015–2017. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Wang, Y.; Li, Z.; Zhou, Y.; Wang, J.; Qu, M.; Liu, T. A network analysis of the long-term quality of life and mental distress of COVID-19 survivors 1 year after hospital discharge. Front. Public. Health 2023, 11, 1223429. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Choh, A.C.; Demerath, E.W.; Knutson, K.L.; Duren, D.L.; Sherwood, R.J.; Sun, S.S.; Chumlea, W.M.; Towne, B.; Siervogel, R.M.; et al. Sleep disturbance in relation to health-related quality of life in adults: The Fels Longitudinal Study. J. Nutr. Health Aging 2009, 13, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.F.; Hagstrom, S.; Mathiason, M.; Wente, S.; Lindquist, R. Emotional, mental health and physical symptom experience of patients hospitalized with COVID-19 up to 3 months post-hospitalization: A longitudinal study. J. Clin. Nurs. 2024, 33, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, J.W. Association of sleep disturbance with risk of cardiovascular disease and all-cause mortality in patients with new-onset type 2 diabetes: Data from the Korean NHIS-HEALS. Cardiovasc. Diabetol. 2020, 19, 61. [Google Scholar] [CrossRef] [PubMed]
| Variable | LC Group (n = 100) | Cardio-LC Group (n = 251) | p-Values | |
|---|---|---|---|---|
| Age [years] | 54 (46–64) | 53 (45–64) | 0.501 | |
| Gender | Male | 20 (20.0) | 58 (23.1) | 0.527 |
| Female | 80 (80.0) | 193 (76.9) | ||
| BMI [kg/m2] | 26.68 (24.21–29.72) | 26.77 (23.52–30.38) | 0.908 | |
| Hypertension | 38 (38.0) | 92 (36.7) | 0.814 | |
| Diabetes | 9 (9.0) | 29 (11.6) | 0.487 | |
| Hyperlipidemia | 27 (27.0) | 48 (19.1) | 0.104 | |
| Venous thromboembolism | 2 (2.0) | 0 (0.0) | 0.066 | |
| Asthma | 5 (5.0) | 31 (12.4) | 0.040 | |
| Chronic obstructive pulmonary disease | 2 (2.0) | 2 (0.8) | 0.338 | |
| Thyroid disease | 18 (18.0) | 50 (19.9) | 0.681 | |
| Any comorbidity | 33 (33.0) | 94 (37.5) | 0.434 | |
| Sleep disturbances | 81 (82.7) | 206 (82.7) | 0.986 | |
| Anxiety score | 2.0 (1.0–3.75) | 2.0 (1.0–4.0) | 0.809 | |
| Depression score | 2.0 (1.0–3.0) | 2.0 (1.0–4.0) | 0.571 | |
| Systolic 24 h [mmHg] | 124.9 (117.0–127.9) | 124.0 (113.0–124.0) | 0.265 | |
| Diastolic, 24 h [mmHg] | 74.6 (69.3–78.0) | 73.7 (68.2–77.8) | 0.519 | |
| Systolic, day [mmHg] | 129.8 (123.3–132.9) | 129.50 (118.0–133.0) | 0.354 | |
| Diastolic, day [mmHg] | 78.4 (72.7–82.1) | 78.0 (72.0–81.9) | 0.595 | |
| Systolic, night [mmHg] | 113.8 (103.9–118.0) | 113.8 (102.60–119.0) | 0.886 | |
| Diastolic, night [mmHg] | 66.0 (60.0–68.8) | 65.2 (60.0–69.0) | 0.801 | |
| Total cholesterol [mmol/L] | 11.2 (9.6–11.6) | 11.2 (10.0–12.4) | 0.062 | |
| HDL cholesterol [mmol/L] | 3.2 2.7–3.6) | 3.2 (2.8–3.5) | 0.634 | |
| LDL cholesterol [mmol/L] | 6.6 (5.3–6.9) | 6.7 (5.9–8.0) | 0.027 | |
| Triglicerides [mmol/L] | 6.4 (4.6–18.3) | 6.4 (4.5–7.3) | 0.154 | |
| Non-HDL cholesterol [mmol/L] | 7.9 (6.6–8.3) | 7.9 (6.9–9.1) | 0.127 | |
| Glucose [mmol/L] | 5.4 (5.0–5.6) | 5.3 (4.9–5.6–100.5) | 0.510 | |
| Insulin resistance [mU/mL] | 2.27 (1.41–2.90) | 2.18 (1.33–2.42) | 0.212 | |
| ESC SCORE2 | 3.9 (1.7–7.4) | 3.4 (1.2–6.5) | 0.356 | |
| ACEI | 15 (16.3) | 40 (18.3) | 0.667 | |
| ARB | 16 (17.4) | 34 (15.6) | 0.695 | |
| Beta-blocker | 34 (37.0) | 70 (32.1) | 0.409 | |
| Calcium blocker | 10 (10.9) | 27 (12.4) | 0.707 | |
| Diuretic | 13 (14.1) | 24 (11.0) | 0.439 | |
| Statin | 15 (16.3) | 25 (11.5) | 0.246 | |
| Variable | Univariable, OR (95% CI) | Multivariable, OR (95% CI) a | Multivariable, OR (95% CI) b |
|---|---|---|---|
| Age [years] | 0.99 (0.97–1.01) | 0.99 (0.97–1.01) | 0.99 (0.97–1.01) |
| Female gender | 0.83 (0.47–1.47) | 0.83 (0.47–1.47) | 0.65 (0.35–1.20) |
| BMI [kg/m2] | 1.00 (0.96–1.05) | 1.00 (0.96–1.05) | 1.02 (0.98–1.08) |
| Hypertension | 0.94 (0.58–1.52) | 1.00 (0.58–1.71) | 1.13 (0.63–2.03) |
| Diabetes | 1.32 (0.60–2.90) | 1.35 (0.61–3.00) | 1.96 (0.80–4.76) |
| Hyperlipidemia | 0.63 (0.37–1.09) | 0.66 (0.38–1.16) | 0.63 (0.35–1.12) |
| Asthma | 2.67 (1.01–7.09) | 2.71 (1.02–7.21) | 2.80 (1.04–7.53) |
| COPD | 0.39 (0.05–2.83) | 0.40 (0.05–2.98) | 0.32 (0.04–2.62) |
| Thyroid disease | 1.13 (0.62–2.05) | 1.22 (0.66–2.25) | 1.28 (0.68–2.40) |
| Any comorbidities | 1.21 (0.74–1.98) | 1.14 (0.67–1.94) | 0.79 (0.44–1.40) |
| Sleep disturbances | 1.00 (0.54–1.86) | 1.04 (0.56–1.95) | 1.25 (0.66–2.38) |
| Anxiety | 0.98 (0.86–1.11) | 0.98 (0.86–1.11) | 0.92 (0.56–1.49) |
| Depression | 1.01 (0.88–1.16) | 1.01 (0.88–1.16) | 1.34 (0.82–2.20) |
| Systolic 24 h [mmHg] | 0.99 (0.97–1.00) | 0.99 (0.97–1.01) | 0.99 (0.97–1.00) |
| Diastolic, 24 h [mmHg] | 0.99 (0.96–1.02) | 0.98 (0.96–1.01) | 0.99 (0.96–1.02) |
| Systolic, day [mmHg] | 0.99 (0.97–1.01) | 0.99 (0.98–1.01) | 0.99 (0.97–1.01) |
| Diastolic, day [mmHg] | 0.99 (0.96–1.02) | 0.99 (0.96–1.02) | 0.99 (0.96–1.02) |
| Systolic, night [mmHg] | 1.00 (0.98–1.01) | 1.00 (0.98–1.01) | 0.99 (0.98–1.01) |
| Diastolic, night [mmHg] | 1.00 (0.97–1.03) | 1.00 (0.97–1.03) | 0.99 (0.967–1.03) |
| Total cholesterol [mmol/L] | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 0.79 (0.55–1.12) |
| HDL cholesterol [mmol/L] | 1.00 (0.98–1.02) | 1.01 (0.98–1.03) | 0.79 (0.46–1.37) |
| LDL cholesterol [mmol/L] | 1.00 (0.99–1.01) | 1.006 (1.00–1.01) | 1.01 (1.00–1.01) |
| Tryglicerides [mmol/L] | 0.99 (0.99–1.00) | 0.99 (0.99–0.99) | 0.89 (0.74–1.07) |
| Non-HDL cholesterol [mmol/L] | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 0.99 (0.97–1.01) |
| Glucose [mmol/L] | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 1.00 (0.99–1.01) |
| Insulin resistance [mU/mL] | 0.88 (0.77–1.00) | 0.85 (0.74–0.99) | 0.84 (0.72–0.99) |
| ESC SCORE2 | 0.98 (0.94–1.03) | 1.01 (0.93–1.09) | 1.02 (0.94–1.11) |
| ACEI | 1.15 (0.60–2.21) | 1.21 (0.61–2.40) | 1.14 (0.57–2.29) |
| ARB | 0.87 (0.45–1.68) | 0.90 (0.46–1.77) | 0.94 (0.46–1.92) |
| Beta-blocker | 0.80 (0.48–1.34) | 0.82 (0.48–1.39) | 0.89 (0.51–1.56) |
| Calcium blocker | 1.15 (0.53–2.50) | 1.22 (0.55–2.72) | 1.27 (0.57–2.85) |
| Diuretics | 0.75 (0.36–1.55) | 0.76 (0.36–1.59) | 0.79 (0.36–1.71) |
| Statin | 0.66 (0.33–1.32) | 0.66 (0.32–1.36) | 0.73 (0.35–1.52) |
| Variables | Non-LC Group (n = 353) | Cardio-LC Group (n = 251) | p-Values | |
|---|---|---|---|---|
| Age [years] | 54 (44–65) | 53 (45–64) | 0.850 | |
| Gender | Male | 120 (34.0) | 58 (23.1) | 0.004 |
| Female | 233 (66.0) | 193 (76.9) | ||
| BMI [kg/m2] | 27.2 (24.0–30.5) | 26.8 (23.5–30.4) | 0.465 | |
| Hypertension | 150 (42.5) | 92 (36.7) | 0.149 | |
| Diabetes | 34 (9.6) | 29 (11.6) | 0.446 | |
| Hyperlipidemia | 77 (21.8) | 48 (19.1) | 0.421 | |
| Venous thromboembolism | 1 (0.3) | 0 (0.0) | 0.347 | |
| Asthma | 42 (11.9) | 31 (12.4) | 0.866 | |
| Chronic obstructive pulmonary disease | 5 (1.4) | 2 (0.8) | 0.483 | |
| Thyroid disease | 61 (17.3) | 50 (19.9) | 0.409 | |
| Any comorbidity | 244 (69.1) | 157 (62.5) | 0.092 | |
| Sleep disturbances | 238 (67.6) | 206 (82.7) | <0.001 | |
| Anxiety score | 2.0 (0.0–3.0) | 2.0 (1.0–4.0) | <0.001 | |
| Depression score | 2.0 (0.0–3.0) | 2.0 (1.0–4.0) | <0.001 | |
| Systolic 24 h [mmHg] | 124.93 (117.0–132.85) | 124.0 (113.0–128.0) | 0.005 | |
| Diastolic, 24 h [mmHg] | 74.56 (70.40–80.90) | 73.70 (68.20–77.80) | 0.001 | |
| Systolic, day [mmHg] | 129.76 (123.00–139.20) | 129.50 (118.0–133.0) | 0.007 | |
| Diastolic, day [mmHg] | 78.40 (74.80–84.05) | 78.0 (72.0–81.90) | 0.002 | |
| Systolic, night [mmHg] | 113.78 (106.0–121.95) | 113.78 (102.60–119.0) | 0.028 | |
| Diastolic, night [mmHg] | 65.96 (61.95–71.55) | 65.20 (60.0–69.0) | 0.008 | |
| Total cholesterol [mmol/L] | 11.17 (9.77–12.0) | 11.17 (9.99–12.43) | 0.043 | |
| HDL cholesterol [mmol/L] | 3.22 (2.83–3.66) | 3.22 (2.83–3.55) | 0.478 | |
| LDL cholesterol [mmol/L] | 6.7 (5.3–7.3) | 6.7 (5.8–8.0) | 0.010 | |
| Triglicerides [mmol/L] | 6.2 (4.2–6.9) | 6.4 (4.4–7.3) | 0.227 | |
| Non-HDL cholesterol [mmol/L] | 7.9 (6.5–8.3) | 7.9 (6.9–9.2) | 0.013 | |
| Insulin resistance [mU/mL] | 2.1 (1.2–2.3) | 2.2 (1.3–2.4) | 0.175 | |
| Glucose [mmol/L] | 5.6 (5.1–5.8) | 5.3 (4.9–5.6) | 0.001 | |
| ESC SCORE2 | 4.0 (1.4–8.7) | 3.4 (1.2–6.5) | 0.135 | |
| ACEI | 44 (14.1) | 40 (18.3) | 0.193 | |
| ARB | 44 (14.1) | 34 (15.6) | 0.644 | |
| Beta-blocker | 101 (32.5) | 70 (32.1) | 0.929 | |
| Calcium blocker | 31 (10.0) | 27 (12.4) | 0.381 | |
| Diuretic | 45 (14.5) | 24 (11.0) | 0.245 | |
| Statin | 40 (12.9) | 25 (11.5) | 0.631 | |
| Variables | Univariable, OR (95% CI) | Multivariable, OR (95% CI) a | Multivariable, OR (95% CI) b |
|---|---|---|---|
| Age [years] | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.99 (0.97–1.01) |
| Female | 1.71 (1.18–2.47) | 1.71 (1.18–2.47) | 1.66 (1.12–2.46) |
| BMI [kg/m2] | 0.99 (0.96–1.02) | 1.00 (0.97–1.03) | 1.00 (0.97–1.03) |
| Hypertension | 0.78 (0.56–1.09) | 0.84 (0.58–1.22) | 0.76 (0.49–1.17) |
| Diabetes | 1.22 (0.72–2.07) | 1.29 (0.75–2.22) | 1.40 (0.78–2.52) |
| Hyperlipidemia | 0.84 (0.56–1.26) | 0.83 (0.55–1.25) | 0.73 (0.47–1.11) |
| Asthma | 1.04 (0.63–1.71) | 1.02 (0.62–1.68) | 0.99 (0.59–1.65) |
| Chronic obstructive pulmonary disease | 0.55 (0.10–2.90) | 0.72 (0.13–3.85) | 0.89 (0.16–5.02) |
| Thyroid disease | 1.19 (0.78–1.80) | 1.05 (0.68–1.61) | 1.06 (0.68–1.65) |
| Any comorbidities | 0.74 (0.53–1.05) | 0.74 (0.51–1.06) | 0.68 (0.46–1.01) |
| Sleep disturbances | 2.29 (1.54–3.41) | 2.18 (1.46–3.26) | 1.87 (1.23–2.85) |
| Anxiety | 1.18 (1.08–1.30) | 1.17 (1.06–1.28) | 1.21 (0.80–1.83) |
| Depression | 1.20 (1.09–1.32) | 1.18 (1.07–1.30) | 1.30 (0.86–1.95) |
| Systolic 24 h [mmHg] | 0.98 (0.97–0.99) | 0.98 (0.97–1.00) | 0.99 (0.97–0.99) |
| Diastolic, 24 h [mmHg] | 0.96 (0.94–0.98) | 0.96 (0.94–0.99) | 0.97 (0.95–0.99) |
| Systolic, day [mmHg] | 0.98 (0.97–1.00) | 0.99 (0.98–1.00) | 0.99 (0.98–1.02) |
| Diastolic, day [mmHg] | 0.96 (0.94–0.98) | 0.97 (0.95–0.99) | 0.97 (0.95–0.99) |
| Systolic, night [mmHg] | 0.98 (0.97–1.00) | 0.99 (0.98–1.00) | 0.99 (0.98–1.01) |
| Diastolic, night [mmHg] | 0.97 (0.95–0.99) | 0.97 (0.95–0.99) | 0.98 (0.96–1.00) |
| Total cholesterol [mmol/L] | 1.08 (1.00–1.168) | 1.08 (1.00–1.17) | 1.00 (1.00–1.01) |
| HDL cholesterol [mmol/L] | 0.87 (0.68–1.11) | 0.73 (0.56–0.96) | 0.98 (0.96–0.99) |
| LDL cholesterol [mmol/L] | 1.12 (1.03–1.22) | 1.13 (1.04–1.23) | 1.12 (1.02–1.22) |
| Triglicerides [mmol/L] | 1.01 (0.96–1.07) | 1.03 (0.98–1.09) | 1.00 (0.99–1.01) |
| Non-HDL cholesterol [mmol/L] | 1.11 (1.02–1.20) | 1.11 (1.03–1.21) | 1.01 (1.00–1.01) |
| Insulin resistance [mU/mL] | 1.03 (0.92–1.16) | 1.090 (0.96–1.23) | 1.09 (0.96–1.25) |
| Glucose [mmol/L] | 0.99 (0.98–1.00) | 0.94 (0.800–1.11) | 0.93 (0.78–1.10) |
| ESC SCORE2 | 0.97 (0.94–1.00) | 0.97 (0.92–1.01) | 0.97 (0.92–1.02) |
| ACEI | 1.36 (0.85–2.17) | 1.65 (1.01–2.71) | 1.60 (0.97–2.64) |
| ARB | 1.12 (0.69–1.82) | 1.18 (0.71–1.94) | 1.37 (0.81–2.32) |
| Beta-blocker | 0.98 (0.67–1.42) | 1.05 (0.71–1.54) | 0.96 (0.64–1.43) |
| Calcium blocker | 1.27 (0.73–2.20) | 1.41 (0.80–2.49) | 1.20 (0.65–2.22) |
| Diuretics | 0.73 (0.43–1.24) | 0.80 (0.46–1.37) | 0.79 (0.45–1.41) |
| Statin | 0.87 (0.51–1.49) | 0.97 (0.56–1.69) | 1.14 (0.63–2.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapusta, J.; Sinnadurai, S.; Babicki, M.; Kałuzińska-Kołat, Ż.; Meijers, W.C.; Kołat, D.; Manintveld, O.C.; Jankowski, P.; Chudzik, M. Predictors of Cardiovascular Symptoms Among Long COVID Patients: Data from the Polish Long COVID Cardiovascular (PoLoCOV-CVD) Study. J. Clin. Med. 2025, 14, 956. https://doi.org/10.3390/jcm14030956
Kapusta J, Sinnadurai S, Babicki M, Kałuzińska-Kołat Ż, Meijers WC, Kołat D, Manintveld OC, Jankowski P, Chudzik M. Predictors of Cardiovascular Symptoms Among Long COVID Patients: Data from the Polish Long COVID Cardiovascular (PoLoCOV-CVD) Study. Journal of Clinical Medicine. 2025; 14(3):956. https://doi.org/10.3390/jcm14030956
Chicago/Turabian StyleKapusta, Joanna, Siamala Sinnadurai, Mateusz Babicki, Żaneta Kałuzińska-Kołat, Wouter C. Meijers, Damian Kołat, Olivier C. Manintveld, Piotr Jankowski, and Michał Chudzik. 2025. "Predictors of Cardiovascular Symptoms Among Long COVID Patients: Data from the Polish Long COVID Cardiovascular (PoLoCOV-CVD) Study" Journal of Clinical Medicine 14, no. 3: 956. https://doi.org/10.3390/jcm14030956
APA StyleKapusta, J., Sinnadurai, S., Babicki, M., Kałuzińska-Kołat, Ż., Meijers, W. C., Kołat, D., Manintveld, O. C., Jankowski, P., & Chudzik, M. (2025). Predictors of Cardiovascular Symptoms Among Long COVID Patients: Data from the Polish Long COVID Cardiovascular (PoLoCOV-CVD) Study. Journal of Clinical Medicine, 14(3), 956. https://doi.org/10.3390/jcm14030956










